Introduction
In developed countries, where the aging population
is on the increase, cancer is a major health concern, in terms of
public welfare and preventive measures, with a cancer-related to
overall mortality ratio of 1:4 in the United States (1). Colorectal cancer (CRC) is one of the
most common malignancies and among the leading causes of
cancer-related mortality. Approximately 1 in 5 patients with CRC
present with distant metastatic disease at diagnosis and the
distant metastases, such as to the liver or lung, are the major
cause of death. A significant proportion of patients with
metastatic CRC are not curable; however, a subset of these patients
with liver- and/or lung-isolated disease is potentially curable
with surgery (2–4).
When treating metastatic CRC, systemic chemotherapy
is the standard approach. Over the last decade, there has been
significant progress in CRC treatment strategies. Compared to the
era when 5-fluorouracil (5-FU) was the only efficient drug against
CRC, the median survival duration has increased over the last few
years, mainly due to the availability of novel agents, such as
irinotecan and oxaliplatin, along with cetuximab and bevacizumab
(5–7).
Although several new drugs are currently used for metastatic CRC,
it is difficult to change the standard treatment with surgical
resection.
The role of synchronous curative resection for CRC
with lung and/or liver metastases is limited. Previous studies on
these treatments were retrospective and included small sample sizes
with short-term follow-up periods (3,4).
Therefore, it is difficult to determine the benefits of curative
resection for primary and metastatic lesions concurrently. In this
study, the treatment outcome of curative resection combined with
standard chemotherapy was evaluated in patients with liver and/or
lung metastatic CRC. Furthermore, the clinical predictive factors
determining the benefits of curative resection for synchronous
metastases were identified.
Materials and methods
Patient characteristics
A total of 103 patients who were diagnosed with
stage IV CRC with liver and/or lung metastases at the Osaka Medical
Center for Cancer and Cardiovascular Diseases between 1983 and 2010
were investigated (Table I). All the
patients had histologically confirmed CRC with distant metastasis
and underwent curative resection for the primary and metastatic
lesions. The surgical specimens were fixed in formalin, processed
through graded ethanols and embedded in paraffin blocks. The
histological sections were stained with hematoxylin and eosin and
elastica-van Gieson's stain and the degree of histological
differentiation, lymphatic invasion and venous invasion were
assessed. Data on patient age and gender, primary tumour site
(rectum or colon), distant metastatic site (liver and/or lung),
pathological stage (histological grade, tumour invasiveness, lymph
node metastases, lymphatic invasion and venous invasion) and
perioperative chemotherapy were retrieved from patient medical
records and retrospectively evaluated.
 | Table I.Clinicopathological factors in
metastatic colorectal cancer patients (n=103). |
Table I.
Clinicopathological factors in
metastatic colorectal cancer patients (n=103).
| Factors | Patient no. (%) |
|---|
| Age, years
(range) | 61 (20–81) |
| Gender |
| Male | 61 (59.2) |
|
Female | 42 (40.8) |
| Primary tumour
location |
|
Rectum | 36 (35.0) |
|
Rectosigmoid | 7 |
|
Upper rectum | 17 |
|
Lower rectum | 11 |
|
Anal region | 1 |
| Colon | 67 (65.0) |
|
Cecum | 6 |
|
Ascending | 15 |
|
Transverse | 8 |
|
Descending | 4 |
|
Sigmoid | 33 |
| N/A | 1 |
| Histological
grade |
| Well
differentiated Ad | 27 (26.2) |
|
Moderately differentiated
Ad | 70 (67.9) |
|
Othersa | 4 (3.9) |
| N/A | 2 (2.0) |
| Tumour invasion |
| T3 | 67 (65.0) |
| T4a | 27 (26.2) |
| T4b | 5 (4.8) |
| N/A | 4 (4.0) |
| Lymph node
metastasis |
| N0 | 31 (30.1) |
| N1 | 34 (33.0) |
| N2a | 27 (26.2) |
| N2b | 9 (8.8) |
| N/A | 2 (1.9) |
| Lymphatic
invasion |
|
Absent | 87 (84.5) |
|
Present | 11 (10.7) |
| N/A | 5 (4.8) |
| Venous invasion |
|
Absent | 89 (86.4) |
|
Present | 9 (8.8) |
| N/A | 5 (4.8) |
| Metastases |
| Liver
(n=90) |
|
Solitary | 42 (40.7) |
|
≥2 | 48 (46.6) |
| Lung (n=20) |
|
Solitary | 12 (11.6) |
| ≥2 | 8 (7.8) |
| Synchronous liver and
lung (n=7) |
Preoperative evaluation
Preoperatively, the extent of tumour spread was
determined by using modalities such as X-ray, computed tomography
(CT), magnetic resonance imaging and/or positron emission
tomography (PET). The intraoperative findings contributed to the
determination of metastatic tumour spread. Following surgery, all
the patients underwent follow-up blood tests measuring the serum
carcinoembryonic antigen (CEA) levels and imaging examinations,
such as abdominal ultrasonography, CT, chest X-ray and/or PET every
3–6 months. In this study, the time-to-recurrence after the first
synchronous curative resection for primary and metastatic lesions
was also evaluated during the postoperative follow-up and is
referred to as ‘recurrence interval’.
Adjuvant therapy
Postoperatively, a proportion of the patients
received chemotherapy following provision of written informed
consent. The adjuvant therapies were administered according to the
the guidelines of the Japanese Society for Cancer of the Colon and
Rectum (8) and included mFOLFOX6
(oxaliplatin 85 mg/m2 and 5-fluorouracil 2,800
mg/m2 per 2 weeks × 12 courses), tegafur + uracil (UFT;
300 mg/m2/day × 28 days per 5 weeks × 5 courses),
capecitabine (2,500 mg/m2/day × 14 days per 3 weeks × 8
courses), or S-1 (80 mg/m2/day × 28 days per 6 weeks × 4
courses). The clinicopathological factors were assessed according
to the tumour node metastasis (TNM) classification of the
International Union Against Cancer (9).
Statistical analysis
Data were analyzed with the Pearson's Chi-square
test or the Fisher's exact test. The Mann-Whitney U test was used
for comparison between different groups. Kaplan-Meier survival
curves were plotted and compared with the generalized log-rank
test. Univariate and multivariate analyses were performed using a
Cox regression model for overall survival (OS) and disease-free
survival (DFS) following final curative resection, to identify
independent factors. Two-sided P-values of <0.05 were considered
to indicate statistically significant differences. All the tests
were analyzed using JMP software, version 11.0 (SAS Institute,
Cary, NC, USA).
This study was designed in accordance with the
Institutional Ethical Guidelines and received approval from the
Ethics Committee of the Osaka Medical Center for Cancer and
Cardiovascular Diseases.
Results
Patient characteristics
The patient characteristics are summarized in
Table I. The median patient age was
61 years (range, 20–81 years) and 61 patients (59.2%) were male.
The primary tumours were located in the rectum (36 patients,
35.0%), or the colon (67 patients, 65.0%). The most common site of
metastases at presentation was the liver (90 patients, 87.3%),
followed by the lung (20 patients, 19.4%). The median number of
liver or lung metastatic sites was 2 (range, 1–7) and 1 (range,
1–3), respectively.
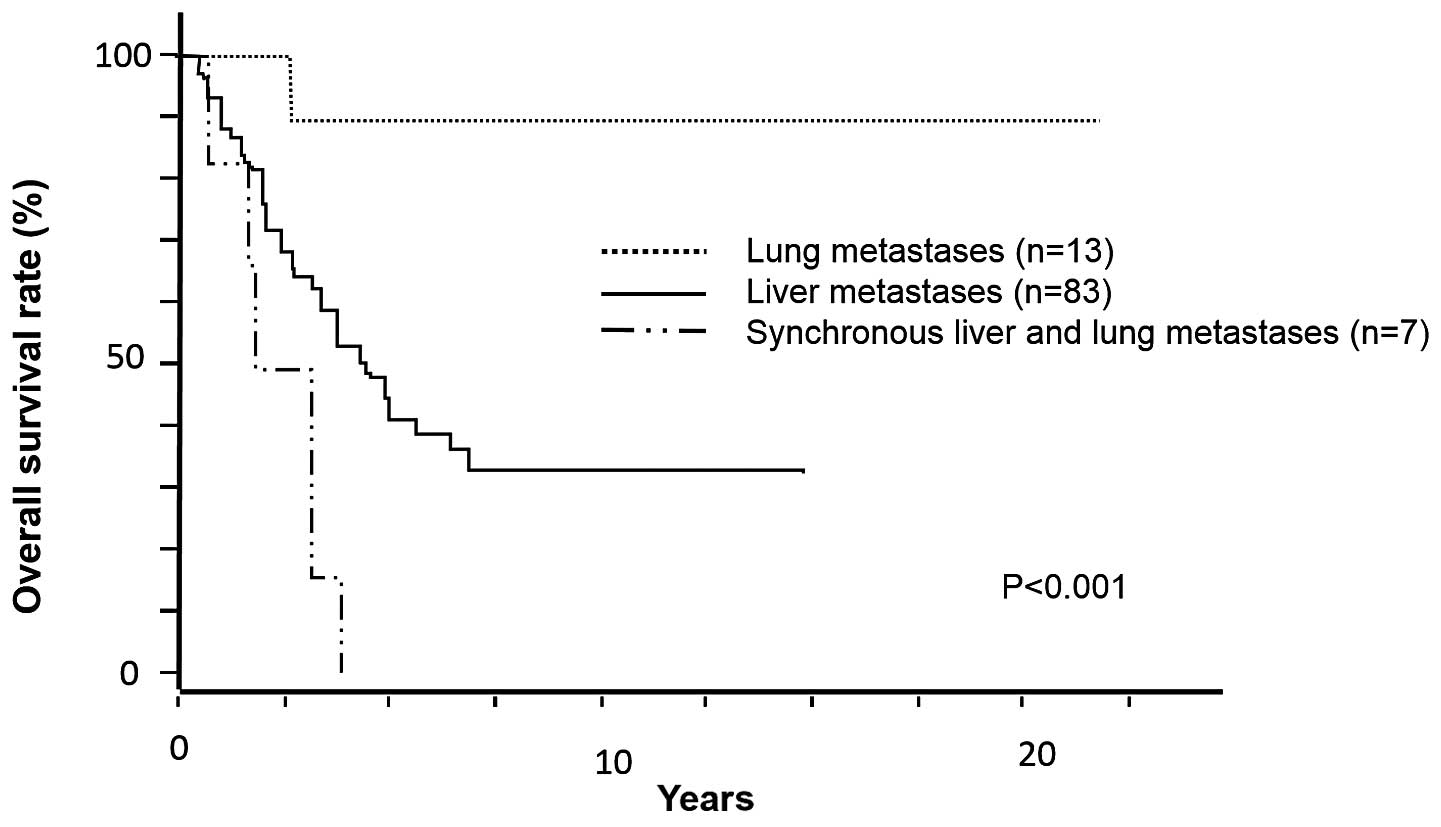
Survival analysis
The median OS in the entire study population was
4.60 years. The cohort of 103 patients underwent curative resection
of primary and metastatic lesions. Curative resection of the liver
or lung was performed in 90 and 20 patients, respectively, whereas
liver and lung resections were concurrently performed in 7
patients. The median OS was 20.7 months in this population
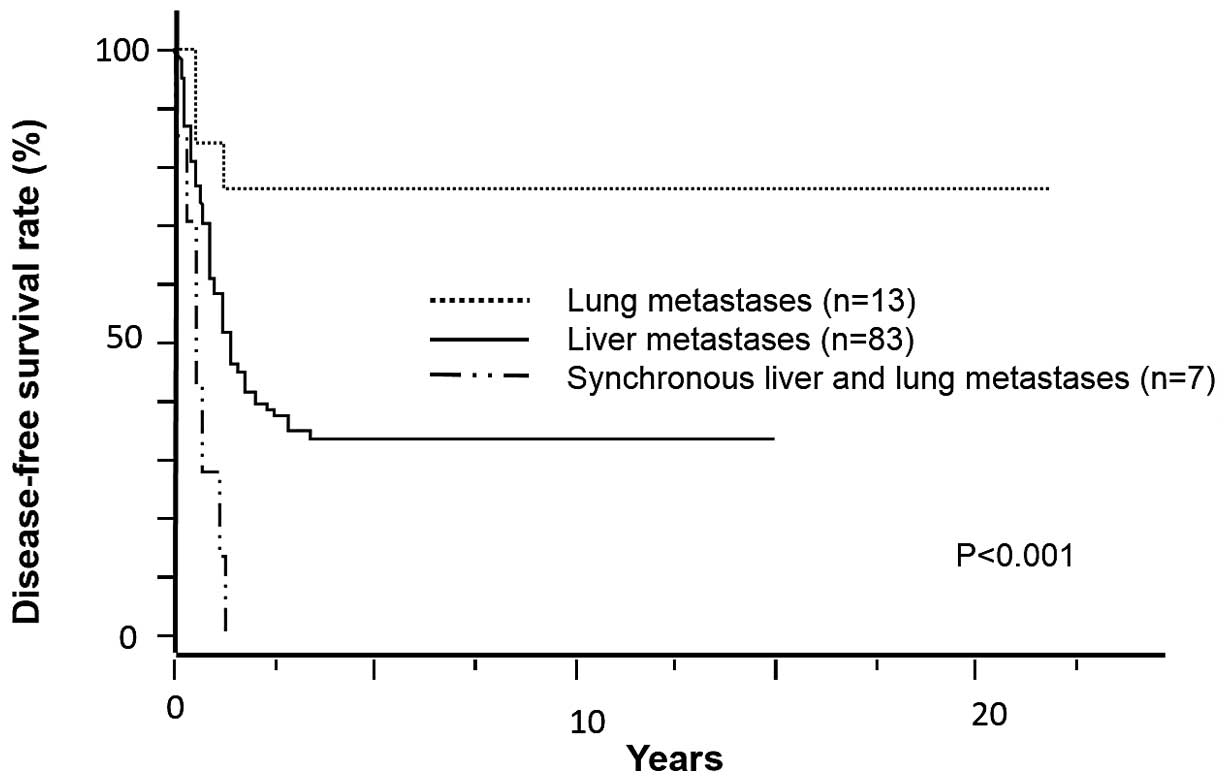
(Fig. 1). Following curative
resection, 25 patients (24.2%) had no recurrence [median DFS, 5.69
years (range, 1.20–21.73 years)], whereas 14 patients (13.5%)
received more than one re-resection for disease recurrence and
survived without any further recurrence thereafter [median DFS,
5.53 years (range, 2.30–11.89 years)]. The DFS curves of liver,
lung and synchronous liver and lung metastatic CRC are plotted in
Fig. 2.
Treatment outcome
Following curative resection, the patients exhibited
several recurrences such as in the liver, lung, bone, brain and
distant lymph nodes (Table II). In
cases with liver or lung metastatic CRC, 17 and 8 patients,
respectively, underwent curative resection of primary and
metastatic lesions without any recurrence. However, in cases with
synchronous liver and lung metastatic CRC, all the patients
developed re-recurrence following curative resection and the median
DFS of this population was 5.46 months.
 | Table II.Clinical results of liver and/or lung
metastases in colorectal cancer (CRC) patients (n=103). |
Table II.
Clinical results of liver and/or lung
metastases in colorectal cancer (CRC) patients (n=103).
| Factors | Liver, no. (%)
(n=83) | Lung, no. (%)
(n=13) | Liver and lung, no.
(%) (n=7) | P-value |
|---|
| CEA (ng/ml) | 17.4
(1.0–2,540.0) | 3.4 (1.0–20.0) | 3.5 (2.4–4.0) |
<0.001a |
| Primary CRC
location |
|
Rectum | 27 (32.5) | 8 (61.5) | 2 (28.6) |
0.304 |
|
Colon | 56 (67.5) | 5 (38.5) | 5 (71.4) |
| Recurrence following
curative resection |
| None | 17 (20.4) | 8 (61.5) | 0 (0.0) | N/A |
|
Liver | 47 (56.6) | 1 (7.7) | 7 (100.0) | N/A |
| Lung | 32 (38.6) | 3 (23.1) | 7 (100.0) | N/A |
|
Bones | 11 (13.2) | 0 (0.0) | 2 (28.6) | N/A |
|
Brain | 3 (3.6) | 0 (0.0) | 2 (28.6) | N/A |
| Distant
lymph nodes | 10 (12.0) | 2 (15.4) | 4 (57.1) | N/A |
|
Othersb | 5 (6.0) | 1 (7.7) | 1 (14.2) | N/A |
Chemotherapy
Of the 103 patients, 81 (78.6%) received adjuvant
chemotherapy following curative resection. Oxaliplatin and
irinotecan were used in 22 (27.1%) and 15 patients (18.5%),
respectively. 5-FU, capecitabine or UFT were administered to 77
patients (74.7%). Finally, 8 patients (7.7%) received bevacizumab.
In our study, the selection of chemotherapy was not found to be
significantly associated with patient outcome (Table III).
 | Table III.Univariate analysis for overall
survival with chemotherapy following curative resection (Cox
proportional hazards regression model). |
Table III.
Univariate analysis for overall
survival with chemotherapy following curative resection (Cox
proportional hazards regression model).
|
| Univariate
analysis |
|---|
|
|
|
|---|
| Chemotherapy | HR | 95% CI | P-value |
|---|
| Oxaliplatin | 0.96 | 0.47–1.89 | 0.919 |
| Irinotecan | 1.92 | 0.95–3.68 | 0.066 |
|
5-FU-baseda | 0.91 | 0.50–1.78 | 0.783 |
| Bevacizumab | 0.65 | 0.15–1.78 | 0.446 |
Factors associated with DFS
The univariate and multivariate analyses of factors
associated with DFS are presented in Table IV. In the univariate analysis, tumour
invasion [hazard ratio (HR)=1.70, 95% confidence interval (CI):
1.01–2.81, P=0.045], synchronous liver and lung metastases
(HR=4.11, 95% CI=1.67–8.70, P=0.003) and recurrence interval after
the first curative resection (HR=6.09, 95% CI: 3.51–11.06,
P<0.001) were significantly correlated with DFS. In the
multivariate analysis, tumour invasion (HR=2.20, 95% CI: 1.27–3.78,
P=0.005), synchronous liver and lung metastases (HR=3.69, 95% CI:
1.44–8.33, P=0.008) and recurrence interval after the first
curative resection (HR=5.65, 95% CI: 3.20–10.41, P<0.001) were
found to be independent predictors of DFS.
 | Table IV.Univariate and multivariate analyses
of factors associated with DFS (Cox proportional hazards regression
model). |
Table IV.
Univariate and multivariate analyses
of factors associated with DFS (Cox proportional hazards regression
model).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | 1.18 | 0.72–1.93 | 0.502 |
|
|
|
| (<61
vs. ≥62) |
| Gender | 1.09 | 0.66–1.82 | 0.727 |
|
|
|
| (Male
vs. female) |
| Primary CRC
location | 1.66 | 0.79–3.13 | 0.163 |
|
|
|
| (Lower
rectum and anus vs. others) |
| Histological
grade | 3.95 | 0.94–11.10 | 0.057 |
|
|
|
|
(Well-modb vs. othersc) |
| CEA, ng/ml | 0.98 | 0.58–1.70 | 0.951 |
|
|
|
| (≥5 vs.
<5) |
| Tumour
invasion | 1.70 | 1.01–2.81 | 0.045 | 2.20 | 1.27–3.78 | 0.005a |
| (T4a-b
vs. T3) |
| Lymph node
metastasis | 1.39 | 0.81–2.49 | 0.232 |
|
|
|
| (N1–2
vs. N0) |
| Lymphatic
invasion | 0.62 | 0.32–1.36 | 0.224 |
|
|
|
|
(Present vs. absent) |
| Venous
invasion | 1.18 | 0.55–3.08 | 0.682 |
|
|
|
|
(Present vs. absent) |
| Metastases | 4.11 | 1.67–8.70 | 0.003a | 3.69 | 1.44–8.33 | 0.008a |
| (Liver
or lung vs. synchronous) |
| Recurrence interval
after the first operationd | 6.09 | 3.51–11.06 |
<0.001a | 5.65 | 3.20–10.41 |
<0.001a |
| (<1
year vs. ≥1 year) |
Factors associated with OS
The univariate and multivariate analyses of factors
associated with OS are presented in Table
V. In the univariate analysis, primary CRC location (HR=3.09,
95% CI: 1.44–6.03, P=0.005), tumour invasion (HR=1.56, 95% CI:
1.01–2.38, P=0.045), synchronous liver and lung metastases
(HR=2.99, 95% CI: 1.23–6.18, P=0.018) and recurrence interval after
the first curative resection (HR=2.69, 95% CI: 1.78–4.11,
P<0.001) were significantly correlated with OS. In the
multivariate analysis, primary CRC location (HR=4.45, 95% CI:
1.91–9.54, P=0.001), tumour invasion (HR=5.99, 95% CI: 3.06–12.34,
P<0.001), synchronous liver and lung metastases (HR=4.03, 95%
CI: 1.44–9.77, P=0.010) and recurrence interval after the first
curative resection (HR=7.95, 95% CI: 3.97–17.14, P<0.001) were
found to be independent predictors of OS.
 | Table V.Univariate and multivariate analyses
of factors associated with OS (Cox proportional hazards regression
model). |
Table V.
Univariate and multivariate analyses
of factors associated with OS (Cox proportional hazards regression
model).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | 1.10 | 0.64–1.90 | 0.707 |
|
|
|
| (<61
vs. ≥62) |
| Gender | 1.42 | 0.81–2.56 | 0.217 |
|
|
|
| (Male
vs. female) |
| Primary CRC
location | 3.09 | 1.44–6.03 | 0.005a | 4.45 | 1.91–9.54 | 0.001a |
| (Lower
rectum and anus vs. others) |
| Histological
grade | 3.18 | 0.77–8.62 | 0.097 |
|
|
|
|
(Well-modb vs. othersc) |
| CEA, ng/ml | 1.38 | 0.90–2.15 | 0.132 |
|
|
|
| (≥5 vs.
<5) |
| Tumour
invasion | 1.56 | 1.01–2.38 | 0.045a | 5.99 | 3.06–12.34 |
<0.001a |
| (T4a-b
vs. T3) |
| Lymph node
metastasis | 1.09 | 0.68–1.61 | 0.860 |
|
|
|
| (N1–2
vs. N0) |
| Lymphatic
invasion | 0.66 | 0.36–1.32 | 0.231 |
|
|
|
|
(Present vs. absent) |
| Venous
invasion | 1.18 | 0.62–2.54 | 0.623 |
|
|
|
|
(Present vs. absent) |
| Metastases | 2.99 | 1.23–6.18 | 0.018a | 4.03 | 1.44–9.77 | 0.010a |
| (Liver
or lung vs. synchronous) |
| Recurrence interval
after the first operationd | 2.69 | 1.78–4.11 |
<0.001a | 7.95 | 3.97–17.14 |
<0.001a |
| (<1
vs. ≥1 year) |
Discussion
CRC patients with isolated liver or lung metastasis
may achieve long-term survival with concurrent curative resection
of the primary and metastatic lesions.
It may be useful to determine the necessity of
intensive follow-up and selective adjuvant therapy for CRC patients
by predicting recurrence and metastases following curative surgical
resection (10,11). In the present study, the
clinicopathological analysis revealed that CRC patients with T4a-4b
disease had a poorer prognosis regarding DFS and OS compared to
those with T3 disease. Additionally, CRC with synchronous liver and
lung metastases was associated with a poorer prognosis compared to
CRC with isolated liver or lung metastasis. The data indicated that
tumour invasiveness and metastatic status (liver and lung) are
independent prognostic factors. As regards OS, primary cancer
location at the lower rectum and anal region was associated with
worse prognosis. It was previously reported that rectal cancer
exhibits early recurrence, resulting in poor prognosis (12). Unlike previous studies indicating
several prognostic factors, such as lymph node metastasis and
vascular invasion, these factors were not found to be
prognostically significant, as all the patients in our study had
stage IV disease (13,14). In the clinical setting, it must be
decided whether re-resection should be selected for recurrence
following curative surgical resection for metastatic CRC. In the
present study, we also evaluated the recurrence interval during the
follow-up period after the first curative surgical resection for
metastatic CRC. Our results indicated that a recurrence interval of
<1 year was associated with a poorer prognosis in terms of DFS
and OS after the final curative resection. Of the 103 patients, 54
developed recurrence within 1 year after the first curative
resection and 46 of those 54 patients (85.1%) were unable to
undergo surgical resection for the recurrence. Of the 54 patients,
2 (3.7%) underwent palliative resection for disease recurrence and
7 (12.9%) underwent curative surgical resection. The remaining 49
patients developed no recurrence within 1 year after the first
curative resection. Of those 49 patients, 25 (53.0%) survived
without any further recurrence [median DFS, 5.75 years (range,
2.17–21.73 years)], whereas 24 patients developed recurrence and 7
underwent curative surgical resection for the recurrence, surviving
without any further recurrence [median DFS, 4.73 years (range,
2.30–11.86 years)]; of the remaining 17 patients, 1 underwent
palliative surgical resection and 16 were unable to undergo
surgical resection. Our results suggested that combination therapy,
namely palliative surgical resection and chemotherapy, may be an
option for recurrent cases that appear within 1 year after the
first curative resection (3). In CRC
therapy, it is essential to prevent metachronous metastasis.
Several adjuvant chemotherapies may be beneficial for advanced
metastatic CRC (10,15). In such cases, predictive markers of
metastasis are crucial, regardless of the traditional TNM
classification, and may contribute to the diagnosis and treatment
of metachronous distant metastases.
In the present study, the combination of adjuvant
chemotherapies did not result in statistically significant
differences in patient outcome. This may be due to the fact that
the reviewed data were collected over the past 20 years, within
which time the strategy of the adjuvant treatment has changed.
Adjuvant chemotherapy for CRC has been the treatment of choice in
highly suspicious metachronous metastatic cases (16,17).
Improving treatments, such as postoperative chemotherapy,
combination therapy with chemotherapy following surgical resection,
or palliative surgical resection with chemotherapy for metastatic
CRC, may contribute to improving patient outcome (3,10,15–17).
In summary, surgical resection may be a potentially
curative option for selected CRC patients with liver or lung
metastasis. If the metastases are diagnosed as potentially
resectable and the patient's performance status is satisfactory,
surgical resection of the primary as well as the metastatic lesions
may be a viable treatment option. Following surgical resection,
tumour invasiveness should be considered. With the combination of
surgery and improved chemotherapy, longer-term survival may be
achieved.
References
|
1
|
Jemal A, Siegel R, Xu J, et al: Cancer
statistics 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Headrick JR, Miller DL, Nagorney DM, et
al: Surgical treatment of hepatic and pulmonary metastases from
colon cancer. Ann Thorac Surg. 71:975–979; discussion 979–980.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park JH, Kim TY, Lee KH, et al: The
beneficial effect of palliative resection in metastatic colorectal
cancer. Br J Cancer. 108:1425–1431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanzaki R, Higashiyama M, Oda K, et al:
Outcome of surgical resection for recurrent pulmonary metastasis
from colorectal carcinoma. Am J Surg. 202:419–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grothey A, Sargent D, Goldberg RM, et al:
Survival of patients with advanced colorectal cancer improves with
the availability of fluorouracil-leucovorin, irinotecan and
oxaliplatin in the course of treatment. J Clin Oncol. 22:1209–1214.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sobrero AF, Maurel J, Fehrenbacher L, et
al: EPIC: phase III trial of cetuximab plus irinotecan after
fluoropyrimidine and oxaliplatin failure in patients with
metastatic colorectal cancer. J Clin Oncol. 26:2311–2319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watanabe T, Itabashi M, Shimada Y, et al:
Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2010 for the treatment of colorectal cancer. Int J Clin
Oncol. 17:1–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sobin LH and Fleming ID: TNM
Classification of Malignant Tumors7th. Union Internationale Contre
le Cancer and the American Joint Committee on Cancer.
Wiley-Blackwell; Oxford, UK: pp. 100–109. 2009
|
|
10
|
Bathe OF, Dowden S, Sutherland F, et al:
Phase II study of neoadjuvant 5-FU + leucovorin + CPT-11 in
patients with resectable liver metastases from colorectal
adenocarcinoma. BMC Cancer. 4:322004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kornmann M, Formentini A, Ette C, et al:
Prognostic factors influencing the survival of patients with colon
cancer receiving adjuvant 5-FU treatment. Eur J Surg Oncol.
34:1316–1321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lan YT, Chang SC, Yang SH, et al:
Comparison of clinicopathological characteristics and prognosis
between early and late recurrence after curative surgery for
colorectal cancer. Am J Surg. 207:922–930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Derkinderen DJ, Boxma OJ, Koten JW, et al:
Stochastic theory of oncogenesis. Anticancer Res. 10:497–504.
1990.PubMed/NCBI
|
|
14
|
Miyoshi N, Ishii H, Mimori K, et al:
Abnormal expression of TRIB3 in colorectal cancer: a novel marker
for prognosis. Br J Cancer. 101:1664–1670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koshariya M, Jagad RB, Kawamoto J, et al:
An update and our experience with metastatic liver disease.
Hepatogastroenterology. 54:2232–2339. 2007.PubMed/NCBI
|
|
16
|
Schwartzberg LS, Rivera F, Karthaus M, et
al: PEAK: A randomized, multicenter phase II study of panitumumab
plus modified fluorouracil, leucovorin and oxaliplatin (mFOLFOX6)
or bevacizumab plus mFOLFOX6 in patients with previously untreated,
unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J
Clin Oncol. 32:2240–2247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Price TJ, Peeters M, Kim TW, et al:
Panitumumab versus cetuximab in patients with
chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal
cancer (ASPECCT): a randomised, multicentre, open-label,
non-inferiority phase 3 study. Lancet Oncol. 15:569–579. 2014.
View Article : Google Scholar : PubMed/NCBI
|