Introduction
Gastric cancer is one of the most prevalent
malignancies and the second leading cause of cancer-related
mortality worldwide (1). Although the
mainstay of treatment is curative surgery (2), several patients develop recurrence, even
following surgery. Various adjuvant therapies have been developed
to prevent postoperative recurrence (3–5). However,
the efficacy of these therapies is limited and there is a need for
more effective treatments for gastric cancer.
Human epidermal growth factor receptor 2 (HER2) is
known to be involved in the complex signaling pathways controlling
cell proliferation, differentiation and apoptosis through the
activation of signaling cascades (6).
The overexpression of HER2 is associated with increased metastatic
potential and poor clinical outcome (7,8).
Trastuzumab, a humanized anti-HER2 antibody, exerts its antitumor
activity via several mechanisms, including blockade of constitutive
HER2 signaling, antibody-dependent cell-mediated cytotoxicity
(ADCC) by tumor-infiltrating FcR-expressing immune effector cells
and suppression of tumor angiogenesis (9–13).
Trastuzumab is widely used as a standard therapy for patients with
HER2-overexpressing metastatic breast cancer (14–16). In
addition to its effect in breast cancer, trastuzumab is reported to
exhibit strong antitumor activity in HER2-overexpressing human
gastric cancer mouse xenograft models (17). In a phase III clinical trial of
trastuzumab in HER2-positive advanced and inoperable gastric cancer
(ToGA trial), compared with chemotherapy alone, treatment with
trastuzumab in combination with chemotherapy (capecitabine plus
cisplatin) significantly prolonged the overall survival of patients
with HER2-positive advanced gastric or gastroesophageal junction
cancer (18). Therefore, anti-HER2
therapy with trastuzumab is highly recommended for
HER2-overexpressing gastric cancer.
Capecitabine
(N4-pentyloxycarbonyl-5′-deoxy-5-fluorocytidine) is an oral
fluoropyrimidine that undergoes a 3-step enzymatic activation
process in hepatic and tumor tissues. The final step that generates
5-fluorouracil (5-FU) occurs selectively within tumor tissues, due
to the higher expression of thymidine phosphorylase (TP) in tumors
compared with that in normal tissues (19–21). As
regards the metabolism of 5-FU, it is deactivated enzymatically by
dihydropyrimidine dehydrogenase (DPD), which is also expressed in
tumor tissues. By using human tumor xenograft models, it has been
demonstrated that the sensitivity to capecitabine correlates with
the expression of TP and DPD, specifically the TP/DPD ratio
(22,23). It has also been demonstrated in
clinical studies that the levels of TP and DPD in tumors correlate
with the efficacy of capecitabine (24–26).
Several antitumor modalities, such as cyclophosphamide, taxanes,
oxaliplatin, erlotinib, or radiation, have been reported to
increase the levels of TP in tumors in xenograft models and, when
these modalities are used in combination with capecitabine, they
exhibit a significantly higher antitumor activity compared with
each agent or treatment used as monotherapy (27–31).
Oxaliplatin is an alkylating drug that forms
compounds between two adjacent guanines or a guanine and an adenine
residue, leading to inhibition of DNA synthesis and repair
(32). Several phase II trials have
demonstrated that the combination of oral capecitabine with
intravenous oxaliplatin (XELOX) is an effective and well-tolerated
treatment for advanced gastric cancer (33–35). In
addition, the CLASSIC trial compared XELOX with surgery alone in
patients who underwent D2 gastrectomy. The interim results of that
study demonstrated that XELOX improved 3-year disease-free survival
compared with surgery alone (36). It
has also been reported that XELOX exhibited a superior antitumor
activity over either single agent in human gastrointestinal cancer
xenograft models (30).
Considering the aforementioned findings, we
hypothesized that a combination treatment with trastuzumab and
XELOX may be a potent therapy for HER2-positive gastric cancer.
However, thus far there have been no preclinical or clinical
studies investigating the efficacy of this combination in gastric
cancer. The aim of the present study was to assess the antitumor
effect of the combination of trastuzumab with XELOX and analyze its
mechanism of action from the aspect of the induction of the
capecitabine-activating enzyme TP.
Materials and methods
Antitumor agents
Capecitabine and trastuzumab were obtained from
Chugai Pharmaceutical Co., Ltd (Tokyo, Japan). Oxaliplatin was
purchased from Wako Pure Chemical Industries (Osaka, Japan). Human
IgG (HuIgG) was purchased from MP Biomedicals, Inc. (Aurora, OH,
USA).
Animals
A total of 254, 5-week-old male
CAnN.Cg-Foxn1nu/CrlCrlj mice were obtained from Charles River
Laboratories Japan, Inc. (Yokohama, Japan). The mice had an
average body weight of 26.3 g on the day of treatment
initiated. The health of the mice was monitored by daily
observation. Chlorinated water and irradiated food (CE-2; Clea
Japan, Inc., Tokyo, Japan) were provided ad libitum and the
animals were kept under a controlled light/dark cycle (12 h light;
12 h dark). All the mice were allowed to acclimatize and recover
from shipping-related stress for at least 1 week prior to the
study. All the animal experiment protocols were reviewed and
approved by the Institutional Animal Care and Use Committee at
Chugai Pharmaceutical Co., Ltd.
Cell lines and culture conditions
The HER2-positive human gastric cancer cell line
NCI-N87 was purchased from the American Type Culture Collection
(Manassas, VA, USA) and maintained in RPMI-1640 medium supplemented
with 10% (v/v) fetal bovine serum (FBS) at 37°C under 5%
CO2. CD16(158V)/NK-92 cells were constructed as
previously described (37) and
maintained in MEMα medium (Wako Pure Chemical Industries)
supplemented with 12.5% FBS, 12.5% horse serum, 0.02 mmol/l folic
acid, 0.1 mmol/l 2-mercaptoethanol, 0.2 mmol/l inositol, 0.5 mg/ml
G418 and 20 ng/ml recombinant human interleukin (IL)-2 at 37°C
under 5% CO2.
In vivo tumor growth inhibition
studies
Each mouse was inoculated subcutaneously into the
right flank with 5×106 NCI-N87 cells. The tumor volumes
(V) were estimated from the equation V = ab2/2, where a
and b are the tumor length and width, respectively. Several weeks
after tumor inoculation and once tumors had reached a volume of
~160 mm3, the mice were randomized into 7–8 mice per
treatment group, and treatment with capecitabine (359 mg/kg),
oxaliplatin (10 mg/kg), trastuzumab (20 mg/kg) or HuIgG (20 mg/kg)
was initiated (day 1). Capecitabine was suspended in 40 mmol/l
citrate buffer (pH 6.0) containing 5% gum arabic as the vehicle and
was administered orally once a day for 14 days. Oxaliplatin was
dissolved in 5% glucose and administered intravenously on day 1.
Trastuzumab and HuIgG were diluted with saline and administered
intraperitoneally once a week for 3 weeks. The tumor volume was
measured twice a week and the degree of tumor growth inhibition was
evaluated on day 22. In order to determine the levels of TP and DPD
in the tumor and for immunohistochemistry (IHC), the mice bearing
NCI-N87 tumors were randomized into 6 mice per treatment group and
treated once with oxaliplatin and once a week with trastuzumab or
HuIgG. The tumors were excised on day 15.
Measurement of TP and DPD protein
levels in tumor tissues
The tumor samples obtained on day 15 were
immediately frozen in liquid nitrogen and stored at −80°C until
use. The tumor tissues were homogenized in 10 mmol/l Tris-buffer
(pH 7.4) containing 15 mmol/l NaCl, 1.5 mmol/l MgCl2 and
50 µmol/l potassium phosphate and were then centrifuged at 10,000 ×
g for 20 min at 4°C. The protein concentration of the supernatant
was determined by using Direct Detect Spectrometer (Merck KGaA,
Darmstadt, Germany). The levels of TP and DPD were measured by
ELISA with monoclonal antibodies specific to human TP and DPD, as
described previously (38,39).
IHC for TP in tumor tissues
The tumors were excised on day 15 and 4-µm sections
were prepared from paraffin-embedded formalin-fixed tissues. IHC
for TP was performed by using anti-TP antibody (anti-TYMP antibody
produced in rabbit; cat. no. HPA001072, Sigma-Aldrich, St. Louis,
MO, USA) and peroxidase-labeled polymer-horseradish peroxidase
(HRP) conjugated goat anti-rabbit immunoglobulins (Envision+ kit,
HRP-DAB; cat. no. K4003; Dako, Tokyo, Japan).
IHC was evaluated by scoring the positive staining
strength in each mouse in the HuIgG-treated control, trastuzumab,
oxaliplatin and oxaliplatin plus trastuzumab groups, and the scores
were as follows: 1, weakly positive; 2, moderately positive; 3,
markedly positive.
In vitro reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
To determine the direct effect of trastuzumab on the
expression of capecitabine-activating enzymes, NCI-N87 cells were
seeded on 6-well plates at 8×105 cells/well and treated
with 100 µg/ml HuIgG or trastuzumab for 6, 24 and 48 h (n=3).
In the co-culture study, NCI-N87 cells were seeded
on 6-well plates at 8×105 cells/well. Following
adhesion, 8×105 CD16(158V)/NK-92 cells/well were
transferred to the NCI-N87 plates and treated with 2 ng/ml HuIgG or
trastuzumab in NCI-N87 medium [CD16(158V)/NK-92 medium without G418
(1:1)] for 6 and 24 h (n=3). Prior to extracting total RNA from
NCI-N87 cells, the CD16(158V)/NK-92 cells were removed by washing
with phosphate-buffered saline.
To evaluate the effects of soluble factors from
CD16(158V)/NK-92 cells, these cells were seeded on 6-well plates at
8×105 cells/well and treated with HuIgG or trastuzumab
(2 ng/ml) in CD16(158V)/NK-92 cell medium without G418. After 24 h,
the culture medium was collected, centrifuged and filtered. NCI-N87
cells were seeded on 6-well plates at 8×105 cells/well.
Following adhesion, the NCI-N87 cells were cultured in the filtered
CD16(158V)/NK-92 cell culture medium [new NCI-N87 medium (1:1)] for
24 h (n=3).
After culturing, total RNA from the cells was
extracted by using an AS2000 Maxwell 16 Instrument (Promega,
Madison, WI, USA). The cDNA was synthesized from total RNA by a
Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics,
Indianapolis, IN, USA). The expression of TP was detected by using
SYBR-Green (Roche Diagnostics). The expression of GAPDH, interferon
(IFN)-γ, tumor necrosis factor (TNF)-α and IL-1α was detected by
using the Universal Probe Library (Roche Diagnostics). GAPDH was
used as control.
Statistical analysis
Data are presented as the mean ± standard deviation.
For in vivo studies with three or four groups, statistical
differences between individual groups were evaluated with
Steel-Dwass tests. For in vivo studies with two groups,
statistical comparisons between the control and trastuzumab groups
were performed by t-tests. In in vitro studies, the mRNA
levels of the control or treatment groups were compared to that at
0 h by using the Dunnett's test. The mRNA levels of the treatment
groups were compared to that of the control group at the same time
point using t-tests. For all the tests, P<0.05 was considered to
indicate statistically significant differences. The statistical
analysis was performed using a SAS preclinical package (SAS
Institute, Cary, NC, USA).
Results
Combination of trastuzumab with
XELOX
The antitumor activity of trastuzumab (20 mg/kg) in
combination with capecitabine [359 mg/kg, 2/3 maximum tolerated
dose (MTD)] (22) and oxaliplatin (10
mg/kg, 2/3 MTD) (30) was evaluated
in a HER2-positive human gastric cancer NCI-N87 xenograft model.
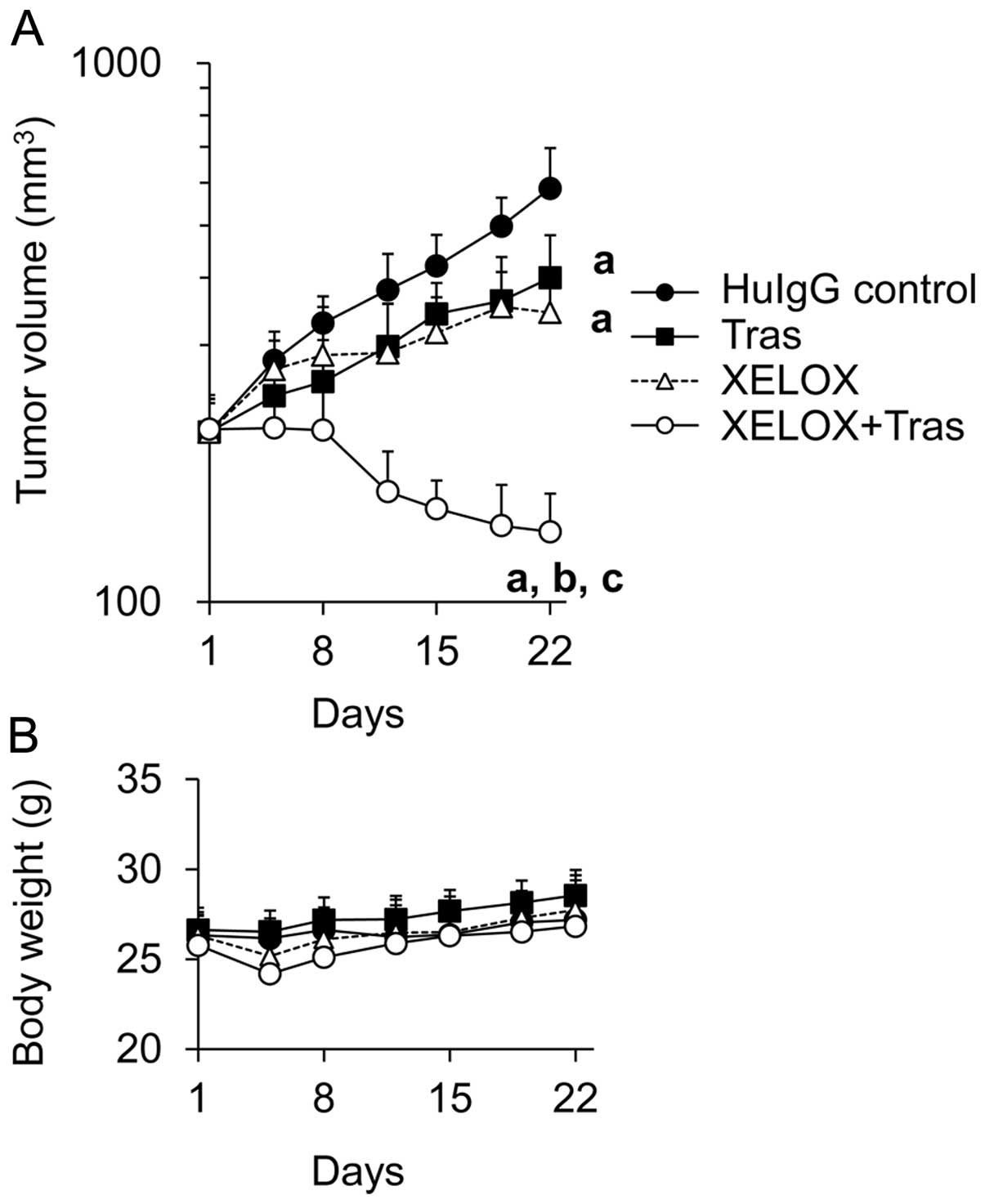
The tumors in all the treatment groups were significantly smaller
compared with those in the HuIgG-treated control group on day 22.
Combined treatment with trastuzumab and XELOX achieved a
significantly stronger inhibition of tumor growth compared with
either trastuzumab or XELOX alone on day 22 (Fig. 1A). In addition, no augmentation of
toxicity, as shown by body weight loss, was observed in any of the
treatment groups (Fig. 1B).
Effect of trastuzumab on TP expression
in tumor tissues
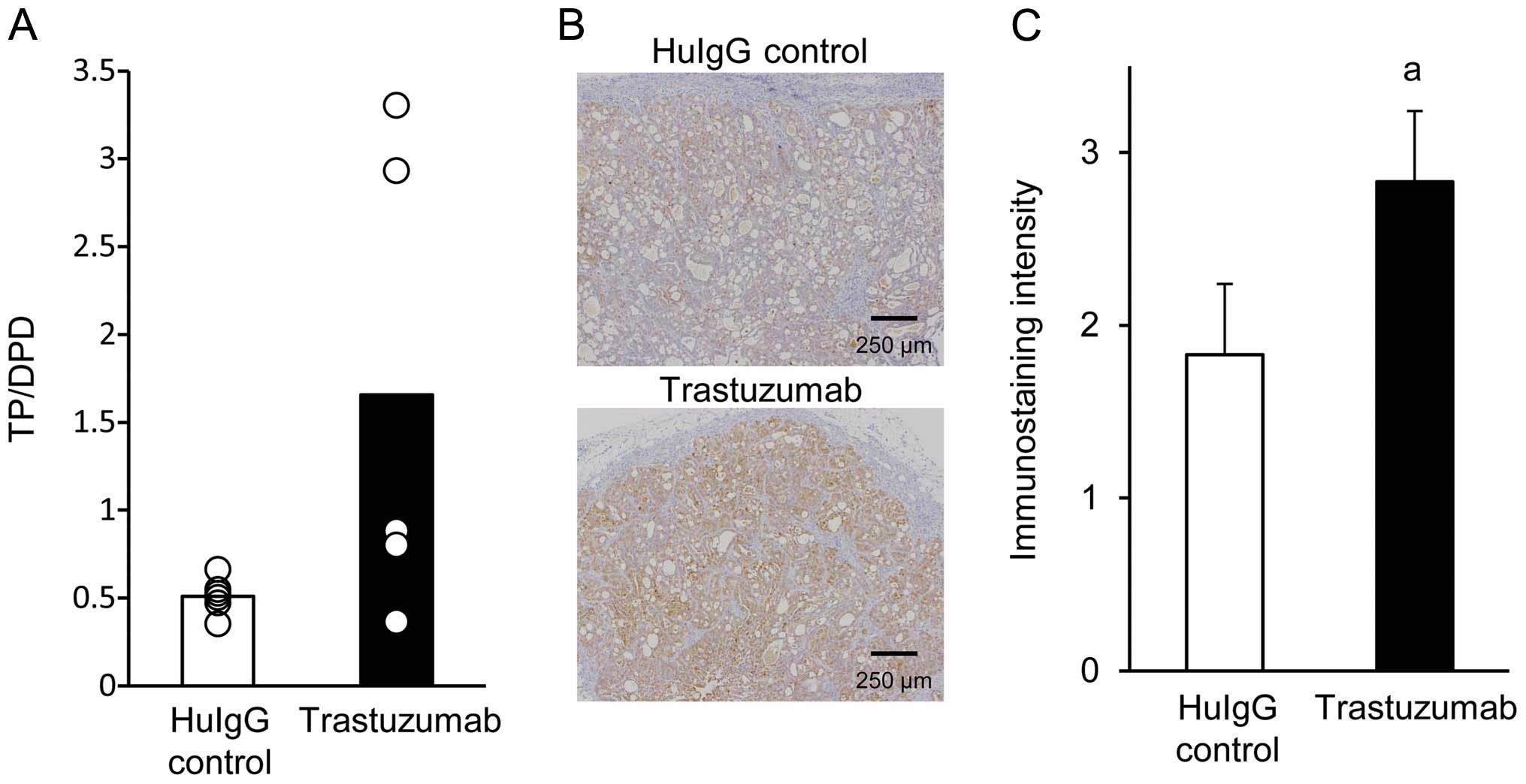
ELISA was used to investigate the effects of
trastuzumab on the TP/DPD protein ratio in whole tumor tissues
sampled from the NCI-N87 xenograft models on day 15. However, there
was no significant difference in the TP/DPD ratio between tumor
tissues obtained from mice treated with trastuzumab and those
treated with HuIgG alone (Fig. 2A).
As whole tumor tissues are composed of vital as well as necrotic
areas, measuring TP/DPD using whole tumor tissues may not return
accurate results. In order to evaluate the TP/DPD ratio more
accurately, we performed an IHC examination of TP and measured the
TP expression level in the vital tumor cell area. The number of
TP-positive cells (brown-tinged cells) in tumors from
trastuzumab-treated mice were increased compared with the
respective number in tumors from HuIgG-treated mice (Fig. 2B). The score of positive staining
strength was significantly higher in the trastuzumab group compared
with that in the HuIgG-treated control group (Fig. 2C).
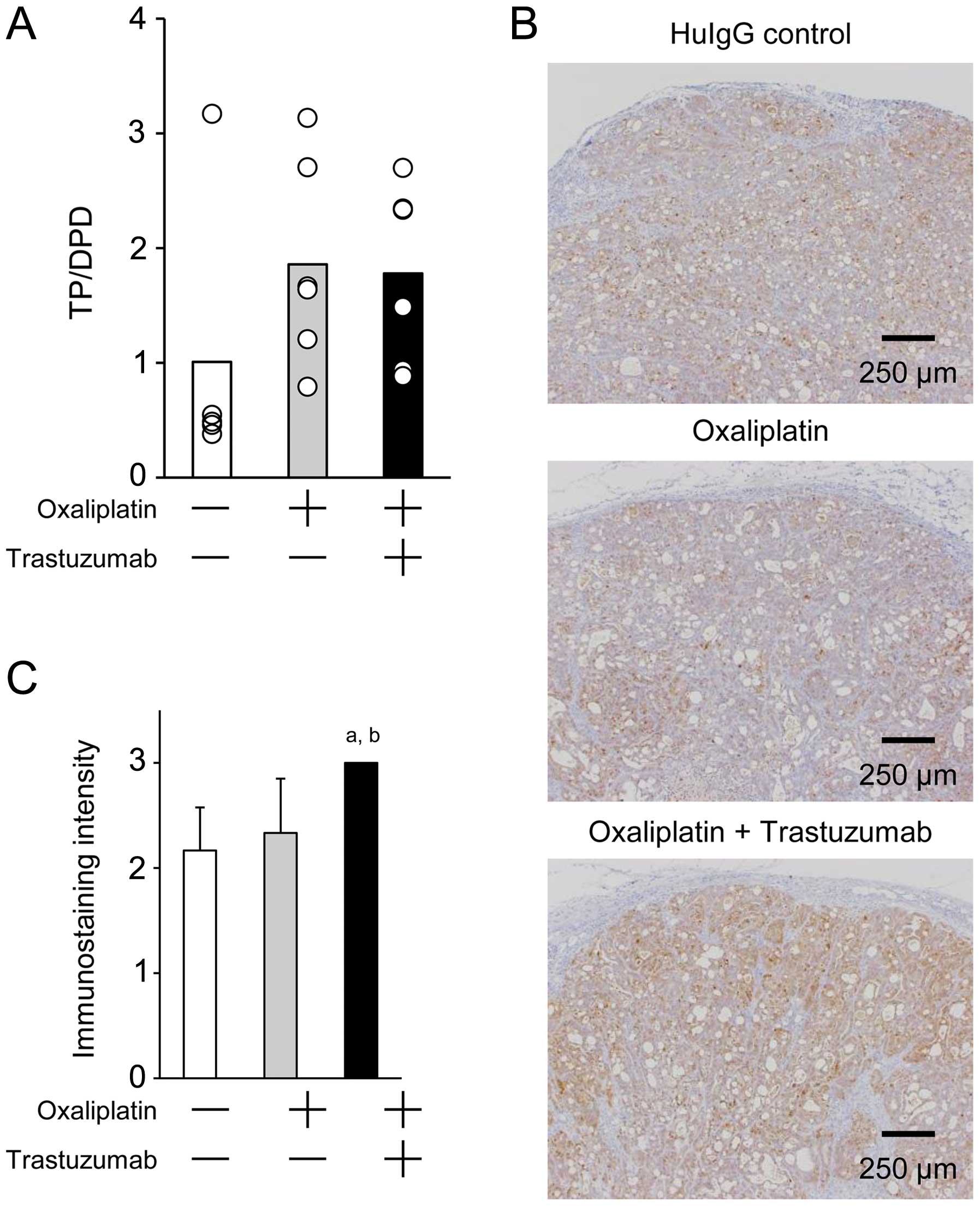
We next used ELISA to investigate the effects of
oxaliplatin monotherapy and oxaliplatin in combination with
trastuzumab on the TP/DPD ratio in whole tumor tissues obtained
from the NCI-N87 models on day 15. The TP/DPD ratio was increased
in the oxaliplatin monotherapy group and in the oxaliplatin plus
trastuzumab combination group, as compared with the ratio in the
HuIgG-treated control group, although the difference was not
significant (Fig. 3A). However, in
IHC, the TP immunostaining intensity was very strong in tumors
obtained from mice treated with the combination of trastuzumab plus
oxaliplatin, whereas the staining intensity of tumor tissues from
oxaliplatin-treated or HuIgG-treated mice was weaker and of a
similar level (Fig. 3B). The score of
positive staining strength for TP was significantly higher in the
combination group compared with that in the HuIgG-treated control
or oxaliplatin groups. There was no significant difference in the
score between the HuIgG-treated control and oxaliplatin groups
(Fig. 3C).
Effects of trastuzumab on TP mRNA
expression
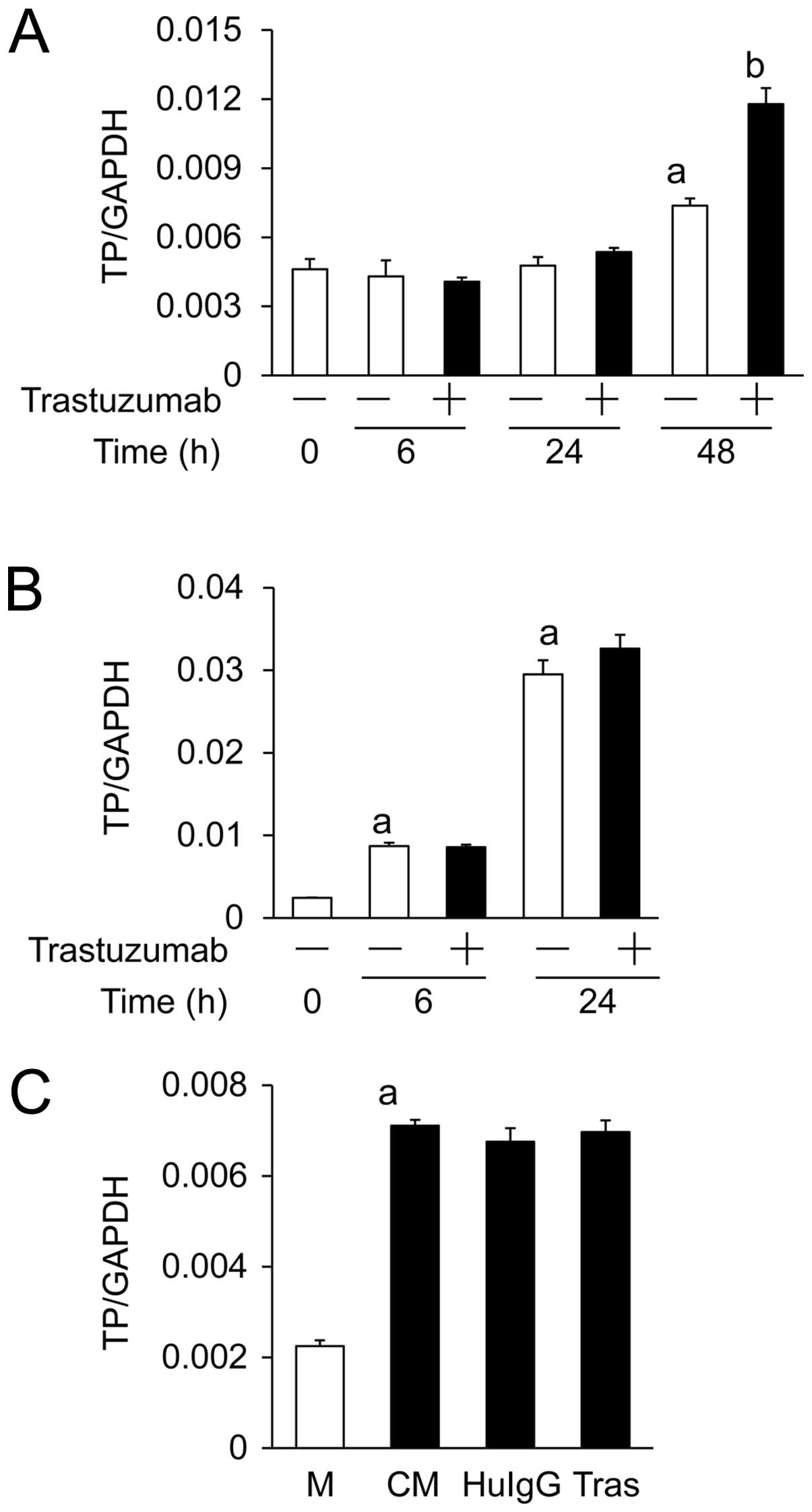
We first examined the effects of trastuzumab
treatment on the expression of TP by using qPCR. In a 48-h culture,
the TP mRNA expression level in NCI-N87 cells was significantly
increased compared with the initial level, irrespective of the
presence of trastuzumab (100 µg/ml). However, trastuzumab treatment
significantly increased the TP mRNA expression compared with that
without trastuzumab (Fig. 4A).
Second, in the in vitro co-culture study of
NCI-N87 and CD16(158V)/NK-92 cells, the TP mRNA expression in
NCI-N87 cells was significantly higher at 6 and 24 h compared with
that at 0 h, regardless of the presence of trastuzumab (2 ng/ml).
The addition of trastuzumab did not affect the TP mRNA expression
at either 6 h or 24 h (Fig. 4B).
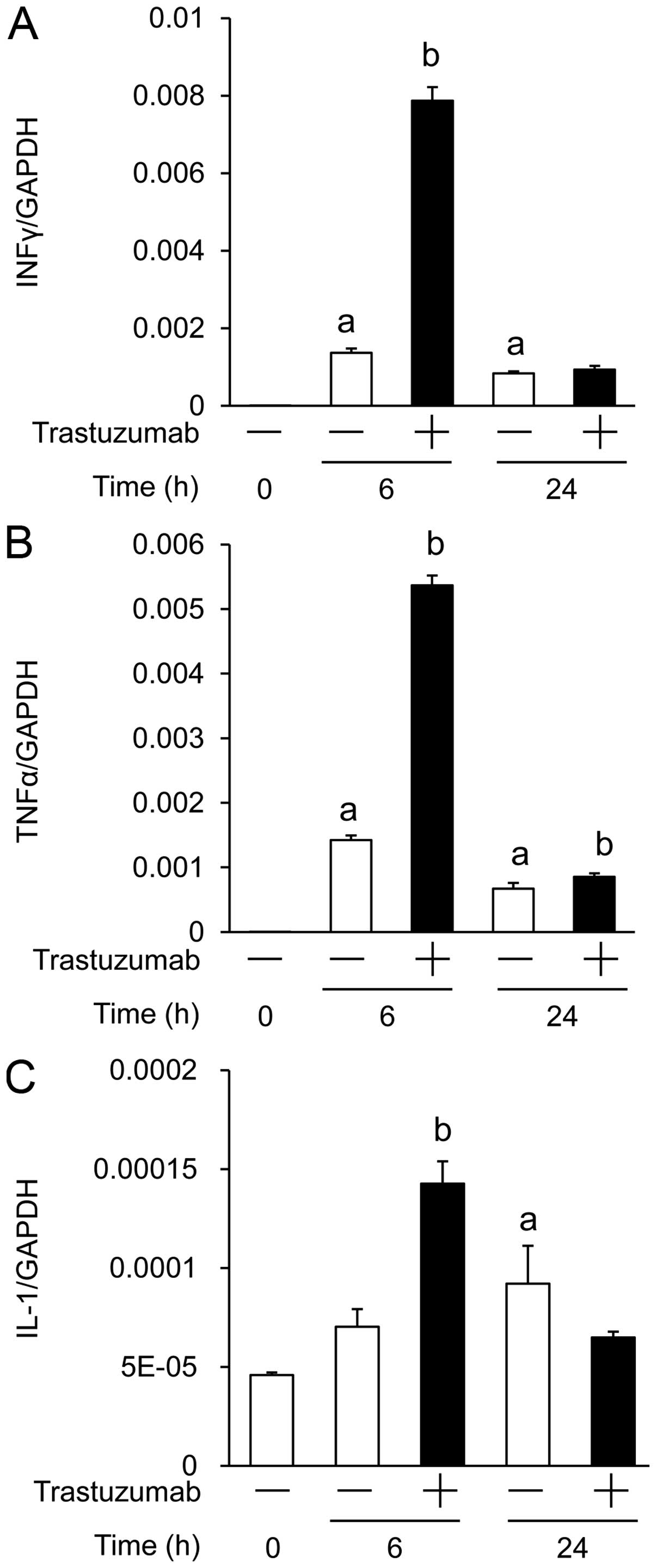
Third, we used the co-culture system to investigate
the effects of trastuzumab on the mRNA expression of three
cytokines known to induce TP in tumor cells; the mRNA expressions
of IFN-γ, TNF-α and IL-1α at 6 h were significantly higher in
trastuzumab-treated NCI-N87 cells compared with the HuIgG-treated
cells (Fig. 5).
Finally, we investigated the effects of soluble
factors from CD16(158V)/NK-92 cells on TP mRNA expression in
NCI-N87 cells. Treatment for 24 h with a medium conditioned by
CD16(158V)/NK-92 cells significantly increased TP mRNA expression
in NCI-N87 cells. There was no significant difference in TP mRNA
expression between conditioned media prepared with trastuzumab,
HuIgG, or without antibodies (Fig.
4C).
Discussion
In the present study, using the HER2-positive human
gastric cancer NCI-N87 xenograft model, we investigated the
efficacy of the combination of trastuzumab with XELOX and analyzed
the mechanism underlying the effects of this combination in terms
of capecitabine activation. We observed that, in this model,
combination treatment with trastuzumab and XELOX exerted a
significantly stronger antitumor effect compared with either agent
alone. This effect was considered to be synergistic. In the
trastuzumab group, there was an obvious antitumor effect similar to
that previously reported (17).
However, XELOX exerted a weaker antitumor effect compared with that
previously reported in a study using human colon cancer and
HER2-negative gastric cancer xenograft models (30). This discrepancy between the results of
that study and ours may be due to the different XELOX
administration schedules and the different types of cancer.
To elucidate the mechanism underlying the potent
antitumor effect of the combination therapy with trastuzumab and
XELOX in our model, we conducted experiments focusing on factors
that facilitate the generation of 5-FU from capecitabine. For this
purpose, we measured TP and DPD levels in the tumors.
In the present study, the results of IHC, which was
performed with the utmost care, revealed that the administration of
trastuzumab alone or trastuzumab plus oxaliplatin significantly
increased the TP levels in the tumor tissues. However, no
significant difference in the TP/DPD protein ratio was observed
between the HuIgG-treated control and the trastuzumab groups
(Fig. 2A), or between the
HuIgG-treated control, oxaliplatin and trastuzumab plus oxaliplatin
groups (Fig. 3A). Similar results
regarding this mismatch between the results obtained by IHC and
those obtained by ELISA have been previously reported (30). The discrepancy between the two methods
may be explained as follows: In ELISA, the TP protein expression is
represented as a value relative to the total protein in whole tumor
tissues. Accordingly, the samples used for ELISA may contain
proteins from connective tissue and necrotic areas, along with the
vital tumor cells. On the other hand, in the IHC assay, evaluation
of TP is performed by means of immunostaining intensity
specifically focusing on the vital tumor cell area in the tumor
tissues. Thus, we consider the results of IHC to be more reliable
compared with those of ELISA for explaining the antitumor mechanism
of the combination therapy. Therefore, the superior antitumor
activity of the trastuzumab plus XELOX combination may be, at least
in part, attributable to the increased TP levels in tumors induced
by trastuzumab or trastuzumab plus oxaliplatin, which consequently
facilitated the generation of 5-FU from capecitabine.
The mechanism through which TP is upregulated by
trastuzumab or trastuzumab plus oxaliplatin is of great interest.
We considered two possible mechanisms through which TP may be
upregulated by trastuzumab: Trastuzumab acting directly on tumor
cells to upregulate the expression of TP; or trastuzumab acting on
other cells to release soluble factors, thereby indirectly
upregulating TP expression in tumor cells. We performed several
in vitro experiments to analyze these mechanisms and
obtained results that may support either or both mechanisms.
When NCI-N87 cells were cultured in the presence of
a relatively high concentration of trastuzumab (100 µg/ml),
upregulation of TP mRNA in NCI-N87 cells was observed after 48 h of
culture compared with TP mRNA in NCI-N87 cells cultured without
trastuzumab, suggesting that trastuzumab acts directly to
upregulate TP in HER2-positive NCI-N87 cells in vitro
(Fig. 4A).
To analyze indirect upregulation of TP by
trastuzumab, we conducted the following experiments focusing on
tumor-infiltrating NK cells, as it is considered that ADCC is one
of the key mechanisms underlying the antitumor activity of
trastuzumab (40). Studies using the
human NK cell line NK-92 and the CD16-transfected human NK cell
line CD16(158V)/NK-92 as effector cells have indicated that
trastuzumab triggers ADCC against HER2-positive human breast cancer
and human gastric cancer cell lines (37,41); in
addition, in clinical studies, increased numbers of
tumor-infiltrating NK cells have been detected in breast cancer
tissues following trastuzumab treatment (42,43). In
light of these reports, we performed a co-culture experiment to
determine whether NK cells affect TP expression in tumor cells.
Specifically, NCI-N87 and CD16(158V)/NK-92 cells were co-cultured
in the presence or absence of 2 ng/ml trastuzumab. The
concentration of trastuzumab used in our study was the
concentration used in the ADCC assay reported previously (37). It was observed that co-culture
significantly increased the expression of TP mRNA in NCI-N87 cells
by as early as 6 h of culture, and more strongly increased TP mRNA
expression at 24 h. However, the effect that co-culture without
trastuzumab exerted on increasing TP mRNA expression was almost
identical to that of co-culture with trastuzumab (Fig. 4B). This may be due to the strong
TP-inducing effect of CD16(158V)/NK-92 cells alone, or due to the
low trastuzumab concentration used in this assay. The results
suggested that CD16(158V)/NK-92 cells spontaneously produce soluble
factors that may act on NCI-N87 cells to upregulate TP. To
investigate this hypothesis, we next examined the TP-inducing
activity of CD16(158V)/NK-92-conditioned medium. As expected, TP
mRNA expression in NCI-N87 cells was significantly increased by
culturing in a medium conditioned by CD16(158V)/NK-92 cells
(Fig. 4C). Interestingly, the
induction of TP mRNA in NCI-N87 cells was similar in the
conditioned medium prepared in the presence as well as in the
absence of trastuzumab. These results suggested that soluble
factors responsible for inducing TP mRNA expression in NCI-N87
cells were constitutively produced by CD16(158V)/NK-92 cells and
their production was not affected by trastuzumab.
As regards the soluble factors that induce TP, it
has been reported that the expression of TP in tumor cells is
induced by treatment with cytokines, such as IL-1α, TNF-α and IFN-γ
(44). Therefore, we measured the
mRNA levels of these cytokines in NCI-N87 cells co-cultured with
CD16(158V)/NK-92 cells (Fig. 5). Of
note, when co-culture was performed with trastuzumab for 6 h, the
mRNA levels of IFN-γ, TNFα and IL-1 were significantly increased
compared with those in the co-culture without trastuzumab
(P<0.05). In the 24-h co-culture, the mRNA level of each of the
cytokines decreased from that in the 6-h co-culture (Fig. 5). There appears to be some
inconsistency between these results and the induction of TP in the
co-culture experiment (Fig. 4B) in
two respects: The first is the lag between cytokine production and
TP induction; the second is the inconsistency in the effects of
trastuzumab, i.e., although the levels of production of the three
cytokines were higher in the presence of trastuzumab, the TP mRNA
expression level was almost identical in the presence as well as in
the absence of trastuzumab. With respect to the first issue, it may
be considered that there is a delay for NCI-N87 cells to produce TP
in response to the cytokines produced after ~6 h of co-culture.
Albanell et al (45) reported
that IFN-γ, TNF-α, or IL-1 upregulated TP when the WiDr and MKN45
human gastric cancer cell lines were cultured for 1 h with these
cytokines, either as single agents or in combination. The second
issue may be interpreted as a result of the co-culture period; the
difference in TP mRNA expression in NCI-N87 cells co-cultured with
or without trastuzumab may become clearer when the culture period
is shorter or longer than 24 h. Taken together, these data suggest
that the tumor-infiltrating NK cells play an important role in the
expression of TP.
In this study, we clearly demonstrated that the
antitumor activity achieved with the combination of trastuzumab and
XELOX was significantly greater compared with that with trastuzumab
or XELOX alone in this HER2-positive human gastric cancer xenograft
model. This synergistic effect was considered to be attributable,
at least in part, to the upregulation of TP levels in tumors caused
by trastuzumab or trastuzumab plus oxaliplatin, as the upregulation
of TP facilitates the conversion of capecitabine into 5-FU in
tumors. In addition, our study revealed two mechanisms through
which trastuzumab upregulated TP in tumor cells: One was a direct
mechanism, in which trastuzumab binding to HER2-positive tumor
cells directly upregulated TP in the target tumor cells; the other
was an indirect mechanism, in which trastuzumab-mediated release of
soluble factors from tumor-infiltrating immune cells promoted the
upregulation of TP in the HER2-positive gastric tumor cells. Thus
far, it remains unclear which of these two is the principal
mechanism and which is the pathway to TP upregulation. Although
these issues remain to be addressed, it is expected that
trastuzumab will be clinically used in combination with XELOX for
HER2-positive gastric cancer in the foreseeable future.
References
|
1
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sasako M, Saka M, Fukagawa T, Katai H and
Sano T: Surgical treatment of advanced gastric cancer: Japanese
perspective. Dig Surg. 24:101–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunningham D, Allum WH, Stenning SP, et
al: MAGIC Trial Participants: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Starling N, Rao S, et al:
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom: Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
J Med. 345:725–730. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
García I, Vizoso F, Martín A, et al:
Clinical significance of the epidermal growth factor receptor and
HER2 receptor in resectable gastric cancer. Ann Surg Oncol.
10:234–241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanner M, Hollmen M, Junttila TT, et al:
Amplification of HER 2 in gastric carcinoma: association with
topoisomerase IIalpha gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Molina MA, Codony-Servat J, Albanell J, et
al: Trastuzumab (herceptin), a humanized anti-Her2 receptor
monoclonal antibody, inhibits basal and activated Her2 ectodomain
cleavage in breast cancer cells. Cancer Res. 61:4744–4749.
2001.PubMed/NCBI
|
|
10
|
Izumi Y, Xu L, di Tomaso E, Fukumura D and
Jain RK: Tumour biology: Herceptin acts as an anti-angiogenic
cocktail. Nature. 416:279–280. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barok M, Isola J, Pályi-Krekk Z, et al:
Trastuzumab causes antibody-dependent cellular
cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1
breast cancer xenografts despite intrinsic drug resistance. Mol
Cancer Ther. 6:2065–2072. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valabrega G, Montemurro F and Aglietta M:
Trastuzumab: mechanism of action, resistance and future
perspectives in HER2-overexpressing breast cancer. Ann Oncol.
18:977–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Musolino A, Naldi N, Bortesi B, et al:
Immunoglobulin G fragment C receptor polymorphisms and clinical
efficacy of trastuzumab-based therapy in patients with
HER-2/neu-positive metastatic breast cancer. J Clin Oncol.
26:1789–1796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buzdar AU, Valero V, Ibrahim NK, et al:
Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil,
epirubicin and cyclophosphamide chemotherapy and concurrent
trastuzumab in human epidermal growth factor receptor 2-positive
operable breast cancer: An update of the initial randomized study
population and data of additional patients treated with the same
regimen. Clin Cancer Res. 13:228–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Romond EH, Perez EA, Bryant J, et al:
Trastuzumab plus adjuvant chemotherapy for operable HER2-positive
breast cancer. N Engl J Med. 353:1673–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith I, Procter M, Gelber RD, et al: HERA
study team: Two year follow-up of trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer: A randomised
controlled trial. Lancet. 369:29–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujimoto-Ouchi K, Sekiguchi F, Yasuno H,
Moriya Y, Mori K and Tanaka Y: Antitumor activity of trastuzumab in
combination with chemotherapy in human gastric cancer xenograft
models. Cancer Chemother Pharmacol. 59:795–805. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishikawa T, Utoh M, Sawada N, et al: Tumor
selective delivery of 5-fluorouracil by capecitabine, a new oral
fluoropyrimidine carbamate, in human cancer xenografts. Biochem
Pharmacol. 55:1091–1097. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pentheroudakis G and Twelves C: The
rational development of capecitabine from the laboratory to the
clinic. Anticancer Res. 22:3589–3596. 2002.PubMed/NCBI
|
|
21
|
Schüller J, Cassidy J, Dumont E, et al:
Preferential activation of capecitabine in tumor following oral
administration to colorectal cancer patients. Cancer Chemother
Pharmaco. 45:291–297. 2000. View Article : Google Scholar
|
|
22
|
Ishikawa T, Sekiguchi F, Fukase Y, Sawada
N and Ishitsuka H: Positive correlation between the efficacy of
capecitabine and doxifluridine and the ratio of thymidine
phosphorylase to dihydropyrimidine dehydrogenase activities in
tumors in human cancer xenografts. Cancer Res. 58:685–690.
1998.PubMed/NCBI
|
|
23
|
Yasuno H, Kurasawa M, Yanagisawa M, Sato
Y, Harada N and Mori K: Predictive markers of capecitabine
sensitivity identified from the expression profile of pyrimidine
nucleoside-metabolizing enzymes. Oncol Rep. 29:451–458.
2013.PubMed/NCBI
|
|
24
|
Ishii R, Takiguchi N, Oda K, Koda K and
Miyazaki M: Thymidine phosphorylase expression is useful in
selecting adjuvant chemotherapy for stage III gastric cancer. Int J
Oncol. 19:717–722. 2001.PubMed/NCBI
|
|
25
|
Nishimura G, Terada I, Kobayashi T, et al:
Thymidine phosphorylase and dihydropyrimidine dehydrogenase levels
in primary colorectal cancer show a relationship to clinical
effects of 5′-deoxy-5-fluorouridine as adjuvant chemotherapy. Oncol
Rep. 9:479–482. 2002.PubMed/NCBI
|
|
26
|
Terashima M, Fujiwara H, Takagane A, et
al: Role of thymidine phosphorylase and dihydropyrimidine
dehydrogenase in tumour progression and sensitivity to
doxifluridine in gastric cancer patients. Eur J Cancer.
38:2375–2381. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Endo M, Shinbori N, Fukase Y, et al:
Induction of thymidine phosphorylase expression and enhancement of
efficacy of capecitabine or 5′-deoxy-5-fluorouridine by
cyclophosphamide in mammary tumor models. Int J Cancer. 83:127–134.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sawada N, Ishikawa T, Fukase Y, Nishida M,
Yoshikubo T and Ishitsuka H: Induction of thymidine phosphorylase
activity and enhancement of capecitabine efficacy by taxol/taxotere
in human cancer xenografts. Clin Cancer Res. 4:1013–1019.
1998.PubMed/NCBI
|
|
29
|
Sawada N, Ishikawa T, Sekiguchi F, Tanaka
Y and Ishitsuka H: X-ray irradiation induces thymidine
phosphorylase and enhances the efficacy of capecitabine (Xeloda) in
human cancer xenografts. Clin Cancer Res. 5:2948–2953.
1999.PubMed/NCBI
|
|
30
|
Sawada N, Kondoh K and Mori K: Enhancement
of capecitabine efficacy by oxaliplatin in human colorectal and
gastric cancer xenografts. Oncol Rep. 18:775–778. 2007.PubMed/NCBI
|
|
31
|
Ouchi KF, Yanagisawa M, Sekiguchi F and
Tanaka Y: Antitumor activity of erlotinib in combination with
capecitabine in human tumor xenograft models. Cancer Chemother
Pharmacol. 57:693–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Woynarowski JM, Faivre S, Herzig MC, et
al: Oxaliplatin-induced damage of cellular DNA. Mol Pharmacol.
58:920–927. 2000.PubMed/NCBI
|
|
33
|
Dong N, Jiang W, Li H, Liu Z, Xu X and
Wang M: Triweekly oxaliplatin plus oral capecitabine as first-line
chemotherapy in elderly patients with advanced gastric cancer. Am J
Clin Oncol. 32:559–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu C, Sun Q, Hang X, Zhong B and Wang D:
Multicenter phase II study of capecitabine plus oxaliplatin as a
first-line therapy in Chinese patients with advanced gastric
cancer. Anticancer Drugs. 19:825–831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park YH, Lee JL, Ryoo BY, et al:
Capecitabine in combination with oxaliplatin (XELOX) as a
first-line therapy for advanced gastric cancer. Cancer Chemother
Pharmacol. 61:623–629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bang YJ, Kim YW, Yang HK, et al: CLASSIC
trial investigators: Adjuvant capecitabine and oxaliplatin for
gastric cancer after D2 gastrectomy (CLASSIC): A phase 3
open-label, randomised controlled trial. Lancet. 379:315–321. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamashita Kashima Y, Iijima S, Yorozu K,
et al: Pertuzumab in combination with trastuzumab shows
significantly enhanced antitumor activity in HER2-positive human
gastric cancer xenograft models. Clin Cancer Res. 17:5060–5070.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mori K, Hasegawa M, Nishida M, et al:
Expression levels of thymidine phosphorylase and dihydropyrimidine
dehydrogenase in various human tumor tissues. Int J Oncol.
17:33–38. 2000.PubMed/NCBI
|
|
39
|
Nishida M, Hino A, Mori K, Matsumoto T,
Yoshikubo T and Ishitsuka H: Preparation of anti-human thymidine
phosphorylase monoclonal antibodies useful for detecting the enzyme
levels in tumor tissues. Biol Pharm Bull. 19:1407–1411. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Spector NL and Blackwell KL: Understanding
the mechanisms behind trastuzumab therapy for human epidermal
growth factor receptor 2-positive breast cancer. J Clin Oncol.
27:5838–5847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kute TE, Savage L, Stehle JR Jr, et al:
Breast tumor cells isolated from in vitro resistance to trastuzumab
remain sensitive to trastuzumab anti-tumor effects in vivo and to
ADCC killing. Cancer Immunol Immunother. 58:1887–1896. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arnould L, Gelly M, Penault-Llorca F, et
al: Trastuzumab-based treatment of HER2-positive breast cancer: An
antibody-dependent cellular cytotoxicity mechanism? Br J Cancer.
94:259–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Varchetta S, Gibelli N, Oliviero B, et al:
Elements related to heterogeneity of antibody-dependent cell
cytotoxicity in patients under trastuzumab therapy for primary
operable breast cancer overexpressing Her2. Cancer Res.
67:11991–11999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Akiyama S, Furukawa T, Sumizawa T, et al:
The role of thymidine phosphorylase, an angiogenic enzyme, in tumor
progression. Cancer Sci. 95:851–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Albanell J, Codony J, Rovira A, Mellado B
and Gascón P: Mechanism of action of anti-HER2 monoclonal
antibodies: Scientific update on trastuzumab and 2C4. Adv Exp Med
Biol. 532:253–268. 2003. View Article : Google Scholar : PubMed/NCBI
|