Introduction
In Japan, the standard chemotherapy for advanced or
recurrent gastric cancer is a combination of S-1 and CDDP. However,
in a phase III clinical trial (1),
the median overall survival following combination therapy was only
13 months, indicating a poor prognosis for patients with advanced
gastric cancer. Furthermore, administration of this combination
therapy requires hospitalization for hydration to prevent
nephrotoxicity from CDDP (2) and is
associated with adverse effects, including severe nausea,
neurotoxicity and allergic reactions (3). Due to these adverse effects,
discontinuation of CDDP treatment is often necessary. As an
alternative, combination therapy with S-1 and oxaliplatin is
considered standard for advanced gastric cancer (4) and it may be administered on an
outpatient basis, as no hydration is required with oxaliplatin
administration. However, peripheral neuropathy is a major adverse
effect of oxaliplatin and often warrants discontinuation of
treatment due to the compromised quality of life.
Drug delivery systems include active and passive
targeting approaches. Passive targeting may be achieved through the
enhanced permeability and retention (EPR) effect (5), which leads to selective accumulation of
micelle-incorporated anticancer agents in tumors and prominent
antitumor effects, while decreasing toxicity of the drug payload
(6). NC-6004, which contains
CDDP-incorporating polymeric micelles, was developed to reduce the
adverse effects of CDDP and enhance its antitumor activity
(7). We previously reported the
improved antitumor effects and reduced toxicity, including reduced
nephrotoxicity, of NC-6004 in vivo (8). Peak urinary concentrations of CDDP
correlate better with toxicity compared with total renal platinum
concentrations (9); hence, CDDP
nephrotoxicity is considered to be dependent on peak urinary
concentrations and maximum CDDP concentrations in the uriniferous
tubules. Therefore, strategies that allow gradual, rather than
sudden, proximal and distal renal tubular CDDP accumulation may
ameliorate nephrotoxicity. NC-6004 requires no concomitant
medications or hydration and was shown to eliminate CDDP toxicity
without attenuating the antitumor effect (10). A phase I study of NC-6004 in patients
with advanced solid tumors demonstrated that delayed and sustained
release of CDDP following intravenous administration significantly
reduced the renal toxicity of NC-6004. In addition,
gastrointestinal (GI) toxicity was significantly reduced, as almost
all patients in the phase I trial only experienced grade 1 or weak
GI toxicity (11).
The aim of the present study was to compare the
effects of combined treatment with NC-6004/S-1 with the effects of
CDDP/S-1 treatment in a human gastric cancer model.
Materials and methods
Chemotherapeutic agents
NC-6004, which consists of polyethylene glycol, a
hydrophilic chain constituting the outer shell of micelles, and the
coordinate complex of poly (glutamic acid) and CDDP (12), was prepared by NanoCarrier Co., Ltd.
(Kashiwa, Japan). CDDP was purchased from Nippon Kayaku Co., Ltd.
(Tokyo, Japan). S-1 was purchased from Taiho Pharmaceutical Co.,
Ltd. (Tokyo, Japan). 5-FU was purchased from Kyowa Hakko Kirin Co.,
Ltd. (Tokyo, Japan).
Cell culture
The 44As3Luc human signet ring cell gastric cancer
cell line, which stably expresses firefly luciferase (12), was kindly provided by Dr K.
Yanagihara (National Cancer Center Hospital East, Kashiwa, Japan).
MKN45 and MKN74 cells were purchased from the JCRB Cell Bank
(Osaka, Japan). The cell lines were maintained in RPMI-1640 medium
(Wako, Osaka, Japan) containing 10% fetal bovine serum (Roche
Diagnostics, Tokyo, Japan), 100 U/ml penicillin, 100 µg/ml
streptomycin and 25 µg/ml amphotericin B (Wako) in a humidified
atmosphere containing 5% CO2 at 37°C.
In vitro growth inhibition assays
The growth inhibitory effects of NC-6004, CDDP and
5-FU were investigated using tetrazolium salt-based proliferation
assays (WST-8 assay; Wako). In these in vitro studies, 5-FU
was used instead of S-1, as tegafur is a fluorouracil prodrug that
is mainly activated in the liver. S-1 consists of tegafur, two
modulators of 5-FU metabolism, 5-chloro-2,4-dihydroxypyridine
(CDHP), a reversible inhibitor of dihydropyrimidine dehydrogenase
(DPD), and potassium oxonate, in a molar ratio of 1: 0.4:1.
Tegafur, an oral prodrug of 5-FU, is gradually converted to 5-FU
and rapidly metabolised by DPD in the liver. CDHP exhibits a
DPD-inhibitory activity that is 180-fold higher compared with that
of uracil, and was confirmed to be an effective DPD inhibitor in
the form of uracil/tegafur (UFT) in vitro. Potassium oxonate
is an orotate phosphoribosyl transferase inhibitor that is
distributed primarily to the gastrointestinal tract. This component
of S-1 decreases the incorporation of 5-fluorouridine triphosphate
into RNA in the gastrointestinal mucosa and reduces the incidence
of diarrhea (13). The 44As3Luc,
MKN45 and MKN74 cells were seeded into 96-well plates at 3,000
cells/well in a final volume of 100 µl, and were incubated for 24 h
at 37°C. Following removal of the media, 100-µl aliquots of medium
containing various concentrations of each drug were added to the
wells, and the cells were incubated for 72 h at 37°C. After
removing the media, 10 µl of WST-8 solution and 90 µl of media were
added to wells, and the cells were incubated for another 3 h at
37°C. Subsequently, absorbance of the formazan product was detected
at 450 nm using a 96-well spectrophotometric plate reader
(SpectraMax 190, Molecular Devices Corp., Sunnyvale, CA, USA). The
experiments were performed in triplicate and repeated at least
three times. Cell viability data are expressed as means and were
normalized to data from non-treated control cells to generate
dose-response curves. Finally, the 50% inhibitory concentrations
(IC50) were determined from the dose-response
curves.
Drug interaction analyses
Interactions between NC-6004 or CDDP and 5-FU in
gastric cancer cell lines were evaluated according to the
combination index (CI) method described by Chou and Talalay
(14). The data were analyzed using
Calcusyn software (Biosoft, New York, NY, USA) and 5-FU was
combined with NC-6004 or CDDP at fixed ratios spanning the
individual IC50 values for each drug. For any given drug
combination, the CI values represent degrees of synergy and
antagonism, and are expressed in terms of the fraction affected
(Fa) values, which represent percentages of cells killed or
inhibited by the drug. Fa/CI plots were constructed using computer
analyses of data generated from median effect analyses.
Experimental mouse models
A total 150 6-week-old female BALB/c nude mice,
weighing ~16 g, were purchased from SLC Japan (Shizuoka, Japan).
The mice were housed in cages under specific pathogen-free
conditions and were provided free access to sterile food and water.
Seven-week-old mice were subcutaneously inoculated in the flank
with 1×106 44As3Luc cells. All animal procedures were
performed in compliance with the guidelines for the care and use of
experimental animals established by the Committee for Animal
Experimentation of the National Cancer Center. These guidelines
meet the ethical standards required by law and comply with the
guidelines for the use of experimental animals in Japan.
In vivo growth inhibition assays
The tumors were grown to a volume of 70
mm3, and the mice were randomly divided into test groups
of 4 or 6 mice (4 for the control and 6 for the treatment group)
(day 0). Subsequently, NC-6004 and CDDP were administered
intravenously via the lateral tail veins, whereas S-1 was
administered orally. The normal control group received a 0.9% NaCl
solution. The body weight and body length (a) and the width of the
tumor mass (b) was measured twice weekly. The tumor volume (TV) was
calculated using the following equation: TV=(axb2)/2.
For humane reasons, animals with TV >2,000 mm3 were
sacrificed.
We initially evaluated the antitumor effects of
NC-6004, CDDP, or S-1 individually. NC-6004 (an equivalent dose of
CDDP) and CDDP were administered at doses of 2.5, 5.0 and 7.5
mg/kg, and S-1 was administered at doses of 5.0, 10.0, and 15.0
mg/kg. NC-6004 and CDDP were administered on days 1 and 8, and S-1
was administered on days 0–4 and 7–11. The antitumor effects of
NC-6004/S-1 or CDDP/S-1 were evaluated following administration of
2.5 mg/kg NC-6004 or 2.5 mg/kg CDDP plus 10 mg/kg S-1. NC-6004,
CDDP and S-1 were administered according to the abovementioned
schedules. In further analyses, enhancement of the antitumor effect
was evaluated following dose escalation of combination therapies as
follows: Mice from the combination therapy groups were treated with
10 mg/kg S-1 plus 5.0 or 7.5 mg/kg NC-6004. Combination S-1/CDDP
therapy was administered as follows: 10 mg/kg S-1 plus 5.0 or
7.5-mg/kg CDDP. NC-6004 and CDDP were administered on days 1, 8 and
15, whereas S-1 was administered on days 0–4, 7–11 and 14–18.
Treatment was discontinued when the mean body weight decreased
>10% from the baseline.
Evaluation of nephrotoxicity and
pathological examination of the small intestine
Female BALB/c nude mice were divided into four
groups of 4–6 mice each (four for control group and six for
treatment group): The S-1/NC-6004 group, the S-1/CDDP group, the
S-1 group and the control group. NC-6004, CDDP and S-1 were
administered according to the dosing schedules described above,
using 10 mg/kg S-1 plus 7.5 mg/kg NC-6004 or 10 mg/kg S-1 plus 7.5
mg/kg CDDP. In this experiment, treatment was continued even when
the mean body weight decreased >10% from the baseline. On day
21, the mice were sacrificed under general anesthesia, blood
samples were collected, and the small intestine was removed for
further investigation. The creatinine levels in the blood samples
were determined by SRL Laboratories (Tokyo, Japan). The small
intestine was excised at the middle portion of the ileum, fixed in
10% formalin, embedded in paraffin, sectioned and stained with
hematoxylin and eosin.
Statistical analysis
All data are expressed as mean ± standard deviation
(SD). Tumor growth inhibition effect, body weight changes and renal
function (serum creatinine level) were compared using Dunnett's
two-tailed t-tests. Statistical analyses were performed
using JMP 10 (SAS Institute, Inc., Cary, NC, USA) and differences
were considered statistically significant when P<0.05.
Results
In vitro growth inhibition assays
The IC50 values of NC-6004 in gastric
cancer cell lines ranged from 21.75 (44As3Luc) to 118.13 µmol/l
(MKN74; Table I). In agreement with
a previous study (8), the cytotoxic
effect of NC-6004 was between 6.1-and 14.0-fold lower compared with
that of CDDP. Hence, molar ratios of 1:1 and 1:10 were used for the
5-FU:CDDP and 5-FU:NC-6004 combination studies, respectively.
 | Table I.IC50 values (µmol/l) of
CDDP and NC-6004 in the 44As3Luc, MKN45 and MKN74 gastric cancer
cell lines. |
Table I.
IC50 values (µmol/l) of
CDDP and NC-6004 in the 44As3Luc, MKN45 and MKN74 gastric cancer
cell lines.
|
| IC50 (µmol/l) ±
standard deviation |
|---|
|
|
|
|---|
| Cell line | 5-FU | CDDP | NC-6004 |
|---|
| 44As3Luc | 3.90±0.15 | 1.94±0.20 |
21.75±0.76 |
| MKN45 | 5.69±0.50 | 9.52±1.32 | 133.13±4.93 |
| MKN74 | 3.21±0.39 | 19.38±1.15 | 118.13±9.15 |
Drug interactions between 5-FU and
CDDP or NC-6004
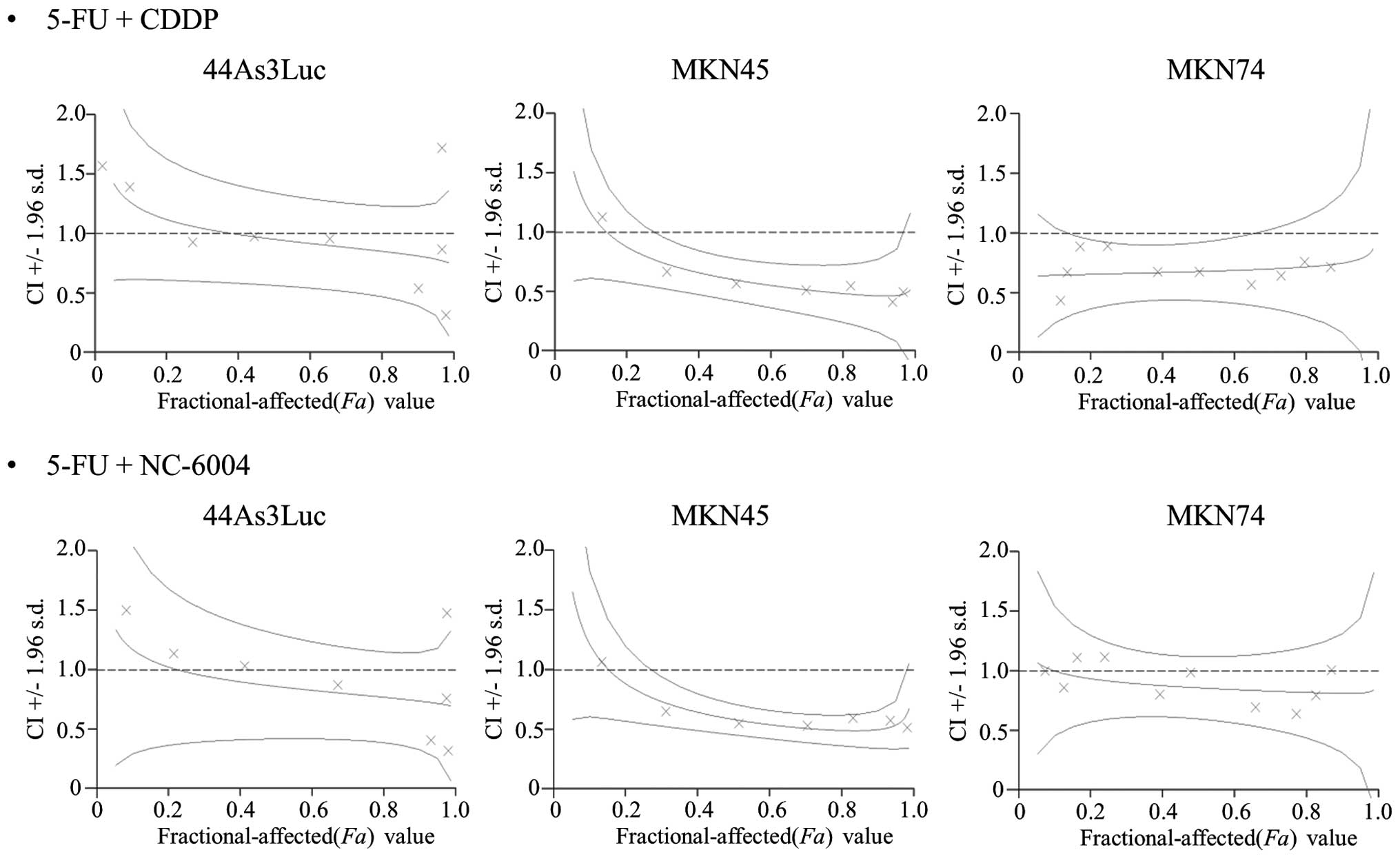
CIs of <1.0, 1.0 and >1.0 are indicative of
synergistic, additive and antagonistic interactions between two
agents, respectively. In all three cell lines, synergy was observed
at Fa values 0.5–1.0 for both combination therapies (Fig. 1), and the synergy between 5-FU and
NC-6004 was almost equivalent to the synergy between 5-FU and CDDP
in 44As3Luc cells.
In vivo antitumor effects of single
agents and combinations in 44As3Luc tumors
The antitumor effects and body weight loss following
administration of NC-6004, CDDP and combinations of 10 mg/kg S-1
plus 2.5 mg/kg CDDP or NC-6004 were similar with the antitumor
effects and body weight loss following administration of NC-6004
and CDDP, and both factors increased in a dose-dependent manner
(data not shown). Combination treatment with S-1 and 7.5 mg/kg CDDP
was discontinued on day 14 due to body weight losses of >10%
from the baseline. However, the therapeutic effect of S-1 plus 5.0
mg/kg NC-6004 did not differ significantly from the effect of S-1
and 5.0 mg/kg CDDP. Similarly, the therapeutic effect of S-1 plus
7.5 mg/kg NC-6004 did not differ significantly from the effect of
S-1 plus 5.0 mg/kg NC-6004 or CDDP until day 21, although a
non-significant trend toward greater efficacy of S-1 plus 7.5 mg/kg
NC-6004 was observed, starting on day 21. By contrast, the body
weight changes were significantly more prominent in the group
treated with S-1 plus 5.0 mg/kg CDDP compared with those in the
group treated with S-1 plus 5.0 mg/kg NC-6004. There were no
significant differences in body weight loss between the S-1 plus
5.0 mg/kg NC-6004 and S-1 plus 7.5 mg/kg NC-6004 groups. However,
the combination of S-1 plus 7.5 mg/kg CDDP led to severe body
weight loss compared with all the other groups.
Nephrotoxicity and intestinal toxicity
of combination therapies
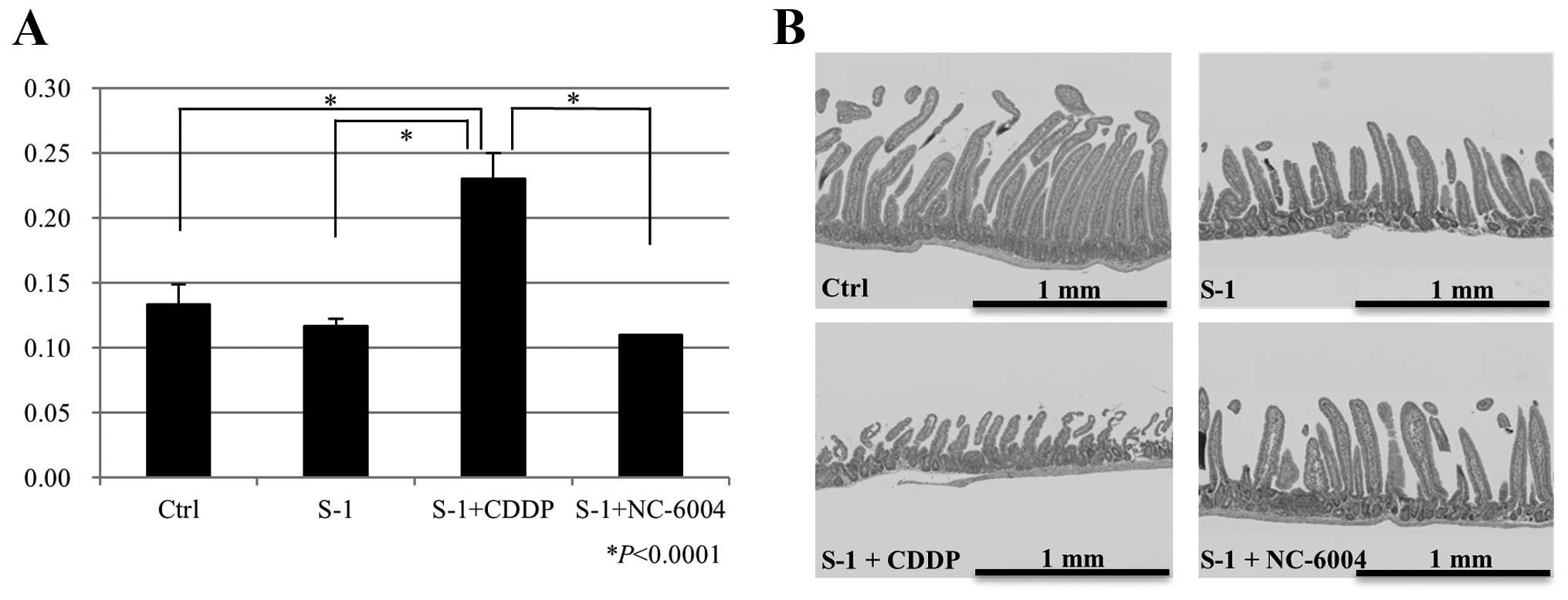
The plasma concentrations of creatinine ± SD on day
21 after administration of 0.9% NaCl, S-1, S-1/CDDP, or S-1/NC-6004
were 0.13±0.02, 0.12±0.01, 0.23±0.02 and 0.11±0.00 mg/dl,
respectively. The S-1 plus 7.5 mg/kg CDDP group had significantly
higher creatinine plasma concentrations compared with the control
group (P<0.0001), the S-1 group (P<0.0001) and the S-1 plus
NC-6004 group (P<0.0001; Fig.
3A).
Regular alignment of normal villi in the jejunal and
ileal mucosa from mice treated with the 0.9% NaCl solution was
observed. By contrast, the jejunal and ileal mucosa from mice in
the S-1, S-1/CDDP and S-1/NC-6004 treatment groups displayed
deformed villi with decreased villus height and width. In
particular, mice treated with S-1/CDDP exhibited more severe
deformations and changes in villus size, and more severe decreases
in villus density compared with mice treated with S-1 and
S-1/NC-6004 (Fig. 3B).
Discussion
In the present study, the antitumor activity and the
toxicity of S-1/NC-6004 combination therapy were compared to those
of S-1/CDDP combination therapy, which is one of the most common
treatments currently used for gastric cancer. Our data indicate
that S-1/NC-6004 therapy is significantly less toxic compared with
S-1/CDDP therapy. Moreover, the antitumor activity of S-1/NC-6004
did not differ significantly from the antitumor activity of
S-1/CDDP in the mouse model of human gastric cancer used in this
study. Furthermore, when administered in combination with S-1, the
antitumor effects of NC-6004 and CDDP increased in a dose-dependent
manner compared with the effects of treatment with a single
therapeutic agent. However, the toxicity of CDDP also increased in
a dose-dependent manner, necessitating the cessation of treatment
with 10 mg/kg S-1 plus 7.5 mg/kg CDDP. By contrast, the toxicity of
NC-6004 did not increase in a dose-dependent manner, and combined
treatment with 10 mg/kg S-1 and 7.5 mg/kg NC-6004 was
well-tolerated. Furthermore, the combination of S-1 and NC-6004 did
not cause nephrotoxicity. It was previously suggested that S-1
dosing should be determined based on renal function, as excessive
concentrations of S-1 in the blood cause severe adverse effects,
including bone marrow suppression and GI toxicity (13). Therefore renal function must be
closely monitored during combination treatment with S-1 and CDDP.
By contrast, NC-6004 is less likely to cause renal dysfunction
compared with CDDP, and myelosuppression and GI toxicity may be
comparatively limited following combination therapy with S-1.
Although we did not measure blood concentrations of the tegafur/S-1
metabolite 5-FU, our data suggest that elevated concentrations of
S-1 in the blood contributed to GI toxicity due to CDDP-induced
renal function impairment.
In the phase I clinical trial of NC-6004 (11), the recommended dose was defined as
the equivalent of a 90 mg/m2 dose of CDDP. This trial
also demonstrated that excessive hydration is not required during
administration of NC-6004, allowing safe administration on an
outpatient basis. Accordingly, a phase III clinical trial in
pancreatic cancer and a phase II trial in non-small-cell lung
cancer are currently underway. Combination chemotherapy with CDDP
has been used as a standard treatment regimen for gastric cancer as
well as several other types of cancer. Replacing CDDP with NC-6004
may lead to reduced toxicity of these treatment regimens and
improve the quality of life of cancer patients.
In conclusion, the present preclinical study
demonstrated that combined treatment with S-1 and NC-6004 is
associated with significantly lower toxicity compared with S-1 and
CDDP, while retaining similar CDDP-mediated antitumor activity.
These data warrant further clinical studies of the combination of
S-1 and NC-6004 in patients with advanced gastric cancer.
Acknowledgements
The present study was supported by the National
Cancer Center Research and Development Fund (Y. Matsumura) and by
the Japan Society for the Promotion of Science (JSPS), through the
‘Funding Program for World-Leading Innovative R&D on Science
and Technology’ (FIRST). The authors would like to thank Mr.
Akifumi Furuya and Ms. Mari Mizoguchi for their technical
assistance, and Ms. Madoka Nakayama for the secretarial
assistance.
Glossary
Abbreviations
Abbreviations:
|
CDDP
|
cisplatin
|
|
5-FU
|
5-fluorouracil
|
|
EPR
|
enhanced permeability and
retention
|
|
GI
|
gastrointestinal
|
|
IC50
|
50% inhibitory concentration
|
|
CI
|
combination index
|
|
Fa
|
fraction affected
|
|
TV
|
tumor volume
|
|
SD
|
standard deviation
|
References
|
1
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pinzani V, Bressolle F, Haug IJ, Galtier
M, Blayac JP and Balmès P: Cisplatin-induced renal toxicity and
toxicity-modulating strategies: A review. Cancer Chemother
Pharmacol. 35:1–9. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartmann JT and Lipp HP: Toxicity of
platinum compounds. Expert Opin Pharmacother. 4:889–901. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamada Y, Higuchi K, Nishikawa K, Gotoh M,
Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al:
Phase III study comparing oxaliplatin plus S-1 with cisplatin plus
S-1 in chemotherapy-naïve patients with advanced gastric cancer.
Ann Oncol. 26:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
6
|
Maeda H and Matsumura Y: EPR effect based
drug design and clinical outlook for enhanced cancer chemotherapy.
Adv Drug Deliv Rev. 63:129–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishiyama N and Kataoka K: Preparation and
characterization of size-controlled polymeric micelle containing
cis-dichlorodiammineplatinum(II) in the core. J Control Release.
74:83–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uchino H, Matsumura Y, Negishi T, Koizumi
F, Hayashi T, Honda T, Nishiyama N, Kataoka K, Naito S and Kakizoe
T: Cisplatin-incorporating polymeric micelles (NC-6004) can reduce
nephrotoxicity and neurotoxicity of cisplatin in rats. Br J Cancer.
93:678–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Levi F, Hrushesky WJ, Borch RF, Pleasants
ME, Kennedy BJ and Halberg F: Cisplatin urinary pharmacokinetics
and nephrotoxicity: A common circadian mechanism. Cancer Treat Rep.
66:1933–1938. 1982.PubMed/NCBI
|
|
10
|
Mizumura Y, Matsumura Y, Hamaguchi T,
Nishiyama N, Kataoka K, Kawaguchi T, Hrushesky WJ, Moriyasu F and
Kakizoe T: Cisplatin-incorporated polymeric micelles eliminate
nephrotoxicity, while maintaining antitumor activity. Jpn J Cancer
Res. 92:328–336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Plummer R, Wilson RH, Calvert H, Boddy AV,
Griffin M, Sludden J, Tilby MJ, Eatock M, Pearson DG, Ottley CJ, et
al: A Phase I clinical study of cisplatin-incorporated polymeric
micelles (NC-6004) in patients with solid tumours. Br J Cancer.
104:593–598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yanagihara K, Takigahira M, Takeshita F,
Komatsu T, Nishio K, Hasegawa F and Ochiya T: A photon counting
technique for quantitatively evaluating progression of peritoneal
tumor dissemination. Cancer Res. 66:7532–7539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Booka E, Imamura CK, Takeuchi H, Hamamoto
Y, Gomi D, Mizukami T, Ichiyama T, Tateishi K, Takahashi T,
Kawakubo H, et al: Development of an S-1 dosage formula based on
renal function by a prospective pharmacokinetic study. Gastric
Cancer. 19:876–886. 2016. View Article : Google Scholar : PubMed/NCBI
|