Introduction
Endometrial cancer is one of the most common
gynecological malignancies. The standard treatment for early-stage
endometrial cancer is primarily total abdominal hysterectomy and
bilateral salpingo-oophorectomy, with additional pelvic and
para-aortic lymph node dissection (1). By contrast, preservation of fertility
for early-stage endometrial cancer has become more important due to
the increasing age of first parity in recent years. Use of
fertility-sparing approaches, such as hormonal therapies using
medroxyprogesterone acetate (MPA) or megestrol acetate,
gonadotropin-releasing hormone-agonist, and intrauterine devices,
which release levonorgestrel, have been reported (2–9).
Of these, treatment with MPA is commonly used as a
fertility-sparing approach in Japan, and various studies have shown
its efficacy (10–13). It has been reported that the complete
response (CR) rate for atypical endometrial hyperplasia by initial
treatment with MPA was >80%. However, only 55–75% of patients
with grade 1 endometrioid adenocarcinoma (EmCa, G1) treated by MPA
achieved CR. Currently, frequent pathological inspections are
required to evaluate the efficacy of MPA treatment; however,
repeated endometrial curettages are invasive, and can reduce
fertility by injury to the endometrium (14,15).
Therefore, establishment of biological markers predicting the
efficacy of MPA is required.
However, there have been few studies that indicate
the predictive factors of the success of hormonal therapies other
than histological markers, such as estrogen and progesterone
receptor (ER, PgR) expression (16,17).
Although sensitivity to MPA is thought to be associated with the
presence of ER and PgR, it is known that patients with
receptor-positive tumors do not necessarily respond completely to
MPA.
Measurement of endometrial thickness using
transvaginal ultrasonography (TVUS) is a convenient and widely-used
technique, and can be used as a non-invasive screening test for
detection of endometrial cancer. Endometrium thicker than 5 or 20
mm for post- or pre-menopausal women, respectively, has been shown
to be a clinically useful predictive marker for endometrial cancer
(18).
Considering the universal use of TVUS and the
clinical significance of endometrial thickness, measurement of
changes in endometrial thickness during treatment may be used as a
non-invasive and useful predictive marker for the therapeutic
efficacy of MPA therapy. In the present study, endometrial
thickness was measured by TVUS during MPA therapy and its
association with pathological response to MPA was assessed.
Materials and methods
Patients
The study was approved by the institutional ethics
committee (The University of Tokyo, Tokyo, Japan). Medical records
and data from the gynecological oncology database of patients who
had undergone treatment with MPA at the University of Tokyo
Hospital between 1999 and 2012 were retrospectively reviewed.
Patients were deemed suitable for this
fertility-sparing treatment provided they met the following
criteria: i) Age <40 years, ii) pathological diagnosis of
endometrioid endometrial adenocarcinoma with grade 1
differentiation (EmCa, G1), iii) absence of myometrial invasion and
extrauterine spread on magnetic resonance imaging, and iv) a desire
of the patient to preserve their fertility and undergo hormonal
therapy following a detailed discussion regarding the current
standard treatment and requirement for follow-up with pathological
examinations.
MPA therapy and clinical
assessment
All the patients who met the inclusion criteria were
administered MPA (600 mg/day with 81–100 mg of aspirin) orally, and
this was continued for 26 weeks. As an interim evaluation,
pathological response to MPA was assessed by histological
examination at weeks 8 and 16 of the treatment period by performing
dilatation and total curettage (D&C). The histological
diagnoses were confirmed by an experienced pathologist (or central
pathological review if it was difficult to diagnose). As an
end-point, total or fractional curettage was performed at week 26
of the treatment for histological assessment. A CR was defined as
the status with evidence of neither carcinoma nor hyperplasia at
week 26 of the treatment.
Measurement of endometrial thickness
by TVUS
Endometrial thickness was measured using TVUS before
D&C at weeks 8 and 16 of the treatment. The measuring procedure
was as follows: i) Detect the uterus in sagittal view, ii) detect
the mid-line view where the endometrium was visible from the fundus
to the cervix of the uterus in succession, iii) keep the line
perpendicular to the mid-line, and iv) measure the endometrium by
keeping the endpoints of the line positioned on the outer edge of
each side of the endometrium (13).
Statistical analysis
Logistic regression was used to calculate any
statistical differences. Receiver operating characteristic (ROC)
curves were used to assess the criterion value. P<0.05 was
considered to indicate a statistically significant difference. JMP®
(SAS Institute, Inc., Cary, NC, USA) was used for statistical
analysis.
Results
Patient characteristics
A total of 32 patients were enrolled in the present
study. Patient backgrounds are described as follows. The mean age
at initiation of treatment was 33 years (range, 19–39 years). Of
them, 31 (97%) patients were nulliparous. The mean body mass index
(BMI) was 20.1 kg/m2 (range, 17.1–37.4 kg/m2)
. Six (19%) patients had polycystic ovary syndrome. One (3%)
patient was positive for carbohydrate antigen 125 (CA125) and 3
(9%) for CA19–9.
CR for patients
CR was achieved in 19 (59%) patients with carcinoma.
The remaining 13 were non-CR patients, of whom seven underwent
curative surgery (hysterectomy + bilateral salpingo-oophorectomy)
at week 16, and four patients at week 26 of treatment. Two patients
refused to undergo curative surgery despite not achieving CR by
week 26 of treatment, and was followed by the administration of
oral estrogen-progestin treatment for at least 6 months, resulting
in no evidence of malignancy.
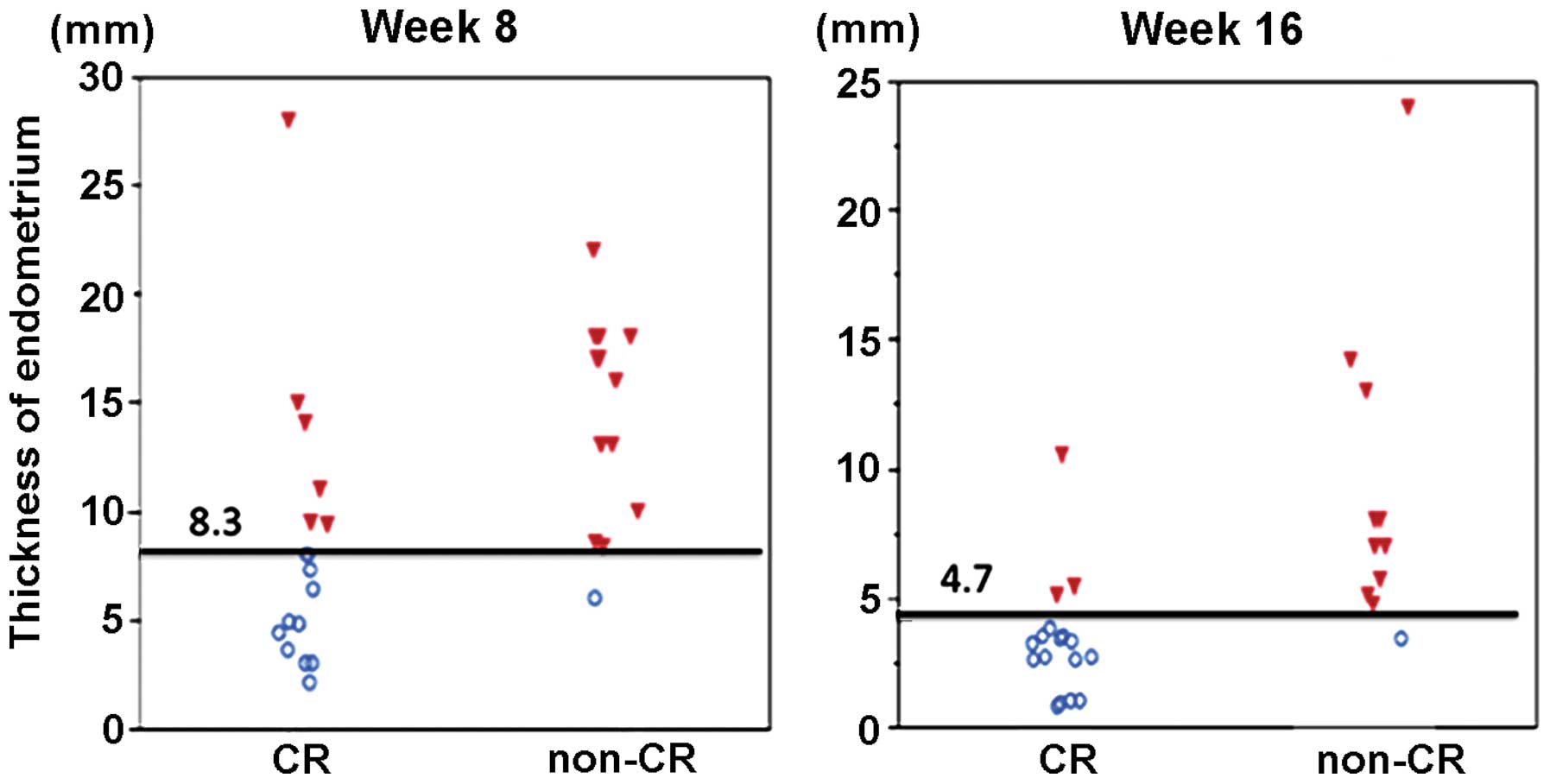
Endometrial thickness
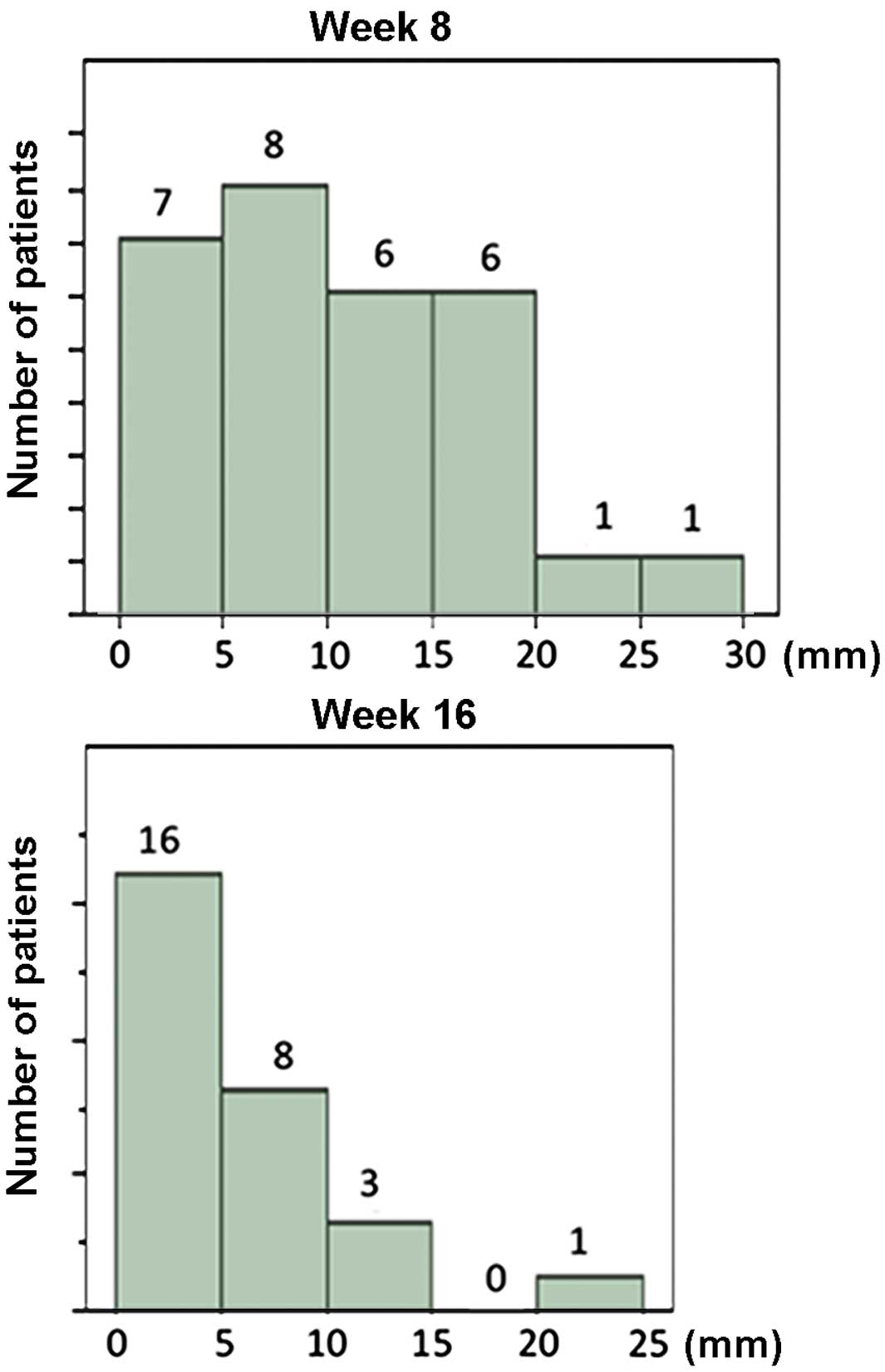
The distribution of endometrial thickness at weeks 8
and 16 of treatment are shown in Fig.
1; all data were included in this analysis. With continuing MPA
therapy, the endometrium tended to become thinner. The regression
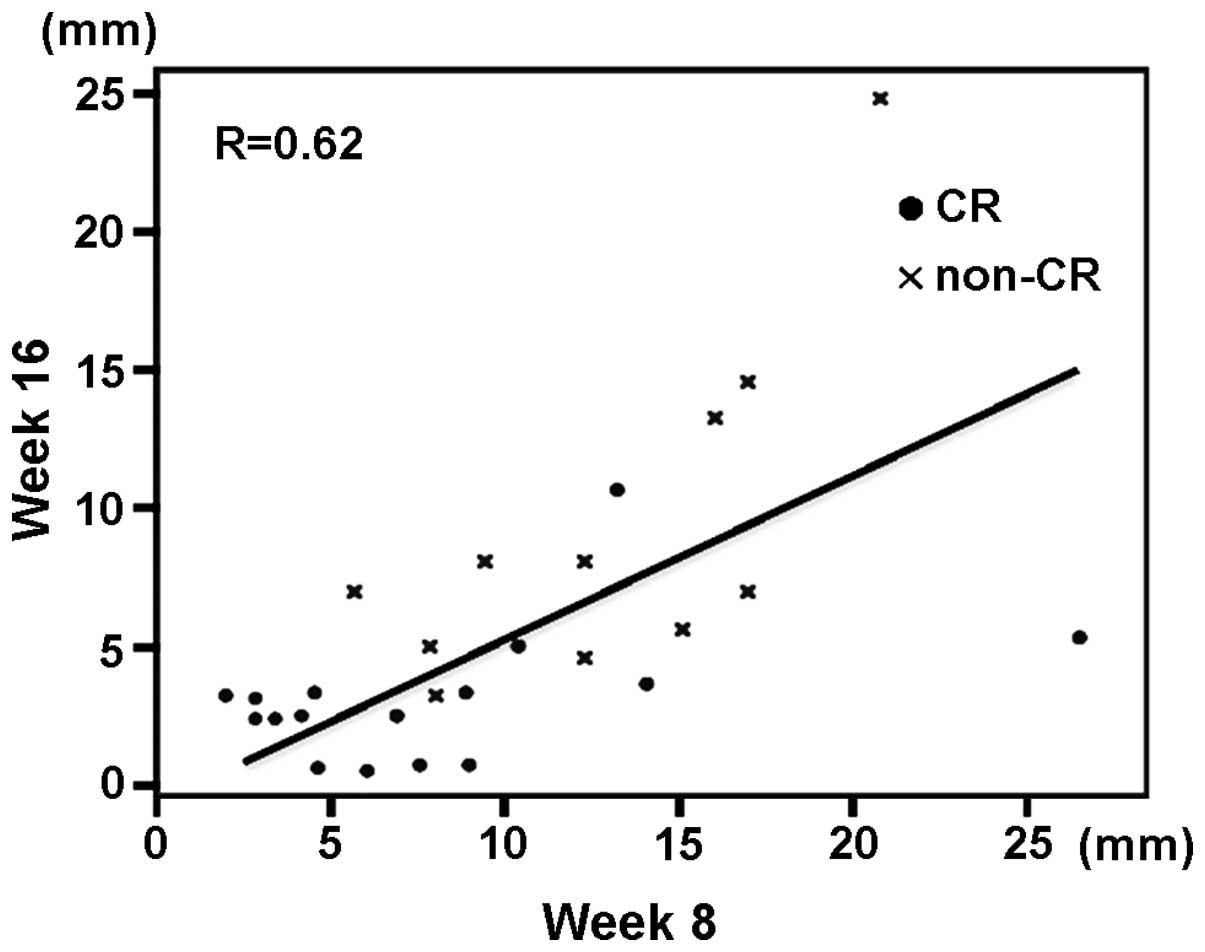
line for endometrial thickness at weeks 8 and 16 is shown in
Fig. 2, with a correlation
coefficient of 0.62. Non-CR patients had significantly thicker
endometrium than that of CR patients at weeks 8 and 16 of
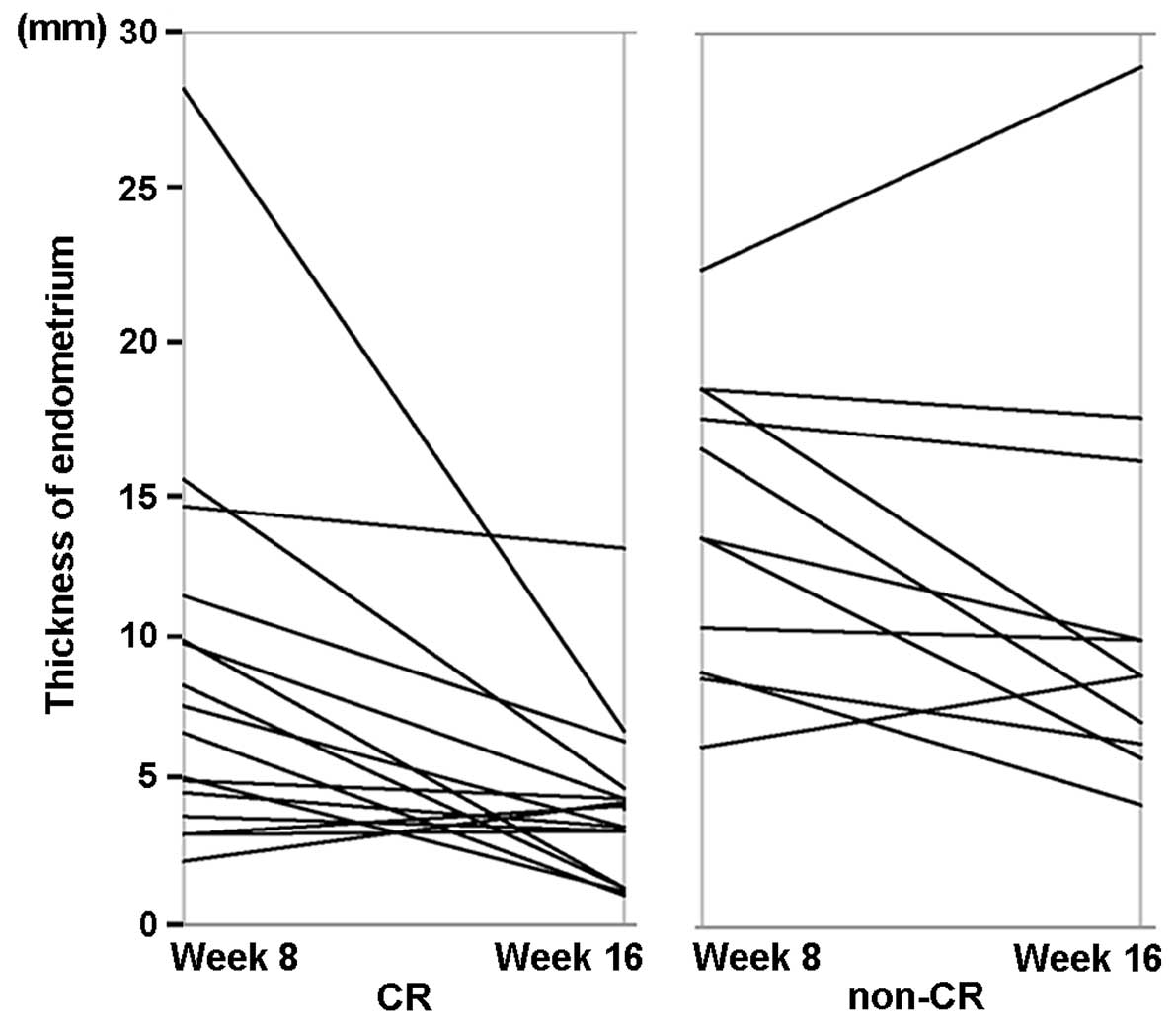
treatment. Changes in endometrial thickness at weeks 8 and 16 in
the same patient are shown in Fig. 3.
CR patients tended to have a more notable reduction of endometrial
thickness from week 8 to week 16, which may be attributable to the
MPA therapy.
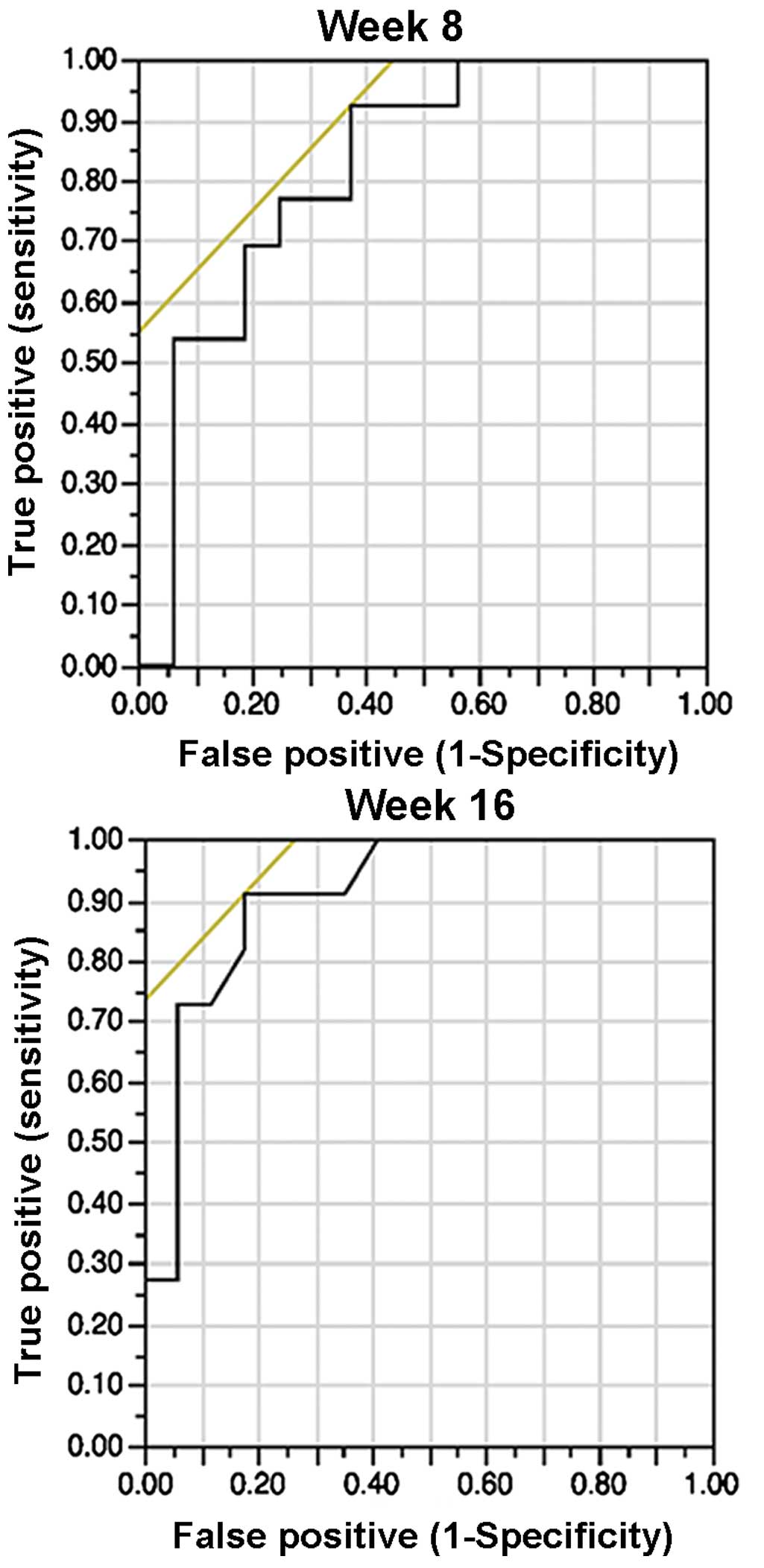
Cut-off
The cut-off points were chosen based on the ROC
curve: 8.3 mm with a sensitivity/specificity of 0.92/0.63 at week
8, and 4.7 mm with a sensitivity/specificity of 0.91/0.81 at week
16 (Fig. 4). Therefore, the values of
8 and 5 mm for weeks 8 and 16, respectively, were chosen as
representative values for clinical significance and easiness to
apply in daily practice.
Univariate analysis
Univariate analysis revealed that endometrial
thickness at weeks 8 and 16 of MPA therapy were significant
predictive markers for non-CR by week 26. Furthermore, multivariate
analysis demonstrated that endometrial thickness at week 16 of
treatment was an independent predictive marker for final non-CR by
week 16 (Table I).
 | Table I.Predictive factors of non-CR with MPA
therapy. |
Table I.
Predictive factors of non-CR with MPA
therapy.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Characteristics | Odds ratio (95%
CI) | P-value | Odds ratio (95%
CI) | P-value |
|---|
| Age >30 years | 4.95
(0.85–28.6) | 0.0533 | 6.68
(0.57–184.54) | 0.1336 |
| >8 mm endometrium
at week 8 | 15.4 (1.59–148) | 0.0038a | – | – |
| >5 mm endometrium
at week 16 | 19.5 (2.69–141) | 0.0008a | 20.52
(3.05–231.16) | 0.0012a |
| BMI >25 | 2.62
(0.35–19.1) | 0.3356 | – | – |
| PCOS positive |
0.68
(0.105–4.41) | 0.6839 | – | – |
Discussion
Fertility-sparing approaches are often sought for
younger patients with early-stage endometrial cancer. In the
present study, endometrial thickness at weeks 8 and 16 of MPA
treatment in patients with EmCa, G1 showed significant correlation
with pathological response to MPA; thicker endometrium (>8 mm at
week 8 or >5 mm at week 16) suggested a higher likelihood of
non-CR by the end-point (week 26). These results are plausible in
the context of daily clinical practice and is in accordance with a
previous study (13). To the best of
our knowledge, only the study by Ushijima et al (13) has suggested the usefulness of TVUS for
assessment of response to MPA treatment. The study concluded that a
thinner endometrium at 8 weeks compared to pretreatment thickness
predicted a 73% possibility of achieving CR at 26 weeks, whereas a
thicker endometrium at 8 weeks was predicted to have a 25%
possibility of achieving CR at 26 weeks. In the present study,
endometrial thickness at pretreatment was not assessed as certain
patients had already undergone D&C prior to initial
consultation, and their endometrium thickness was thought to be
thinner than prior to curettage.
The present results suggested that endometrial
thickness at weeks 8 and 16 in the same patient tended to be
correlated. In all cases, no clear residual endometrium was
observed by TVUS after each D&C, and thereby endometrial
thickness at weeks 8 and 16 was thought to not be influenced by
residual tissues prior to D&C. In addition, the absolute change
of thickness from weeks 8 to 16 was not correlated with
pathological response (CR or non-CR) (P=0.861, data not shown). The
reciprocal change of endometrial thickness at weeks 8 and 16 may
suggest that endometrial thickness reflects the continuous
suppression of cancer expansion by MPA.
Endometrial thickness at week 16 was a more useful
predictive marker for pathological non-CR than that at week 8,
although the thicknesses at weeks 8 and 16 were correlated
(Fig. 5). Endometrial thickness at
week 8 may be influenced by thickness prior to MPA therapy, while
thickness at week 16 was not likely to be so due to complete
dissection of the endometrium by D&C at week 8. In certain
patients, thickness had decreased significantly between weeks 8 and
16.
The most reliable marker for clinical efficacy of
MPA in EmCa patients is a histological approach by total curettage
(D&C). In the present series, certain patients showed an
unexpected outcome association with endometrial thickness. One
patient had an endometrial thickness of 28 mm (>8 mm) at week 8
and 5.4 mm (>5 mm) at week 16; however, the patient exhibited
pathological CR by the end-point. Another patient had an
endometrial thickness of 3.4 mm (<5 mm) at week 16, but did not
show pathological CR at the end-point (Fig. 3). Therefore, the present data cannot
conclude that TVUS can be used as a complete substitute for biopsy
or D&C. These results warrant a future prospective study, in
which patients with endometrium thicker than 8 mm at week 8 or 5 mm
at week 16 of MPA treatment are recommended to undergo D&C at
each time point, while the remaining patients undergo D&C at
the end-point of the treatment.
In conclusion, endometrial thickness by TVUS during
MPA therapy (at week 16) can be a predictive marker for
pathological response to MPA in patients with EmCa, G1. Considering
the side effects of multiple D&C procedures on reproduction,
non-invasive TVUS for endometrial thickness assessment may be
useful as primary screening for efficacy of MPA therapy, and may
assist in the decision making process of whether D&C should be
performed or not.
References
|
1
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park H, Seok JM, Yoon BS, Seong SJ, Kim
JY, Shim JY and Park CT: Effectiveness of high-dose progestin and
long-term outcomes in young women with early-stage,
well-differentiated endometrioid adenocarcinoma of uterine
endometrium. Arch Gynecol Obstet. 285:473–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalogiannidis I and Agorastos T:
Conservative management of young patients with endometrial
highly-differentiated adenocarcinoma. J Obstet Gynaecol. 31:13–17.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laurelli G, Di Vagno G, Scaffa C, Losito
S, Del Giudice M and Greggi S: Conservative treatment of early
endometrial cancer: Preliminary results of a pilot study. Gynecol
Oncol. 120:43–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bovicelli A, D'Andrilli G, Giordano A and
De Iaco P: Conservative treatment of early endometrial cancer. J
Cell Physiol. 228:1154–1158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morelli M, Di Cello A, Venturella R,
Mocciaro R, D'Alessandro P and Zullo F: Efficacy of the
levonorgestrel intrauterine system (LNG-IUS) in the prevention of
the atypical endometrial hyperplasia and endometrial cancer:
Retrospective data from selected obese menopausal symptomatic
women. Gynecol Endocrinol. 29:156–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cade TJ, Quinn MA, Rome RM and Neesham D:
Progestogen treatment options for early endometrial cancer. BJOG.
117:879–884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramirez PT, Frumovitz M, Bodurka DC, Sun
CC and Levenback C: Hormonal therapy for the management of grade 1
endometrial adenocarcinoma: A literature review. Gynecol Oncol.
95:133–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gunderson CC, Fader AN, Carson KA and
Bristow RE: Oncologic and reproductive outcomes with progestin
therapy in women with endometrial hyperplasia and grade 1
adenocarcinoma: A systematic review. Gynecol Oncol. 125:477–482.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang WH, Chen CH and Yu MH: Conservative
therapy of stage I endometrial adenocarcinoma and atypical
endometrial hyperplasia for the preservation of fertility. Int J
Gynaecol Obstet. 92:137–138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujiwara H, Jobo T, Takei Y, Saga Y, Imai
M, Arai T, Taneichi A, Machida S, Takahashi Y and Suzuki M:
Fertility-sparing treatment using medroxyprogesterone acetate for
endometrial carcinoma. Oncol Lett. 3:1002–1006. 2012.PubMed/NCBI
|
|
12
|
Yahata T, Fujita K, Aoki Y and Tanaka K:
Long-term conservative therapy for endometrial adenocarcinoma in
young women. Hum Reprod. 21:1070–1075. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ushijima K, Yahata H, Yoshikawa H, Konishi
I, Yasugi T, Saito T, Nakanishi T, Sasaki H, Saji F, Iwasaka T, et
al: Multicenter phase II study of fertility-sparing treatment with
medroxyprogesterone acetate for endometrial carcinoma and atypical
hyperplasia in young women. J Clin Oncol. 25:2798–2803. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujimoto A, Ichinose M, Harada M, Hirata
T, Osuga Y and Fujii T: The outcome of infertility treatment in
patients undergoing assisted reproductive technology after
conservative therapy for endometrial cancer. J Assit Repord Genet.
31:1189–1194. 2014. View Article : Google Scholar
|
|
15
|
Shufaro Y, Simon A, Laufer N and Fatum M:
Thin unresponsive endometrium-a possible complication of surgical
curettage compromising ART outcome. J Assit Repord Genet.
25:421–425. 2008. View Article : Google Scholar
|
|
16
|
Yamazawa K, Hirai M, Fujito A, Nishi H,
Terauchi F, Ishikura H, Shozu M and Isaka K: Fertility-preserving
treatment with progestin and pathological criteria to predict
responses, in young women with endometrial cancer. Hum Reprod.
22:1953–1958. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Minaguchi T, Nakagawa S, Takazawa Y, Nei
T, Horie K, Fujiwara T, Osuga Y, Yasugi T, Kugu K, Yano T, et al:
Combined phospho-Akt and PTEN expressions associated with
post-treatment hysterectomy after conservative progestin therapy in
complex atypical hyperplasia and stage Ia, G1 adenocarcinoma of the
endometrium. Cancer Lett. 248:112–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minagawa Y, Sato S, Ito M, Onohara Y,
Nakamoto S and Kigawa J: Transvaginal ultrasonography and
endometrial cytology as a diagnostic schema for endometrial cancer.
Gynecol Obstet Invest. 59:149–154. 2005. View Article : Google Scholar : PubMed/NCBI
|