Introduction
The efficient ‘killer’ in the form of advanced
urothelial carcinoma (UC) is often underestimated at present:
5-year overall survival (OS) rates rarely exceed 40–50%, even
following surgical treatment and adjuvant chemotherapy, with
disappointing progression-free survival (PFS) rates observed of 16
to 18 months (1,2). Following the failure of primary
cisplatin-based combination chemotherapy (CBCC), in general, there
are limited choices for second- or third-line therapies (3). The aim of the present study was to offer
a comparatively safe additional maintenance treatment for patients
with stable disease following CBCC, with the objective of improving
survival rates and the time to first progression. Gemcitabine (GEM)
was selected as the treatment for the present study. After having
noted the promising response rates (4,5) and
encouraging case reports (6),
together with low reported rates of adverse events (4–7), 38
patients, predominantly after having achieved stable disease
post-chemotherapy, were treated quarterly with two consecutive
injections of 1,250 mg/m2 GEM as previously described
(4). Subsequently, OS,
cancer-specific survival (CSS) and PFS rates were determined, in
comparison with a matched control group that received best
supportive care alone.
Patients and methods
Patient characteristics and GEM
monotherapy
The present study included 38 patients suffering
from advanced UC of the lower or upper urinary tract, who were
surgically treated by radical cystoprostatectomy with extended
lymphadenectomy or nephroureterectomy between 1999 and 2013 at our
institution (the ‘maintenance collective’). Patients who did not
receive surgical care prior to adjuvant chemotherapy were excluded
from the study. Indications for adjuvant primary chemotherapy were
an advanced pathological tumor stage of pT3 or above (8), positive nodal status (N+), lymphangiosis
(L1) or haemangiosis (V1) carcinomatosa, or perineural invasion
(Pn1). Following the completion of primary CBCC [a treatment of
methotrexate, vinblastin, adriamycin and cisplatin, or
methotrexate, vinblastin, epirubicin and cisplatin, or GEM and
cisplatin (GC), administered in a standardized regimen (9)], patients were staged by performing
computed tomography (CT; Siemens SOMATOM Force; Siemens Healthcare
AG, Erlangen, Germany) of the thorax and the abdomen. Of the
patients examined, 33 were staged stable disease (n=30) or
remission (n=3), whereas five patients were staged as mixed
response (n=3) or progressive disease (n=2). For one patient, no
information concerning the disease status was available. Further
follow-up was performed every four months by CT of the thorax and
the abdomen, or by magnetic resonance imaging (MRI; Siemens
MAGNETOM Prisma MRI Scanner, Siemens Healthcare AG) of the abdomen
with simultaneous chest X-ray scanning (Philips Bucky DIAGNOST
X-ray system; Philips Medical Systems, Hamburg, Germany).
Progression of the disease was defined as the appearance of any new
lesion and/or a 25% increase in the sum of the pre-existing
lesions. The time to progression was defined as the time from the
initial diagnosis to the first event of clinical progression.
After checking inclusion criteria [sufficient renal
function, i.e., glomerular filtration rate (GFR) >30 ml/min,
hepatic function and the haemogram], all the patients were offered
GEM maintenance therapy, and written informed consent was obtained
from all the patients. The patients were treated quarterly with two
consecutive (on days 1 and 8 of the cycle) short, 30 min infusions
of 1,250 mg/m2 GEM on an outpatient basis. As supportive
agents, the patients received 8 mg orally administered ondansetron
prior to the GEM infusion. Blood tests were performed by the
general practitioner of the patients prior to and following the
therapy to monitor adverse events. GEM monotherapy was discontinued
in the event of a marked clinical progression with rapid decline of
the general health status, and severe renal (GFR <30 ml/min) or
hepatic (>2-fold increase in the level of transaminases)
dysfunction, in addition to severe anaemia, leukopenia or
thrombopenia. Two patients discontinued GEM monotherapy for
personal reasons. Following the discontinuation of GEM monotherapy
due to progression of the disease, a further two patients received
second-line treatment [320 mg/m2 vinflunine in one
patient, and dose-reduced GC in the other (to 50% of the standard
dose in the other patient]. There were no dose reductions of GEM
when administered alone; in patients not qualifying for the full
dose of the drug (1,250 mg/m2), therapy was
discontinued.
Data analysis, matching approach and
the control collective
The present study made use of cases of patients with
UC who had received surgical attention at the Departments of
Urology and Paediatric Urology at the University Medical Center of
Würzburg, and at the University of Marburg (n=62) between 1993 and
2009. These patients receiving the best supportive care alone
following first- or second-line chemotherapy were set as controls
(the ‘control collective’). To improve comparability, the GEM
monotherapy patient collective (n=38) was matched with 38 patients
of the control collective by propensity score matching, using the
bioconductor package ‘MatchIt’ for R, version 3.10 (the type of
matching employed was the nearest neighbour method; http://www.r-project.org). The matching criteria were
age at diagnosis, gender, location of the primary tumor (lower or
upper urinary tract), the tumor/lymph node/metastasis (TNM) status
[using the version of the TNM classification of the International
Union Against Cancer revised in 2009 (8)] and the number of initial chemotherapy
cycles. Kaplan-Meier estimates for the OS, CSS and PFS rates were
realized with the open-source software ‘R’, version 3.10, and the
bioconductor package ‘survival’, as well as Cox proportional hazard
models concerning CSS in the two collectives. The log-rank test was
used to determine statistical differences between survival
estimates, and Pearson's chi-squared test was used to realize
intergroup comparisons. On occasions where two means of normally
distributed data were to be compared, the two-sided unpaired
student's t-test was used. More than two group means were
differentiated by analysis of variance with post hoc testing
(Tukey's test) where significant differences occurred. P<0.05
was taken to indicate a statistically significant difference.
Results
The characteristics of the patient collectives are
shown in Tables I and II. Following matching, no statistical
differences were identified with respect to the age at diagnosis,
gender, site of the primary tumor, the TNM status or the number of
initial chemotherapy cycles. The follow-up was also comparable.
Patients in the monotherapy collective received GC more often than
patients in the control collective did (P<0.01). However, no
significant statistical differences were identified regarding the
efficacy of primary chemotherapy (P=0.06).
 | Table I.Unmatched collectives. |
Table I.
Unmatched collectives.
|
| Patient
characteristics: Unmatched collectives |
|
|---|
|
|
|
|
|---|
| Variable | ‘Maintenance
collective’ | ‘Control
collective’ | P-value |
|---|
| Number of
patients | 38 | 62 |
|
| Follow-up
(months) | 9–148 (37) | 1–99 (35) | 0.062 |
| Age | 33–80 (66) | 26–84 (67) | 0.201 |
| Initial cycles | 1–6 (3.1) | 1–6 (2.8) | 0.25 |
| Mono cycles | 1–48 (5.1) | NA |
|
| Sex |
|
|
|
| Male | 30 (78.9%) | 48 (77.4%) |
|
|
Female | 8 (21.1%) | 14 (22.5%) | 0.84 |
| Localization |
|
|
|
| Lower
urinary tract | 29 (76.3%) | 42 (67.8%) |
|
| Upper
urinary tract | 9 (23.7%) | 20 (32.2%) | <0.01 |
| pTa |
|
|
|
| Tis | 1 (2.6%) | 0 (0.0% |
|
| 1 | 5 (13.1%) | 3 (4.8%) |
|
| 2a | 4 (10.5%) | 6 (9.7%) |
|
| 02b | 4 (10.5%) | 7 (11.3%) |
|
| 3a | 9 (23.7%) | 11 (17.7%) |
|
| 3b | 4 (10.5%) | 20 (32.3%) |
|
| 4a | 8 (21.0%) | 14 (22.6%) | pT1+2 vs.
pT3+4 |
| 4b | 0 (0.0%) | 1 (1.6%) | 0.04 |
| pNa |
|
|
|
| 0/x | 11 (28.9%) | 3 (4.8%) |
|
| 1 | 11 (28.9%) | 20 (32.3%) |
|
| 2 | 13 (34.2%) | 36 (58.1%) | pN0+1 vs.
pN2+3 |
| 3 | 3 (7.8%) | 3 (48.4%) | <0.01 |
| pMa |
|
|
|
| 0 | 32 (84.2%) | 55 (88.7%) |
|
| 1 | 6 (15.7%) | 7 (11.3%) | <0.01 |
 | Table II.Matched collectives. |
Table II.
Matched collectives.
|
| Patient
characteristics: Matched collectives |
|
|---|
|
|
|
|
|---|
| Variable | ‘Maintenance
collective’ | ‘Control
collective’ | P-value |
|---|
| Number of
patients | 38 | 38 |
|
| Follow-up
(months) | 9–148 (37) | 1–97 (34.5) | 0.562 |
| Age (years) | 33–80 (66) | 28–83 (68) | 0.24 |
| Initial cycles | 1–6 (3.1) | 1–5 (2.4) | 0.06 |
| Mono cycles | 1–48 (5.1) | NA |
|
| Gender |
|
|
|
| Male | 30 (78.9%) | 29 (76.3%) |
|
|
Female | 8 (21.1%) | 9 (23.7%) | 0.7 |
| Localization |
|
|
|
| Lower
urinary tract | 29 (76.3%) | 30 (78.9%) |
|
| Upper
urinary tract | 9 (23.7%) | 8 (21.1%) | 0.69 |
| pTa |
|
|
|
|
Tis | 1 (2.6%) | 0 (0.0% |
|
| 1 | 5 (13.1%) | 0 (0.0%) |
|
| 2a | 4 (10.5%) | 6 (15.8%) |
|
| 2b | 4 (10.5%) | 5 (13.2%) |
|
| 3a | 9 (23.7%) | 9 (23.7%) |
|
| 3b | 4 (10.5%) | 16 (42.1%) |
|
| 4a | 8 (21.0%) | 0 (0.0%) | pT 1+2 vs. pT
3+4 |
| 4b | 0 (0.0%) | 1 (2.6%) | 0.18 |
| pNa |
|
|
|
|
0/X | 11 (28.9%) | 3 (7.9%) |
|
| 1 | 11 (28.9%) | 13 (34.2%) |
|
| 2 | 13 (34.2%) | 22 (57.9%) | pN 0/X+1 vs. pN
2+3 |
| 3 | 3 (7.8%) | 0 (0.0%) | 0.135 |
| pMa |
|
|
|
| 0 | 32 (84.2%) | 34 (89.5%) |
|
| 1 | 6 (15.7%) | 4 (10.5%) | 0.29 |
| Efficacy of primary
chemotherapy |
|
|
|
|
Regression | 3 (7.9%) | 1 (2.6%) |
|
|
Stable | 30 (78.9%) | 23 (605%) |
|
|
Progression | 2 (5.2%) | 3 (7.9%) |
|
| Mixed
response | 3 (7.9%) | 3 (7.9%) | reg+stable vs.
prog+mixed |
|
N/A | 1 (2.6%) | 8 (21.1%) | 0.06 |
| Type of primary
chemotherapy |
|
|
|
| GC | 35 (92.2%) | 20 (52.6%) |
|
|
MVAC/MVEC | 1 (2.6%) | 18 (47.4%) |
|
|
Other | 2 (5.2%) | 0 (0.0%) | <0.01 |
| Adverse events
monotherapy |
|
|
|
|
Yes | 3 (7.8%) | NA |
|
| No | 35 (92.2%) | NA | NA |
| Discontinuation of
monotherapy |
|
|
|
|
Personal | 2 (5.2%) | NA |
|
| Side
effects | 3 (7.8%) | NA | NA |
| Second-line
therapy |
|
|
|
|
Yes | 2 (5.2%) | 1 (2.6%) |
|
| No | 36 (94.8%) | 37 (97.4%) | 0.243 |
Regarding oncological outcomes, 22 patients died in
the maintenance collective, 21 mortalities of which were
cancer-specific. In the control collective, mortality was recorded
in 29 patients and cancer-specific mortality in 23 patients,
results which were statistically significant (P=0.0076 for
mortality from any cause, and P=0.046 for cancer-specific
mortality). Clinical failure was noted in 18 patients in the
maintenance collective and in 21 patients in the control
collective, results which were comparable (P=0.33).
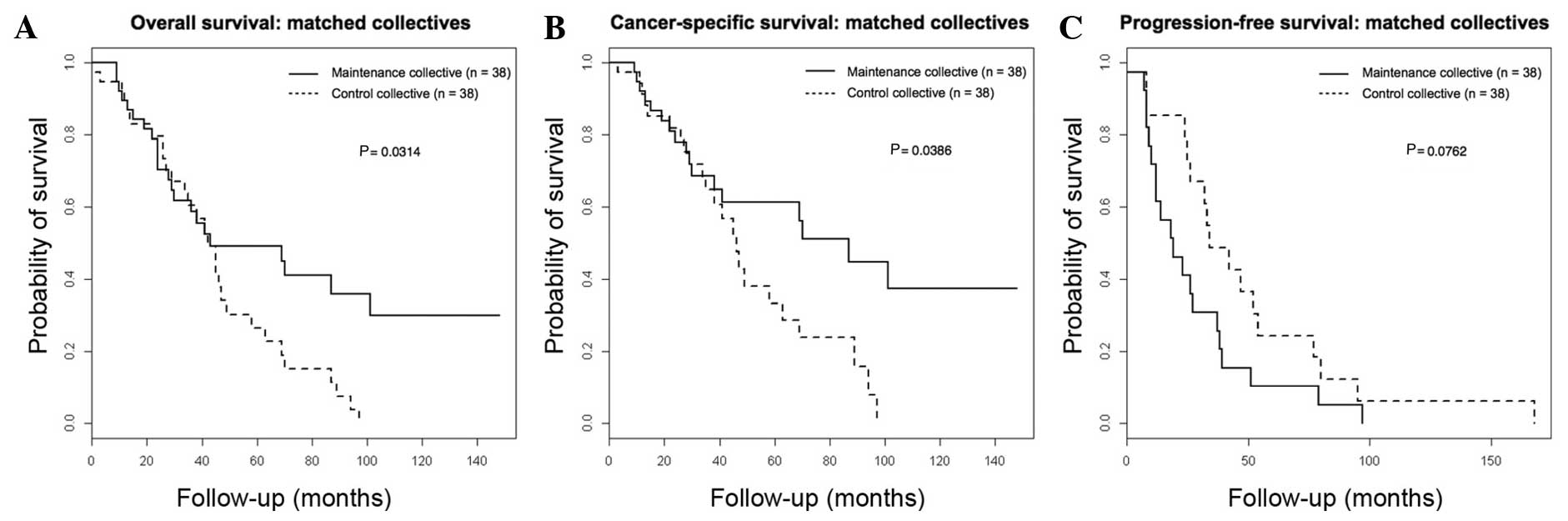
The 5-year OS rate was significantly higher (49.2%)
in the maintenance collective compared with the control collective
(26.5%, log-rank P=0.0314), in addition to the 5-year CSS rate
(61.3% in the maintenance collective vs. 33.4% in the control
collective; log-rank P=0.0386). The 5-year PFS rate did not differ
significantly between the control and the maintenance collectives
(10.3% in the maintenance collective vs. 16.1% in the control
collective; log-rank P=0.134). Fig. 1
summarizes these results. Prognostic factors that influence the CSS
rate were also identified in all patients by compiling Cox
proportional hazard models. In addition, the present study revealed
that the efficacy of primary chemotherapy (P=0.0168) and GEM
maintenance therapy (P=0.0396) were independent prognostic factors
in uni- and multivariate analysis (Table III). All the other examined factors
(age, gender, the location of primary tumor, the TNM status and the
number of initial chemotherapy cycles) were not significantly
predictive of the CSS rate.
 | Table III.Regression analysis. |
Table III.
Regression analysis.
|
| Regression
analysis |
|---|
|
|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable |
X2 | P-value | Coefficient | HR | CI 95%
(low-high) | P-value |
|---|
| Age | 2.6 |
0.1066 |
|
|
|
|
| Sex |
0.78 | 0.377 |
|
|
|
|
| Location
(lut/uut) |
0.27 |
0.6043 |
|
|
|
|
| Initial cycles
(<3 vs. >3) |
1.31 |
0.2527 |
|
|
|
|
| Efficacy of primary
chemotherapy |
5.72 |
0.01681 | 0.8865 | 2.4270 | 0.41–5.14 | 0.0207 |
| GEM
maintenance |
4.24 |
0.03957 | 0.6756 | 1.9653 | 0.51–3.78 | 0.0434 |
| pTa (pT 1+2 vs. pT 3+4) | 3.4 |
0.06539 |
|
|
|
|
| pNa (pN 0+1 vs. pN 2+3) |
0.47 |
0.4918 |
|
|
|
|
| pMa |
0.18 |
0.6728 |
|
|
|
|
Discussion
Uro-oncologists find that their available options
soon become limited with respect to advancing UC following primary
CBCC (3). Rapid rates of clinical
failure (on average, 16–18 months) quickly exhaust secondary or
third-line treatment options (2);
what remains is supportive therapy (e.g. radiation therapy or
polymodal pain management) and, ultimately, following palliative
care, mortality.
To improve the survival rate and the time to first
progression, maintenance therapy is required. GEM monotherapy has
been assessed in specific solid and haematological cancers
(10–12) and, although no established maintenance
therapy for UC is available at present, predominantly Japanese
researchers have gained experience in this cancer entity with GEM
monotherapy, demonstrating comparably low, although potentially
promising response rates when used as a monotherapy alone,
Following primary CBCC, a novel study published by Muto et
al (7) demonstrated that GEM
appeared to have a prognostic benefit in an UC patient collective
(n=33) of palliative, non-surgically treated patients. The authors
showed marked advantages in the maintenance group with respect to
CSS and PFS rates compared with a control group receiving best
supportive care alone, accompanied by a low rate of adverse
events.
This feature provided the foundational basis for the
present study. Knowing that, after disease stabilization following
primary CBCC, the time to first progression rarely exceeds 24
months even in surgically treated patients with advanced UC
(2), it became desirable to study the
oncological effects of a GEM monotherapy in this particular patient
group. In agreement with the study by Muto et al (7), a marked improvement in CSS and OS rates
for the GEM maintenance patients was observed in the present study.
It was also demonstrated that the efficacy of primary CBCC and GEM
maintenance therapy were independent prognostic factors for the CSS
rate in all patients. However, in contrast to Muto et al
(7), presence of visceral metastasis
was not identified as a prognosticator of note for the patients in
the present study, predominantly due to the limited number of
cases. Additionally, and again in contrast to Muto et al
(7), the PFS rate was not affected by
GEM monotherapy in the patient collective in the present study.
Since GEM is a drug which the majority of the patients in the
maintenance collective will have received prior to the study (GC is
considered standard care at our institution), it may be
hypothesized that the survival benefits observed in the present
study are not the result of the prolonged exposure to the drug
itself, but are due to a selection bias resulting from the
retrospective nature of the study.
However, it is reasonable to surmise that more than
merely a selection bias accounted for the particular observations
in the present study. Adjuvant therapies prolonging the OS and CSS
rates, while having no effect upon the PFS rate, are well known in
the treatment of other cancer entities, predominantly vaccination
studies in prostate cancer, but also including studies in
haematological malignancies (13,14).
Explanations for these effects may include delayed drug response,
as well as changes in immunological and/or tumor-biological
features. As GEM is known for changing humoral as well as tumor
immunology in solid cancers (15–17), a
combination of immunological and tumor-biological modifications in
UC leading to the observed survival benefits in the maintenance
group in the present study would be imaginable. However, the
underlying molecular mechanisms leading to a positive response
towards GEM monotherapy in UC have yet to be fully elucidated, and
should therefore be explored in future studies for alleviated and
personalized patient selection regarding this type of maintenance
therapy (e.g., establishment of predictive biomarkers for the GEM
treatment response). This consideration is highly supported by one
particular patient of the present maintenance collective: After
having received an unpleasant diagnosis in the year 2002 at the age
of 49 with a pT3b pN3 M0 UC following anterior exenteration and
creation of an ileocoecal pouch, the patient enjoys good health
with no detectable sign of the disease in May 2015 in the 48th
cycle of GEM monotherapy. Prior to monotherapy, the patient had
received three cycles of GC, and was staged stable disease
thereafter. At present, neither primary CBCC nor monotherapy have
elicited any adverse effects with respect to either haematological
effects or a decline of renal function in the patient.
Another aspect of GEM monotherapy as a maintenance
treatment concept is the incorporation of this additive therapy
regime with reference to vinflunine being approved for second-line
chemotherapy following CBCC (18). We
consider that GEM monotherapy (with its low demands on renal
function and favourable adverse event rates) could be scheduled
either prior to or following Vinflunine therapy, although this
question will need to be definitively addressed through
comprehensive and prospective clinical studies.
There were limitations to the present study. Being a
retrospective analysis, sampling bias must be considered.
Additionally, it has to be acknowledged that information concerning
the efficacy of primary CBCC was not available in ~20% of the
control collective. Furthermore, in view of the comparatively small
number of patients, changes in the time to progression, as
previously mentioned, will need to be examined in prospective
studies to come, including larger patient groups.
Despite these limitations, the present study
indicates that GEM monotherapy is a good choice for young,
surgically treated UC patients with stable disease, in the search
for an active maintenance therapy following primary CBCC; in our
experience, such a therapy often encourages patients mentally,
thereby improving the quality of life (and ultimately, as
demonstrated in the present study, the survival rate).
In conclusion, additional GEM maintenance therapy
may improve the survival rate in stable-disease, surgically treated
patients with UC following primary platinum-based chemotherapy, and
should therefore be validated in prospective studies.
Acknowledgements
This study was supported by the Open Access
Publication Fund of the University of Wuerzburg.
Glossary
Abbreviations
Abbreviations:
|
UC
|
urothelial carcinoma
|
|
LUT
|
lower urinary tract
|
|
UUT
|
upper urinary tract
|
|
OS
|
overall survival
|
|
CSS
|
cancer-specific survival
|
|
PFS
|
progression-free survival
|
|
CT
|
computed tomography
|
|
CBCC
|
cisplatin-based combination
chemotherapy
|
|
GFR
|
glomerular filtration rate
|
|
GEM
|
Gemcitabine
|
|
GC
|
Gemcitabine/cisplatin
|
|
MVAC
|
methotrexate (M), vinblastin (V),
adriamycin (A) and cisplatin (C)
|
|
MVEC
|
methotrexate (M), vinblastin (V),
epirubicin (E) and cisplatin (C)
|
References
|
1
|
Advanced Bladder Cancer (ABC)
Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive
bladder cancer: Update of a systematic review and meta-analysis of
individual patient data advanced bladder cancer (ABC) meta-analysis
collaboration. Eur Urol. 48:202–205; discussion 205–206. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Freiha F, Reese J and Torti FM: A
randomized trial of radical cystectomy versus radical cystectomy
plus cisplatin, vinblastine and methotrexate chemotherapy for
muscle invasive bladder cancer. J Urol. 155:495–499; discussion
499–500. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sonpavde G, Sternberg CN, Rosenberg JE,
Hahn NM, Galsky MD and Vogelzang NJ: Second-line systemic therapy
and emerging drugs for metastatic transitional-cell carcinoma of
the urothelium. Lancet Oncol. 11:861–870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albers P, Siener R, Härtlein M, Fallahi M,
Haeutle D, Perabo FG, Steiner G, Blatter J and Müller SC: German
TCC Study Group of the German Association of Urologic Oncology:
Gemcitabine monotherapy as second-line treatment in
cisplatin-refractory transitional cell carcinoma-prognostic factors
for response and improvement of quality of life. Onkologie.
25:47–52. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akaza H, Naito S, Usami M, Miki T,
Miyanaga N and Taniai H: Japanese Gemcitabine Study Group: Efficacy
and safety of gemcitabine monotherapy in patients with transitional
cell carcinoma after Cisplatin-containing therapy: A Japanese
experience. Jpn J Clin Oncol. 37:201–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsushima M, Kikuchi E, Masugi Y, Akita
H, Miyajima A, Jinzaki M and Oya M: Efficacy and safety of
gemcitabine monotherapy in an elderly patient with penile
metastasis from bladder carcinoma: A case report. Int Canc Conf J.
2(4): 255–260. 2013. View Article : Google Scholar
|
|
7
|
Muto S, Abe H, Noguchi T, Sugiura SI,
Kitamura K, Isotani S, Ide H, Yamaguchi R, Kamai T and Horie S:
Maintenance monotherapy with gemcitabine after standard
platinum-based chemotherapy in patients with advanced urothelial
cancer. Int J Urol. 22:490–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng L, Montironi R, Davidson DD and
Lopez-Beltran A: Staging and reporting of urothelial carcinoma of
the urinary bladder. Mod Pathol. 22:S70–S95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ and Kerbrat P: Gemcitabine and
cisplatin versus methotrexate, vinblastine, doxorubicin, and
cisplatin in advanced or metastatic bladder cancer: Results of a
large, randomized, multinational, multicenter, phase III study. J
Clin Oncol. 18:3068–3077. 2000.PubMed/NCBI
|
|
10
|
Karasek P, Skacel T, Kocakova I, Bednarik
O, Petruzelka L, Melichar B, Bustova I, Spurny V and Trason T:
Gemcitabine monotherapy in patients with locally advanced or
metastatic pancreatic cancer: A prospective observational study.
Expert Opin Pharmacother. 4:581–586. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ricci S, Antonuzzo A, Galli L, Tibaldi C,
Bertuccelli M, Pegna Lopes A, Petruzzelli S, Algeri R, Bonifazi V,
Fioretto ML, et al: Gemcitabine monotherapy in elderly patients
with advanced non-small cell lung cancer: A multicenter phase II
study. Lung Cancer. 27:75–80. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duvic M, Talpur R, Wen S, Kurzrock R,
David CL and Apisarnthanarax N: Phase II evaluation of gemcitabine
monotherapy for cutaneous T-cell lymphoma. Clin Lymphoma Myeloma.
7:51–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Madan RA, Gulley JL, Fojo T and Dahut WL:
Therapeutic cancer vaccines in prostate cancer: The paradox of
improved survival without changes in time to progression.
Oncologist. 15:969–975. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hernandez-Ilizaliturri FJ and Czuczman MS:
Therapeutic options in relapsed or refractory diffuse large B-cell
lymphoma. Part 1. Current treatment approaches. Oncology (Williston
Park). 23:546–553. 2009.PubMed/NCBI
|
|
15
|
Soeda A, Morita-Hoshi Y, Makiyama H,
Morizane C, Ueno H, Ikeda M, Okusaka T, Yamagata S, Takahashi N,
Hyodo I, et al: Regular dose of gemcitabine induces an increase in
CD14+ monocytes and CD11c+ dendritic cells in patients with
advanced pancreatic cancer. Jpn J Clin Oncol. 39:797–806. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki E, Kapoor V, Jassar AS, Kaiser LR
and Albelda SM: Gemcitabine selectively eliminates splenic
Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and
enhances antitumor immune activity. Clin Cancer Res. 11:6713–6721.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nowak AK, Robinson BW and Lake RA:
Gemcitabine exerts a selective effect on the humoral immune
response implications for combination chemo-immunotherapy. Cancer
Res. 62:2353–2358. 2002.PubMed/NCBI
|
|
18
|
Bellmunt J, Théodore C, Demkov T, Komyakov
B, Sengelov L, Daugaard G and Delgado FM: Phase III trial of
vinflunine plus best supportive care compared with best supportive
care alone after a platinum-containing regimen in patients with
advanced transitional cell carcinoma of the urothelial tract. J
Clin Oncol:. 27:4454–4461. 2009. View Article : Google Scholar : PubMed/NCBI
|