Introduction
Keratocystic odontogenic tumors (KCOTs) are one of
the most common odontogenic tumors of the jaw. Previously known as
odontogenic keratocysts (OKCs), these tumors were reclassified by
the World Health Organization in 2005 (1). KCOTs generally appear as unilocular or
multilocular radiolucencies with a smooth border or honeycomb
appearance, and they are characterized by an aggressive behavior
and a high rate of recurrence. Currently, decompression or
marsupialization, combined with stage-two curettage or enucleation,
represent the commonly accepted treatment approach for KCOTs; the
advantage of this therapeutic approach is its minimally invasive
nature, which allows for preservation of jaw function and
appearance. Following decompression, the size of the lesion may
decrease (2). Furthermore, a previous
study (3) showed that the
histological features of KCOTs markedly changed following
decompression, as evidenced by the thickening of the epithelial
layer and enhanced inflammation in the fibrous layer. However, the
mechanisms responsible for these changes in clinical features
remain to be elucidated.
Inducible nitric oxide synthase (iNOS) is a
cytosolic enzyme that has been closely associated with the
pathophysiological process of inflammatory diseases, such as
periodontal disease (4) and
periapical inflammatory lesion (5).
NO is produced mainly by iNOS. Watanuki et al (6) showed that NO generated by iNOS in
osteoblasts has a critical role in regulating bone turnover and
elevated osteogenic activity, and Chen et al (7) reported iNOS expression in KCOT. However,
to the best of our knowledge, no studies have investigated how iNOS
expression changes in response to decompression treatment. In the
present study, immunohistochemistry was used to detect the
expression of iNOS in KCOT samples obtained prior and subsequent to
decompression and to assess the possible roles of iNOS in mediating
changes in clinical features.
Materials and methods
Patients and tissue samples
A total of 16 histologically verified KCOT specimens
collected between 2004 and 2009 were obtained from the Stomatology
Hospital of Jiangsu Province (Jiangsu, China) and from the Shanghai
Ninth People's Hospital (Shanghai, China). Recurrent cases or those
associated with nevoid basal cell carcinoma syndrome were excluded
from the study. All the patients were treated by decompression
followed by enucleation. The clinical information of the patients
is shown in Table I. Postoperative
follow up consisted of clinical and radiographic examinations. The
mean duration before stage II surgery was 19.5 months (range,
6.5–44.0 months).
 | Table I.Clinical information and the
expression intensity of iNOS. |
Table I.
Clinical information and the
expression intensity of iNOS.
|
|
|
|
|
|
| iNOS expression,
n |
|---|
|
|
|
|
|
|
|
|
|---|
| Patient | Gender | Age, years | Duration, months | Location | Radiographic
features | BC | AC |
|---|
| 1 | Male | 22 | 27 | Mandible;
Mol-Ram | Solitary;
unilocular | 0 | 9 |
| 2 | Male | 15 | 10 | Mandible;
Ang-Ram | Multiple;
unilocular | 0 | 4 |
| 3 | Male | 20 | 21 | Mandible;
Ang-Ram | Solitary;
unilocular | 0 | 9 |
| 4 | Male | 42 | 17 | Mandible;
Mol-Ram | Multiple;
unilocular | 0 | 6 |
| 5 | Female | 20 | 16 | Mandible;
Ang-Ram | Solitary;
unilocular | 0 | 4 |
| 6 | Female | 13 | 12 | Mandible;
Mol-Ram | Solitary;
unilocular | 0 | 4 |
| 7 | Female | 38 | 3 | Mandible;
Ang-Ram | Solitary;
multilocular | 0 | 6 |
| 8 | Female | 34 | 16 | Mandible;
Mol-Ram | Solitary;
unilocular | 2 | 9 |
| 9 | Female | 49 | 18 | Mandible;
Ang-Ram | Solitary;
unilocular | 0 | 4 |
| 10 | Male | 55 | 15 | Maxilla; Ant | Multiple;
unilocular | 0 | 4 |
| 11 | Female | 25 | 15 | Mandible;
Mol-Ram | Solitary;
unilocular | 0 | 4 |
| 12 | Female | 33 | 9 | Mandible;
Ang-Ram | Solitary;
unilocular | 0 | 6 |
| 13 | Male | 35 | 23 | Mandible;
Ang-Ram | Solitary;
multilocular | 1 | 4 |
| 14 | Female | 24 | 31 | Mandible;
Ang-Ram | Solitary;
multilocular | 0 | 6 |
| 15 | Male | 29 | 23 | Maxilla; Ant | Solitary;
unilocular | 0 | 6 |
| 16 | Female | 28 | 27 | Maxilla; Ant | Multiple;
unilocular | 0 | 6 |
Immunohistochemistry
Paraffin specimens obtained at the time of
decompression and enucleation were collected from the Department of
Oral Pathology at the Stomatology Hospital of Jiangsu Province and
the Shanghai Ninth People's Hospital. Each of the 16 pairs of
paraffin-embedded samples was sectioned serially into two 4-µm
slices; one slice was prepared for immunohistochemical analysis,
while the other was used as a negative control by substituting
phosphate-buffered saline (PBS) for the specific antibody. Briefly,
the deparaffinized sections were immersed in absolute methanol
containing 3% hydrogen peroxide for 15 min at room temperature to
block endogenous peroxidase activity. Following washing with PBS,
the sections were immersed in 0.01 M citrate buffer (pH 6.0), and
heated in a microwave oven at 95°C for 5 min. Subsequently, diluted
(1:50) mouse monoclonal anti-iNOS antibody (cat. no. ab195661;
mouse anti-human; Abcam, Cambridge, UK) was applied to the sections
at 4°C overnight. The sections were subsequently incubated with
rabbit-anti-mouse secondary antibody (1:5,000; Abcam) for 30 min at
room temperature. The sections were immersed for 8 min in 0.03%
3,3-diaminobenzidine tetrahydrochloride in 0.05 M Tris-HCl buffer
(pH 8.5) containing 0.01% hydrogen peroxide and counterstained with
hematoxylin.
Immunohistochemical evaluation
The immunohistochemical staining pattern of iNOS in
KCOT samples appeared as brown granules on the cell membrane and in
the cytoplasm. Immunohistochemical reactivity for iNOS was defined
as the proportion score multiplied by the intensity score. The
proportion score was defined as 0, <5%; 1, 6–25%; 2, 26–75%; or
3, >76% positive cells. The intensity score was defined as 0,
negative; 1, weak; 2, moderate; or 3, strong. The total score
ranged from 0 to 9. The immunoreactivity scores were used to
classify the samples into one of the following three groups based
on the final score: Negative immunoreactivity, defined as a total
score of 0; low expression, defined as a total score of 1–3;
moderate expression, defined as a total score of 4–6; and high
expression, defined as a total score of >6.
Statistical analyses
Statistical analyses were performed using the paired
t-test to evaluate differences in iNOS immunoreactivity in KCOTs at
the time of enucleation compared to the time of decompression.
P<0.05 was considered to indicate a statistically significant
difference.
Results
iNOS staining
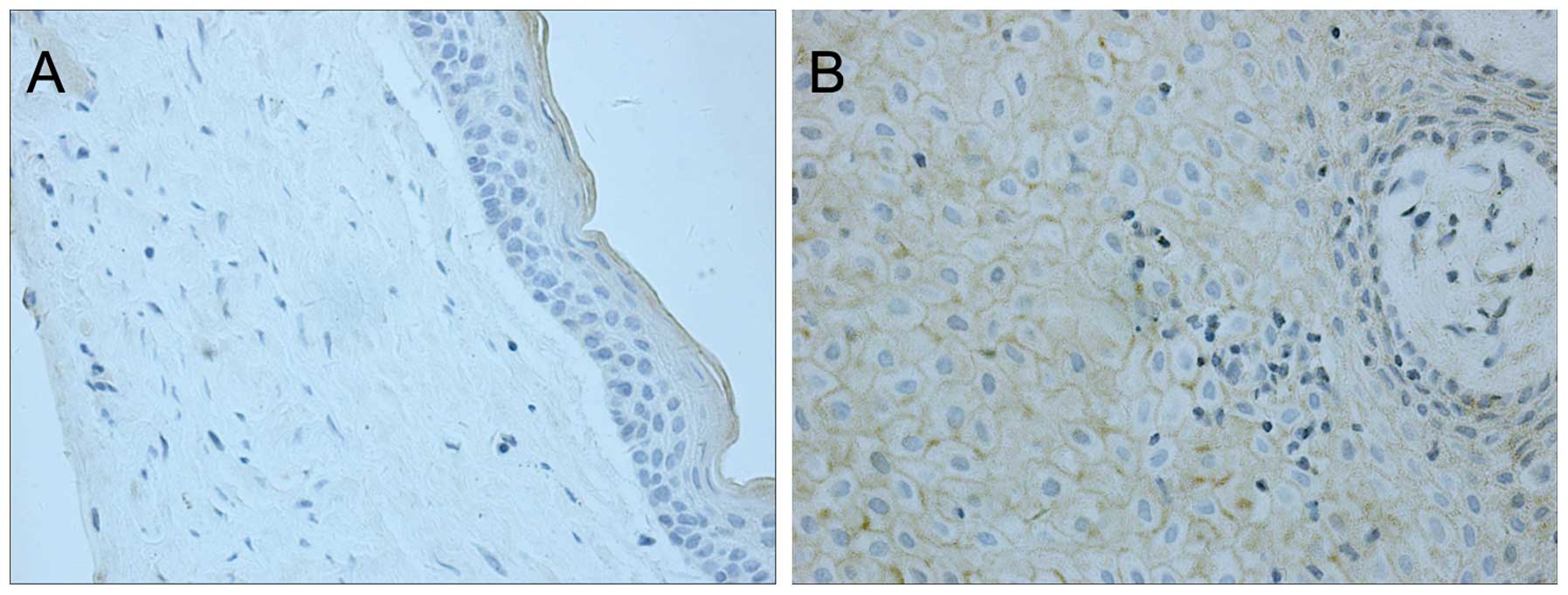
Prior to decompression, only slight iNOS staining,
which was restricted to the cytoplasm, was observed in 2 (12.5%) of
the 16 cases; no immunostaining was observed in the other samples
(Fig. 1A, Table I). Following decompression, all the
samples exhibited moderate to strong staining for iNOS in the
cytoplasm and on the cell membrane of cells in the epithelial layer
(Fig. 1B, Table I). In addition, the fibrous layer also
showed positive iNOS expression following decompression. This
increase in iNOS expression following decompression was
statistically significant (P<0.01).
Discussion
KCOTs are one of the most frequent odontogenic
tumors and they receive significant attention due to their
aggressive biological behavior and tendency for recurrence
(8). Decompression is commonly
employed as a conservative treatment for KCOT. Following
decompression, the size of the tumor is significantly decreased. In
particular, Nakamura et al (9)
reported that 96% of the cases in their study showed a cystic
reduction >50%. Furthermore, it was reported that the typical
features of KCOTs are significantly altered following decompression
treatment (9). In our previous study
(3) we observed that subsequent to
decompression, the typical presentation of KCOT was altered to one
marked by hyperplastic epithelium, thickened fibrous lamina and
increased inflammatory infiltration. Numerous studies have shown
that biomarkers typically expressed at high levels in KCOTs, such
as interleukin (IL)-1α, collagenase, Ki-67, B-cell lymphoma-2 and
keratinocyte growth factors, are notably decreased following
decompression (10–12) indicating the attenuation of cell
proliferation, survival and local invasion. Ninomiya et al
(13) further showed that the
expression of IL-1α mRNA and protein in epithelial cells of KCOTs
was significantly decreased following marsupialization and that the
Ki-67 labeling index decreased proportionally with the expression
of IL-1α. These results suggest that marsupialization may reduce
the size of KCOTs by inhibiting IL-1α expression and epithelial
cell proliferation.
iNOS is a cytosolic enzyme induced by cytokines and
bacterial lipopolysaccharide during inflammation (14). Furthermore, NO is generated primarily
by iNOS and has been shown to regulate inflammation (15). Numerous studies have demonstrated that
the activation of iNOS is associated with the pathophysiological
characteristics of inflammatory diseases (4,5). NO may
also participate in the regulation of bone reconstruction. For
instance, Ralston et al (16)
showed that higher concentrations of NO inhibited bone resorption,
whereas lower concentrations of NO stimulated bone resorption.
However, the mechanisms underlying the expression of iNOS in
inflammation and the regulation of bone metabolism following
decompression remain to be elucidated.
To date, few studies have investigated the
differential expression of iNOS in KCOTs prior and subsequent to
decompression. In the present study, 87.5% of KCOT samples showed
no immunohistochemical reactivity for iNOS prior to decompression,
and only 12.5% of samples showed slight staining in the cytoplasm
of cells in the epithelial layer. Similarly, Chen et al
(7) observed iNOS expression in 10%
of OKC samples. Following decompression, all the samples in the
present study exhibited moderate to intense staining for iNOS in
the cytoplasm and on the cell membrane of cells in the epithelial
and fibrous layers. Thus, the significantly distinct expression of
iNOS prior and subsequent to treatment suggests an important role
for this enzyme in the progression of inflammation and the effects
of KCOT decompression. However, more samples are required to
further validate these results in future studies.
References
|
1
|
Barnes L, Eveson JW, Reichart P, et al:
World Health Organization Classification of Tumors. Pathology and
Genetics of Head and Neck Tumors (Lyon). IARC Press. 2005.
|
|
2
|
Shudou H, Sasaki M, Yamashiro T,
Tsunomachi S, Takenoshita Y, Kubota Y, Ninomiya T, Kawazu T and
Mori Y: Marsupialisation for keratocystic odontogenic tumours in
the mandible: Longitudinal image analysis of tumour size using 3D
visualised CT scans. Int J Oral Maxillofac Surg. 41:290–296. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Morais Melo W, Pereira-Santos D, Sonoda
CK and Hochuli-Vieira E: Decompression for management of
keratocystic odontogenic tumor in the mandible. J Craniofac Surg.
23:e639–e640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Güllü C, Ozmeric N, Tokman B, Elgün S and
Balos K: Effectiveness of scaling and root planing versus modified
Widman flap on nitric oxide synthase and arginase activity in
patients with chronic periodontitis. J Periodontal Res. 40:168–175.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki T, Kumamoto H, Ooya K and Motegi K:
Expression of inducible nitric oxide synthase and heat shock
proteins in periapical inflammatory lesions. J Oral Pathol Med.
31:488–493. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watanuki M, Sakai A, Sakata T, Tsurukami
H, Miwa M, Uchida Y, Watanabe K, Ikeda K and Nakamura T: Role of
inducible nitric oxide synthase in skeletal adaptation to acute
increases in mechanical loading. J Bone Miner Res. 17:1015–1025.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen WL, Ouyang KX, Li HG, Huang ZQ, Li JS
and Wang JG: Expression of inducible nitric oxide synthase and
vascular endothelial growth factor in ameloblastoma. J Craniofac
Surg. 20:171–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhargava D, Deshpande A and Pogrel MA:
Keratocystic odontogenic tumour (KCOT)-a cyst to a tumour. Oral
Maxillofac Surg. 16:163–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura N, Mitsuyasu T, Mitsuyasu Y,
Taketomi T, Higuchi Y and Ohishi M: Marsupialization for
odontogenic keratocysts: Long-term follow-up analysis of the
effects and changes in growth characteristics. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 94:543–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suyama Y, Kubota Y, Yamashiro T, Ninomiya
T, Koji T and Shirasuna K: Expression of keratinocyte growth factor
and its receptor in odontogenic keratocysts. J Oral Pathol Med.
38:476–480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pogrel MA and Jordan RC: Marsupialization
as a definitive treatment for the odontogenic keratocyst. J Oral
Maxillofac Surg. 62:651–655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
August M, Faquin WC, Troulis MJ and Kaban
LB: Dedifferentiation of odontogenic keratocyst epithelium after
cyst decompression. J Oral Maxillofac Surg. 61:678–683. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ninomiya T, Kubota Y, Koji T and Shirasuna
K: Marsupialization inhibits interleukin-1alpha expression and
epithelial cell proliferation in odontogenic keratocysts. J Oral
Pathol Med. 31:526–533. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bredt DS, Hwang PM, Glatt CE, Lowenstein
C, Reed RR and Snyder SH: Cloned and expressed nitric oxide
synthase structurally resembles cytochrome P-450 reductase. Nature.
351:714–718. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kendall HK, Marshall RI and Bartold PM:
Nitric oxide and tissue destruction. Oral Dis. 7:2–10. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ralston SH, Ho LP, Helfrich MH, Grabowski
PS, Johnston PW and Benjamin N: Nitric oxide: A cytokine-induced
regulator of bone resorption. J Bone Miner Res. 10:1040–1049. 1995.
View Article : Google Scholar : PubMed/NCBI
|