Introduction
Despite improvements in the clinical outcome of
patients with esophageal cancer, their prognosis remains generally
very poor. Surgery alone is the standard treatment for early-stage
disease, however, patients with locally advanced disease undergo a
multimodality regimen encompassing combined radiochemotherapy (RCT)
and surgery (1,2). RCT is administered prior to a planned
operation or is used as a definitive procedure if surgery is not
possible due to co-morbidity, or because complete resection is not
feasible for technical reasons. Whether or not surgery is possible
is always decided by an interdisciplinary team.
The tumor response to neoadjuvant RCT is a major
determinant of further therapeutic strategies, whether surgery or a
continuation of RCT is used, and therefore, also the overall
prognosis of the patient. A significant response (complete or
subtotal tumor regression) has a major bearing on the prognosis.
Therefore, one option is to measure response by imaging with
computed tomography (CT) or positron emission tomography/CT
(PET/CT) following the administration of a sufficient radiation
dose (e.g., 45 Gy), and then decide how to proceed (3).
From a clinician's perspective, the evaluation of
treatment response using CT imaging alone may be difficult in
numerous cases owing to its relatively low sensitivity (4,5) and the
irritating co-variants of PET/CT (6–8). This is a
challenge to clinicians an, therefore, the identification of
biomarkers that predict the response to RCT may assist with
optimizing treatment for advanced esophageal carcinoma.
The association between inflammation and cancer is
widely accepted as a reliable concept (9–11).
However, the complex interaction between inflammatory cascades and
cancer progression is not well understood and is currently being
investigated (12–14). Chronic inflammatory processes affect
all stages of tumor development, as well as therapy.
Cancer-associated inflammation (15)
or, as it has also been referred to, inflammation-induced cancer
(11), encompasses a wide array of
factors that coordinate the tumor-promoting and tumor-antagonizing
effects of inflammation and enable crosstalk between cancer
progression and inflammatory processes.
C-reactive protein (CRP) is a representative
biomarker of an acute immunogenic inflammatory response to
infection or tissue damage. In addition to its well-established
pathophysiological functions, CRP has been shown to mediate the
complex interactions between a tumor and its inflammatory
microenvironment, which in turn can promote tumor growth (16–18).
Furthermore, CRP expression is upregulated by inflammatory lipid
sphingosine-1-phosphate, a bioactive sphingolipid metabolite
involved in cancer-associated inflammation. Increased CRP levels
can then activate extracellular signal-regulated kinase, which in
turn leads to the transcriptional activation of matrix
metaloproetinase-9, promoting tumor invasion and metastasis
(14).
A large body of evidence has emerged over the last
few years for a pivotal role of CRP in cancer progression and
treatment response (19–27), and there is also level I evidence for
CRP having prognostic value in a number of different cancer types
(28–34).
CRP dynamics in the serum have been shown to
correlate with progression and poor prognosis in patients with
esophageal carcinoma (35–38), and the level of preoperative CRP is an
independent prognostic factor in patients with potentially
resectable esophageal carcinoma (39). However, the clinical significance of
CRP levels in patients with unresectable tumors and those requiring
induction or even definitive CRT has not been fully investigated
with respect to treatment response and prognosis (40,41), and
little data are available regarding the association between CRP and
the response to RCT (42,43).
The present study investigated the possible
association between CRP and the response to RCT, and that between
CRP and prognosis, by measuring CRP dynamics in serum the during
the induction of RCT for patients with clinical T3 or T4 esophageal
squamous cell carcinoma, all of whom underwent subsequent
esophagectomy. The present study assessed the potential of serum
CRP as a biomarker for the prediction of response to RCT.
Materials and methods
Patient selection and data
acquisition
The present study included patients with
histologically proven squamous cell esophageal carcinoma,
predominantly located in middle or upper third of the esophagus,
and staged II or III, according to the UICC classification (w).
Patients with distant metastases were excluded.
The present study was approved by the responsible
ethics committees and was performed in accordance with the Helsinki
agreement. Each individual case was discussed at a
multidisciplinary team meeting involving radiation oncologists,
dedicated surgeons, medical oncologists, pathologists, and
radiologists. Pre-therapeutic CRP levels were measured. The data
were prospectively collected and stored in databases, from which
the demographics, co-morbidity, tumor pathology, morbidity and
mortality were subsequently retrieved. Case records were
subsequently searched for any missing data. Survival data were
obtained either from Cancer Registries or by direct contact with
physicians.
Staging, neoadjuvant treatment and
surgery
Pre-therapeutic staging included upper endoscopy
with biopsy and endoscopic ultrasound, abdominal and thoracic CT
and, in all cases, a PET/CT scan. Neoadjuvant RCT consisted of 5
cycles of carboplatin (AUC 2) and paclitaxel (45 mg/m2)
in combination with 45–50 Gy of radiotherapy using the volumetric
modulated arc therapy technique. The treatment was delivered as an
in-patient regimen. Surgery was performed 6 weeks following the
completion of RCT.
RCT was delivered by the same radiation oncologists
and the delineation of target volumes was consistently supervised
by two experienced radiation oncologists, as well as the
preparation of chemotherapy and lab work-up. Surgery was performed
by the same three experienced surgeons, using an abdominal and
transthoracic approach. The stomach was used as the conduit for
reconstruction in all cases. The standard UICC classification
(44) was used to evaluate
pathology.
CRP measurement
The CRP level was measured prior to and following
the completion of neoadjuvant RCT. Only CRP measurements taken
within 2 weeks of the start of RCT were analyzed. Further
measurements were then taken at 6, 12, 18, 24, 30, 36 and 40 weeks
after RCT.
CRP measurements were performed in serum samples
with an automated immunoturbidimetric analyzer (Cobas® Integra 800;
Roche Diagnostics, Basel, Switzerland). Normal reference values of
<10 and <8 mg/l were provided by the manufacturers,
respectively.
Statistical analysis
Statistical analysis was performed using SPSS®
(version 22) for Windows (IBM SPSS, Chigago, IL, USA). The data are
expressed as either the mean ± standard deviation or the median
with their 95% confidence intervals (CI). A comparison of data
between the two patient groups was performed using χ2
tests for categorical data and Mann-Whitney U tests for continuous
data. Correlations between variables were tested for using the
Pearson test, survival was calculated using the Kaplan-Meier method
and differences between groups were evaluated using the log rank
test. In order to determine the influence of different variables on
the outcome, Cox regression analyses were performed. P<0.05 was
considered to indicate a statistically significant difference.
Results
Response to RCT
All patient characteristics and tumor variables are
summarized in Table I. All patients
received RCT, which was delivered as a neoadjuvant therapy in 34
cases (73.91%) and as a definitive treatment in 12 cases (26.08%).
A response was observed in 34 cases (73.91%), of which, 6 (17.6%)
were a complete response and 28 (82.3%) were a partial remission.
No response was reported in 12 cases (26%).
 | Table I.Characteristics of the 46 patients
undergoing radiochemotherapy followed by surgery. |
Table I.
Characteristics of the 46 patients
undergoing radiochemotherapy followed by surgery.
| Variable | No. of patients |
|---|
| Mean age in years
(range) | 65.3 (51–81) |
| Gender |
|
|
Female |
11 (23.9%) |
| Male | 35 (76%) |
| Tumor location |
|
|
Upper | 6
(13%) |
|
Middle | 38
(82.6%) |
|
Lower | 2
(4.3%) |
| Tumor depth |
|
| T1 | 0 (0%) |
| T2 |
8 (17.3%) |
| T3 | 30
(65.2%) |
| T4 |
8 (17.3 %) |
| Nodal status |
|
| N1 | 25
(54.3%) |
| N2 | 18
(39.1%) |
| N3 |
3 (17.3%) |
| Tumor stage |
|
| IB | 1
(2.1%) |
|
II–III | 45
(97.8%) |
| IV | 0 (0%) |
CRP levels during and following RCT,
and the association with response to RCT
Table II shows pre-
and post-RCT measurements of CRP. CRP levels were high prior to
treatment, however, eventually decreased and normalized following
therapy. In univariate analysis, pre-therapeutic CRP levels had a
significant influence on the response rate (P=0.033), whilst
post-therapeutic CRP levels had no significant influence
(P=0.383).
 | Table II.CRP measurement prior to and
following radiochemotherapy (n=42 patients). |
Table II.
CRP measurement prior to and
following radiochemotherapy (n=42 patients).
| Measurement | Normal | High | Low |
|---|
| Pre-therapy |
|
|
|
|
CRP | 22 (47.9%) | 24 (52.2%) | 0 |
| Post-therapy |
|
|
|
|
CRP | 26 (56.5%) | 20 (43.5%) | 0 |
CRP levels during and after RCT, and
correlation with response to RCT
Pre-therapeutic CRP levels, however, not
post-therapeutic CRP levels were significantly correlated with the
response rate (P=0.045 and P=0.444, respectively), and no
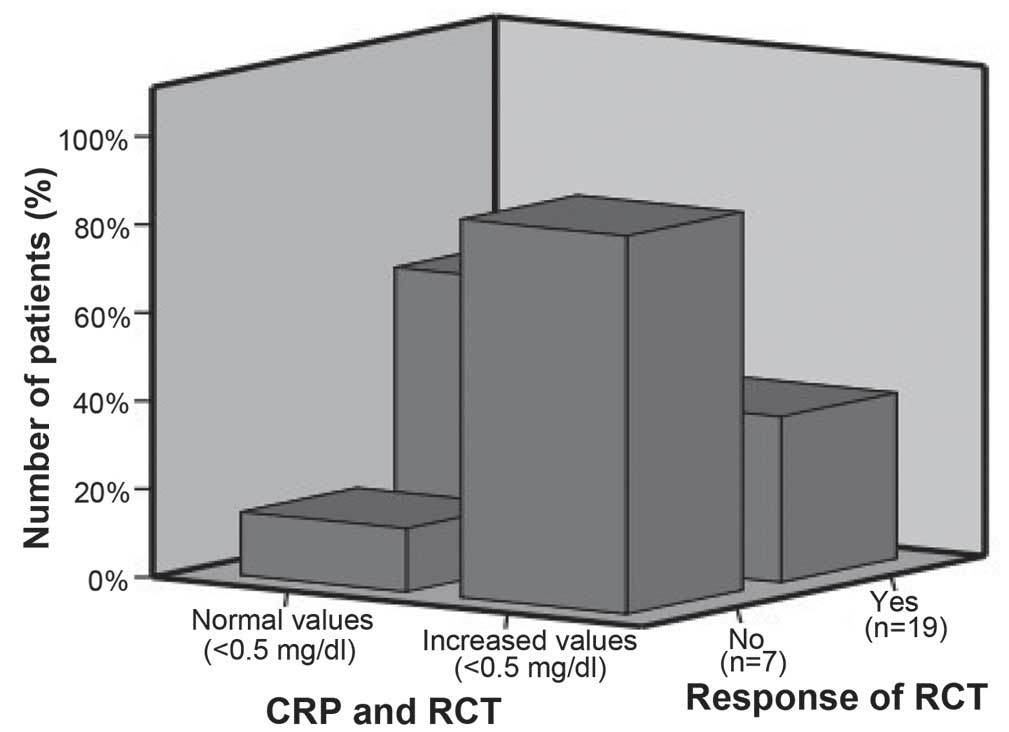
association was observed between CRP levels and survival. Fig. 1 summarizes the association between the
levels of CRP and response to RCT.
Discussion
The present study an association between the
response of squamous cell esophageal carcinoma to neoadjuvant RCT
and the pre-therapeutic levels of CRP. A significant response to
treatment, either complete or subtotal tumor regression, is a key
indicator of prognosis. However, it is difficult to evaluate
response by CT imaging alone due to its relatively low sensitivity
(4,5),
and clinical evaluation remains challenging. An inflammatory
biomarker such as CRP, which is easy to measure and reflects the
response to treatment, can help guide clinical decision making.
Guillem and Triboulet (35) revealed that patients with CRP levels
>6 mg/l more frequently failed to respond to RCT (P=0.035),
tended to have a shorter overall survival (P=0.061), and had a
significantly shorter disease-free survival (P=0.016). The authors
concluded that the pretreatment measurement of serum CRP levels in
esophageal cancer patients can be used in routine practice as an
indicator of prognosis. This was the first attempt to associated
CRP serum levels to neoadjuvant RCT response. A subsequent study
demonstrated that, amongst patients with cancer of the
gastroesophageal junction, the pre-therapeutic CRP level was a
prognostic indicator, in agreement with the results of our pilot
study. A multivariate analysis revealed that only the positive to
total lymph node ratio [hazard ratio (HR), 2.02; 95% CI, 1.44–2.84;
P<0.001] and preoperative CRP concentration (HR, 3.53; 95% CI,
1.88–6.64; P<0.001) were independent predictors of
cancer-specific survival. Patients with no evidence of a
preoperative systemic inflammatory response (CRP ≤10 mg/l) had a
median survival of 79 months compared with 19 months for those with
an elevated systemic inflammatory response (P<0.001). Together
with these findings, the present study indicated that CRP is a
potentially predictive cancer biomarker (36). The same research team subsequently
reported a possible association between CRP and Albumin levels, and
the response of cancer to treatment. Another attempt to associated
inflammation with cancer was the development of the
inflammation-based prognostic score (37), however, the usefulness of this score
has not been confirmed, and was not supported by the present
data.
Motoyama et al (38) revealed evidence for an association
between CRP polymorphisms and CRP levels following
esophagectomy for thoracic esophageal cancer. The authors
demonstrated that, 12 h following surgery, CRP levels were
significantly higher in patients harboring the CRP 1,059 G/C
genotype (0.0266), and that this difference remained for 36 h after
surgery (217±63 vs. 140±51 mg/l; P=0.0020). Logistic regression
models revealed that patients harboring the CRP 1,059 G/G
genotype had a significantly higher likelihood of a post-surgery
increase in serum CRP (38).
Zingg et al (43) analyzed data for 70 patients exhibiting
normal CRP levels, and 20 patients with raised CRP. The groups
revealed no difference with respect to in descriptive,
co-morbidities, white cell counts, pathological data or morbidity.
In-hospital mortality was more frequent in the raised CRP group (3
vs. 1 patient; P=0.048), and Kaplan-Meier survival analysis
revealed a significant survival advantage for patients with a
normal CRP levels compared with those with raised CRP levels
(median survival, 65.4 vs. 18.7 months; log rank test, P=0.027).
The Cox regression analysis identified three independent prognostic
factors for survival: UICC stage (IIB/III vs. I/IIA; HR, 3.48;
P=0.007), extent of resection (HR, 6.33; P=0.002) and CRP levels
(raised vs. normal; HR, 5.07; P=0.001). The authors concluded that
pre-therapeutic CRP levels are an independent prognostic marker for
survival following neoadjuvant treatment in patients with
esophageal cancer and may be of value in the re-staging process
following neoadjuvant treatment (43). This is in agreement with the findings
reported in the present study.
In conclusion, the present preliminary data
indicated that the pre-therapeutic serum CRP level is a possible
indicator of treatment response, however, this finding requires
confirmation in a prospective trial with a larger patient
number.
Acknowledgements
The authors would like to thank staff members,
nurses and technologists.
References
|
1
|
Gebski V, Burmeister B, Smithers BM, Foo
K, Zalcberg J and Simes J: Australasian Gastro-Intestinal Trials
Group: Survival benefits from neoadjuvant chemoradiotherapy or
chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet
Oncol. 8:226–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blum MA, Taketa T, Sudo K, Wadhwa R,
Skinner HD and Ajani JA: Chemoradiation for esophageal cancer.
Thorac Surg Clin. 23:551–558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blom RL, Steenbakkers IR, Lammering G,
Vliegen RF, Belgers EJ, de Jonge C, Schreurs WM, Nap M and Sosef
MN: PET/CT-based metabolic tumour volume for response prediction of
neoadjuvant chemoradiotherapy in oesophageal carcinoma. Eur J Nucl
Med Mol Imaging. 40:1500–1506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hulshoff JB, Smit JK, van der Jagt EJ and
Plukker JT: Evaluation of progression prior to surgery after
neoadjuvant chemoradiotherapy with computed tomography in
esophageal cancer patients. Am J Surg. 280:73–79. 2014. View Article : Google Scholar
|
|
5
|
Krasna MJ: Radiographic and
endosonographic staging in esophageal cancer. Thorac Surg Clin.
23:453–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lutz MP, Zalcberg JR, Ducreux M, Ajani JA,
Allum W, Aust D, Bang YJ, Cascinu S, Hölscher A, Jankowski J, et
al: Highlights of the EORTC St. Gallen International Expert
Consensus on the primary therapy of gastric, gastroesophageal and
oesophageal cancer-differential treatment strategies for subtypes
of early gastroesophageal cancer. Eur J Cancer. 48:2941–2953. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi W, Wang W, Wang J, Cheng H and Huo X:
Meta-analysis of 18FDG PET-CT for nodal staging in patients with
esophageal cancer. Surg Oncol. 22:112–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marzola MC, De Manzoni G, Grassetto G,
Cordiano C, Al-Nahhas A, Alavi A and Rubello D: Extended staging of
oesophageal cancer using FDG-PET - a critical appraisal. Eur J
Radiol. 81:21–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantovani A, Marchesi F, Portal C,
Allavena P and Sica A: Linking inflammation reactions to cancer:
Novel targets for therapeutic strategies. Adv Exp Med Biol.
610:112–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mantovani A: Molecular pathways linking
inflammation and cancer. Curr Mol Med. 10:369–373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aldinucci D and Colombatti A: The
inflammatory chemokine CCL5 and cancer progression. Mediators
Inflamm. 2014:2923762014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ham M and Moon A: Inflammatory and
microenvironmental factors involved in breast cancer progression.
Arch Pharm Res. 36:1419–1431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim ES, Cha Y, Ham M, Jung J, Kim SG,
Hwang S, Kleemann R and Moon A: Inflammatory lipid
sphingosine-1-phosphate upregulates C-reactive protein via C/EBPβ
and potentiates breast cancer progression. Oncogene. 33:3583–3593.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantovani A and Pierotti MA: Cancer and
inflammation: A complex relationship. Cancer Lett. 267:180–181.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morrison WB: Inflammation and cancer: A
comparative view. J Vet Intern Med. 26:18–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lukaszewicz-Zając M, Mroczko B, Kozłowski
M, Nikliński J, Laudański J, Siewko M and Szmitkowski M:
Comparative evaluation of serum C-reactive protein (CRP) levels in
the different histological subtypes of esophageal cancer (squamous
cell carcinoma and adenocarcinoma of esophagus). J Clin Lab Anal.
26:73–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watanabe T, Shibata M, Nishiyama H, Soeda
S, Furukawa S, Gonda K, Takenoshita S and Fujimori K: Serum levels
of rapid turnover proteins are decreased and related to systemic
inflammation in patients with ovarian cancer. Oncol Lett.
7:373–377. 2014.PubMed/NCBI
|
|
20
|
Swede H, Hajduk AM, Sharma J, Rawal S,
Rasool H, Vella AT, Tobet RE and Stevens RG: Baseline serum
C-reactive protein and death from colorectal cancer in the NHANES
III cohort. Int J Cancer. 134:1862–1870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu M, Zhu M, Du Y, Yan B, Wang Q, Wang C
and Zhao J: Serum C-reactive protein and risk of lung cancer: A
case-control study. Med Oncol. 30:3192013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tibau A, Ennis M and Goodwin PJ:
Post-surgical highly sensitive C-reactive protein and prognosis in
early-stage breast cancer. Breast Cancer Res Treat. 141:485–493.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McMillan DC: The systemic
inflammation-based Glasgow Prognostic Score: A decade of experience
in patients with cancer. Cancer Treat Rev. 39:534–540. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laird BJ, Kaasa S, McMillan DC, Fallon MT,
Hjermstad MJ, Fayers P and Klepstad P: Prognostic factors in
patients with advanced cancer: A comparison of clinicopathological
factors and the development of an inflammation-based prognostic
system. Clin Cancer Res. 19:5456–5464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kersten C, Louhimo J, Algars A, Lahdesmaki
A, Cvancerova M, Stenstedt K, Haglund C and Gunnarsson U: Increased
C-reactive protein implies a poorer stage-specific prognosis in
colon cancer. Acta Oncol. 52:1691–1698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mohri Y, Tanaka K, Ohi M, Toiyama Y,
Yasuda H, Inoue Y, Uchida K and Kusunoki M: Inflammation-based
prognostic score as a predictor of postoperative gastric cancer
recurrence. Anticancer Res. 32:4581–4584. 2012.PubMed/NCBI
|
|
27
|
Jiang X, Hiki N, Nunobe S, Kumagai K,
Kubota T, Aikou S, Sano T and Yamaguchi T: Prognostic importance of
the inflammation-based Glasgow prognostic score in patients with
gastric cancer. Br J Cancer. 107:275–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu ZQ, Chu L, Fang JM, Zhang X, Zhao HX,
Chen YJ and Xu Q: Prognostic role of C-reactive protein in prostate
cancer: A systematic review and meta-analysis. Asian J Androl.
16:467–471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu Q, Yu XF, Zhang SD, Wang HH, Wang HY
and Teng LS: Prognostic role of C-reactive protein in gastric
cancer: A meta-analysis. Asian Pac J Cancer Prev. 14:5735–5740.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo YZ, Pan L, Du CJ, Ren DQ and Xie XM:
Association between C-reactive protein and risk of cancer: A
meta-analysis of prospective cohort studies. Asian Pac J Cancer
Prev. 14:243–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou B, Liu J, Wang ZM and Xi T:
C-reactive protein, interleukin 6 and lung cancer risk: A
meta-analysis. PLoS One. 7:e430752012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qayyum T, McArdle PA, Lamb GW, Going JJ,
Orange C, Seywright M, Horgan PG, Oades G, Aitchison MA and Edwards
J: Prospective study of the role of inflammation in renal cancer.
Urol Int. 88:277–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han Y, Mao F, Wu Y, Fu X, Zhu X, Zhou S,
Zhang W, Sun Q and Zhao Y: Prognostic role of C-reactive protein in
breast cancer: A systematic review and meta-analysis. Int J Biol
Markers. 26:209–215. 2011.PubMed/NCBI
|
|
34
|
Heikkilä K, Harris R, Lowe G, Rumley A,
Yarnell J, Gallacher J, Ben-Shlomo Y, Ebrahim S and Lawlor DA:
Associations of circulating C-reactive protein and interleukin-6
with cancer risk: Findings from two prospective cohorts and a
meta-analysis. Cancer Causes Control. 20:15–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guillem P and Triboulet JP: Elevated serum
levels of C-reactive protein are indicative of a poor prognosis in
patients with esophageal cancer. Dis Esophagus. 18:146–150. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Crumley AB, McMillan DC, McKernan M, Going
JJ, Shearer CJ and Stuart RC: An elevated C-reactive protein
concentration, prior to surgery, predicts poor cancer-specific
survival in patients undergoing resection for gastro-oesophageal
cancer. Br J Cancer. 94:1568–1571. 2006.PubMed/NCBI
|
|
37
|
Crumley AB, Stuart RC, McKernan M,
McDonald AC and McMillan DC: Comparison of an inflammation-based
prognostic score (GPS) with performance status (ECOG-ps) in
patients receiving palliative chemotherapy for gastroesophageal
cancer. J Gastroenterol Hepatol. 23:e325–e329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Motoyama S, Miura M, Hinai Y, Maruyama K,
Usami S, Nakatsu T, Saito H, Minamiya Y, Suzuki T and Ogawa J:
C-reactive protein 1059G>C genetic polymorphism influences serum
C-reactive protein levels after esophagectomy in patients with
thoracic esophageal cancer. J Am Coll Surg. 209:477–483. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shimada H, Nabeya Y, Okazumi S, Matsubara
H, Shiratori T, Aoki T, Sugaya M, Miyazawa Y, Hayashi H, Miyazaki S
and Ochiai T: Elevation of preoperative serum C-reactive protein
level is related to poor prognosis in esophageal squamous cell
carcinoma. J Surg Oncol. 83:248–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang CY, Hsieh MJ, Chiu YC, Li SH, Huang
HW, Fang FM and Huang YJ: Higher serum C-reactive protein
concentration and hypoalbuminemia are poor prognostic indicators in
patients with esophageal cancer undergoing radiotherapy. Radiother
Oncol. 92:270–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Groblewska M, Mroczko B, Sosnowska D and
Szmitkowski M: Interleukin 6 and C-reactive protein in esophageal
cancer. Clin Chim Acta. 413:1583–1590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miyata H, Yamasaki M, Kurokawa Y,
Takiguchi S, Nakajima K, Fujiwara Y, Mori M and Doki Y: Prognostic
value of an inflammation-based score in patients undergoing
pre-operative chemotherapy followed by surgery for esophageal
cancer. Exp Ther Med. 2:879–885. 2011.PubMed/NCBI
|
|
43
|
Zingg U, Forberger J, Rajcic B, Langton C
and Jamieson GG: Association of C-reactive protein levels and
long-term survival after neoadjuvant therapy and esophagectomy for
esophageal cancer. J Gastrointest Surg. 14:462–469. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM: Classification of Malignant Tumours (7th). Hoboken, NJ:
Wiley-Blackwell. 2009.
|