Introduction
Extraurothelial recurrence (EUR) is difficult to
predict in patients with upper tract urothelial carcinoma (UTUC)
prior to radical nephroureterectomy (RNU). Previously reported
predictors for EUR, such as pathological T (pT) stage and
lymphovascular invasion (LVI) (1–4), cannot be
determined prior to surgery. Identification of high-risk patients
for EUR using preoperative factors, may allow neoadjuvant
chemotherapy followed by RNU with extended lymph node dissection
(ELND) to improve the survival of these patients. By contrast,
determining which patients have a low-risk for EUR, may allow
minimally-invasive surgery such as laparoscopic RNU without lymph
node dissection (LND) to be used safely in these individuals.
A previous study was performed to identify the
preoperative predictors for recurrence in UTUC patients, and
clinical T stage and a neutrophil counts of ≥4,000/µl were
independent predictors of recurrence (5). Additional previous studies (6–8) have tried
to identify the preoperative predictors of muscle-invasive or
non-organ-confined disease, as patients with these conditions are
considered to be at very high risk of UTUC recurrence. In these
previous reports, tumor location, cytology, the presence of
hydronephrosis, local invasion observed on imaging, a high-grade
tumor confirmed by ureteroscopic biopsy, architecture observed on
ureteroscopy were identified for predictors for muscle-invasive or
non-organ-confined disease in UTUC patients (6–8). However,
the authors performed ureteroscopy in all patients. Routine use of
ureteroscopy before RNU appeared to be an invasive procedure for
some UTUC patients. Predicting EUR by using more simple
preoperative factors would reduce the invasiveness of the
diagnostic methods for the patients.
Due to anatomical differences, it did not appear to
be appropriate to categorize preoperative radiological findings of
renal pelvic cancer (RPC) and those of ureteral cancer (UTC) using
the same classification criteria. For example, RPC located at renal
calyx is unlikely to invade the Gerota's fascia because of the
presence of renal parenchyma. By contrast, UTC easily invades the
surrounding tissues. Furthermore, invasive UTC easily leads to the
full dilation of the renal pelvis and renal calyces, whereas RPC
does not easily lead to the full dilation of the renal pelvis and
renal calyces, even when RPC invades the renal parenchyma. In
addition, some authors reported that patients with UTC had a worse
prognosis than that of patients with RPC, when tumor grades or
stages are comparable (9,10). Therefore, patients with RPC and UTC
were analyzed separately, with a focus on patients with RPC.
We previously analyzed patients with UTC to
determine preoperative risk factors for EUR and demonstrated that
clinical T (cT) stage, ureteral tumor length along the ureter ≥3
cm, positive urine cytology, and an estimated glomerular filtration
rate (eGFR) <60 µl/min/1.73 m2 were independent
predictors for EUR, and risk classification for EUR could be
established in patients with UTC (11). Furthermore, patients in the high-risk
group rapidly developed recurrence, and therefore appeared to be
potential candidates for neoadjuvant chemotherapy. In addition,
patients in the low-risk group had a low risk for EUR, and appeared
to be candidates for omission of LND.
The aim of the present study was to identify
preoperative predictors for EUR in patients with RPC.
Patients and methods
The medical records of 150 N0M0 patients with
pathologically diagnosed urothelial carcinoma, and treated by
unilateral RNU at our institute between April 1999 and July 2013
were retrospectively reviewed. Of the 150 patients, 76 had RPC,
including one with concomitant invasive bladder cancer who
underwent both RNU and radical cystectomy, and another with RPC who
underwent radical cystectomy 1 year prior to RNU, due to muscle
invasive bladder cancer. Therefore, these two patients were
excluded from the present study. Three patients with concomitant
UTC with radiologically confirmed invasive growth were also
excluded from the present study. Finally, a total of 71 patients
with RPC were evaluated in the present study. Among these patients,
noninvasive UTC was suspected in three according to preoperative
radiological examinations. The median follow-up period was 50.3
months (range, 1–160 months). The study protocol was approved by
the institutional review board (approval no. 2086).
Forty patients underwent open nephroureterectomy,
while 31 were treated by laparoscopic nephroureterectomy. Regional
LND was performed in 13 patients (18.3%) with suspected enlarged
LNs, detected during intraoperative inspection, or with suspected
advanced clinical stage by preoperative radiological examinations.
The extent of regional LND was often limited (e.g., only the renal
hilus) and ELND was not routinely performed. One of the 13 patients
who underwent LND was found to have LNM. Of the remaining 12
patients with pN0 disease, recurrence developed in two (lymph node
metastasis revealed in 1 of the 2 patients). No patients in the
present study received neoadjuvant chemotherapy. Cisplatin-based
adjuvant chemotherapy was administered to 12 patients with
pathologically confirmed lymph node metastasis or muscle invasive
disease. Local recurrence and metastasis were monitored by
examining each patient every 3–6 months for the first 5 years
following surgery, and every 6–12 months thereafter. Intravesical
recurrence and recurrence in the contralateral upper urinary tract
(UUT) were not considered as EUR in the current study. No patient
developed recurrence in the contralateral UUT.
Radiological findings, including cT stage, presence
of hydrocalyx, and maximal tumor size on an axial view were
determined by computed tomography (CT) and/or magnetic resonance
imaging (MRI). Clinical T stage was determined according to the TNM
classification (12) and classified
as T ≤2 or ≥3. Contrast-enhanced CT and/or contrast-enhanced MRI
were used for the determination of cT stage. If RPC patients had
decreased renal function, both CT and MRI without contrast media
were used. The lesion was considered to be a clinical T3 tumor when
the tumor obviously extended into the adjacent renal parenchyma or
when the margin of the tumor was irregular and the invasion toward
peripelvic fat tissue was strongly suspected. The lesion without
obvious invasion toward renal parenchyma or peripelvic fat tissue
was considered to be clinical T2 or less.
Urine cytology was evaluated by Papanicolaou
staining. Positive cytology was defined as the presence of
malignant or atypical cells in voided specimens. Grading of
Papanicolaou staining was determined using a 5-grade system.
Inflammatory indices (white blood cell count, neutrophil count,
C-reactive protein level, and neutrophil-to-lymphocyte ratio)
(13) were evaluated using laboratory
tests. The neutrophil-to-lymphocyte ratio (NLR) was calculated by
dividing the absolute neutrophil count by the absolute lymphocyte
count. The eGFR was calculated using the following equation: eGFR
(ml/min/1.73 m2) = 194 × (0.739, if female) × (serum
creatinine)−1.094x age−0.287. The cut-off
values for these laboratory tests are shown in Table I. Upper normal limits were used as
cut-off values for C-reactive protein (CRP) levels. For white blood
cell (WBC) count, neutrophil count, NLR, and lactate dehydrogenase
(LDH), the values best discriminating between good and poor
survival was determined by testing all possible cut-off values
within the central 85% of the distribution of values. Laboratory
data were obtained by performing blood tests within one month prior
to RNU.
 | Table I.Preoperative variables in N0M0
patients with renal pelvic cancer (n=71). |
Table I.
Preoperative variables in N0M0
patients with renal pelvic cancer (n=71).
| Variables | Categories | No. of patients |
|---|
| Age (years) | ≥65 vs. <65 | 51/20 |
| Gender | Male vs. Female | 58/13 |
| Tumor side | Right vs. Left | 32/39 |
| Past history of BT
and/or concomitant BT | Yes vs. No | 18/53 |
| Symptomatic | Yes vs. No | 50/21 |
| Concomitant ureteral
lesion (radiological) | Presence vs.
Absence | 5/66 |
| Hydrocaryx (at least
1 calyx) | Presence vs.
Absence | 26/45 |
| Clinical T stage | cT ≤2 vs. cT ≥3 | 52/19 |
| Maximal tumor
sizea (cm) | <1.5 | 8 |
|
| 1.5–3.0 | 28 |
|
| 3.0–4.0 | 21 |
|
| ≥4.0 | 14 |
| Urine cytology | Positive vs. Negative
(NDb) | 51/19 (1) |
| WBC count (/µl) | >7600 vs.
≤7600 | 64/7 |
| Neutrophil count
(/µl) | >4,500 vs.
≤4,500 | 58/13 |
| CRP (mg/dl) | >0.3 vs. ≤0.3 | 54/17 |
| NLR | >2.0 vs. ≤2.0 | 41/30 |
| LDH (IU/l) | >210 vs. ≤210 | 52/19 |
| eGFR (ml/min./1.73
m2) | >60 vs. ≤60 | 27/44 |
Statistical analysis
Results are presented as means ± standard
deviations. Survival curves were constructed using the Kaplan-Meier
method, and differences between them were assessed using the
log-rank test. To identify preoperative predictors of EUR,
univariate and multivariate analyses were performed using the Cox
proportional hazards model. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics, radiological findings, and
laboratory test results are listed in Table I. Twelve (16.9%) of 71 patients were
diagnosed with EUR. Of these 12 patients, initial recurrence
developed in the LNs in seven, the distant organs in nine, and both
LNs and distant organs in four. The median time to EUR was 6.5
months (range, 1.0–13.0) in the 12 patients.
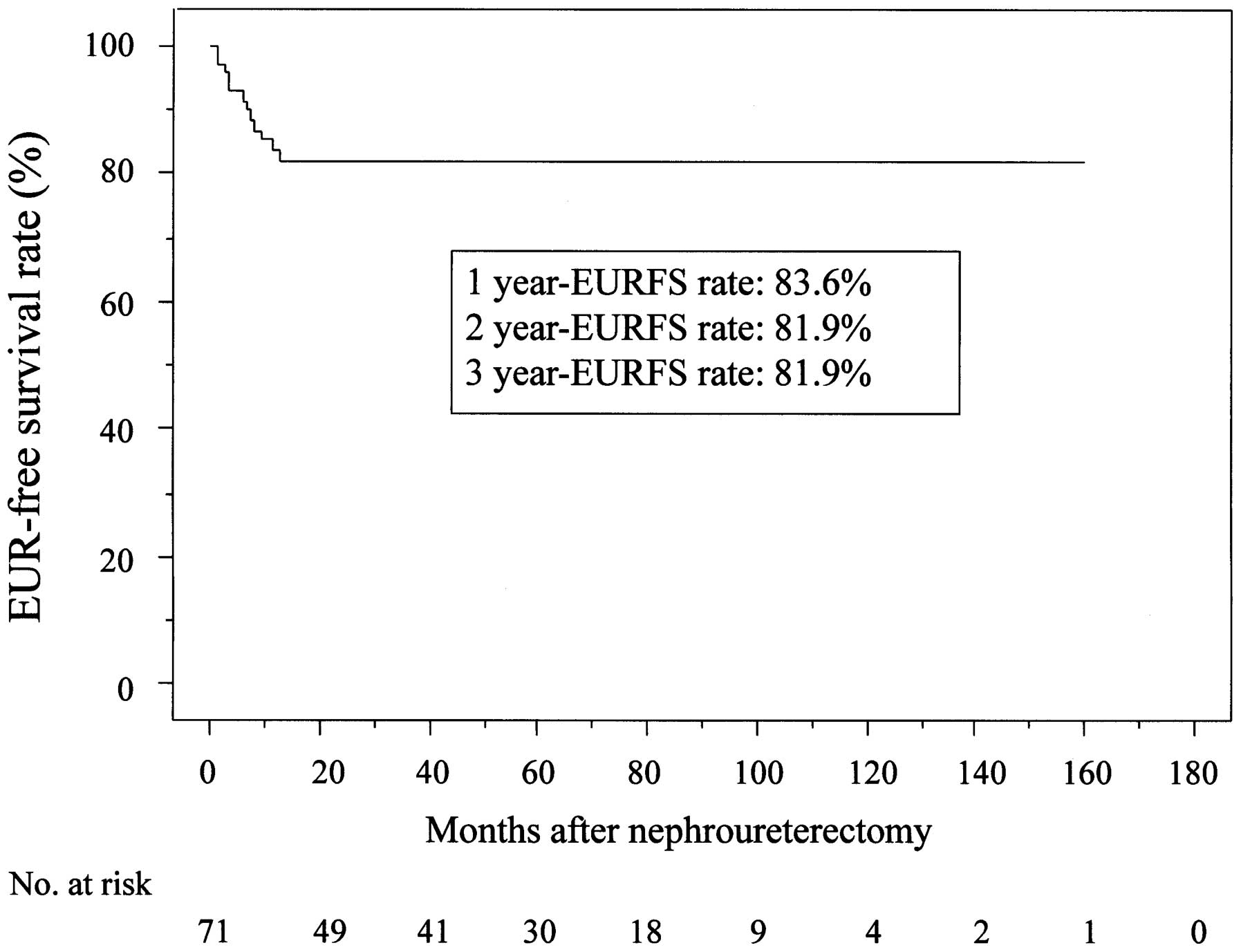
The 3-year EUR-free survival (EURFS) rate was 81.9%
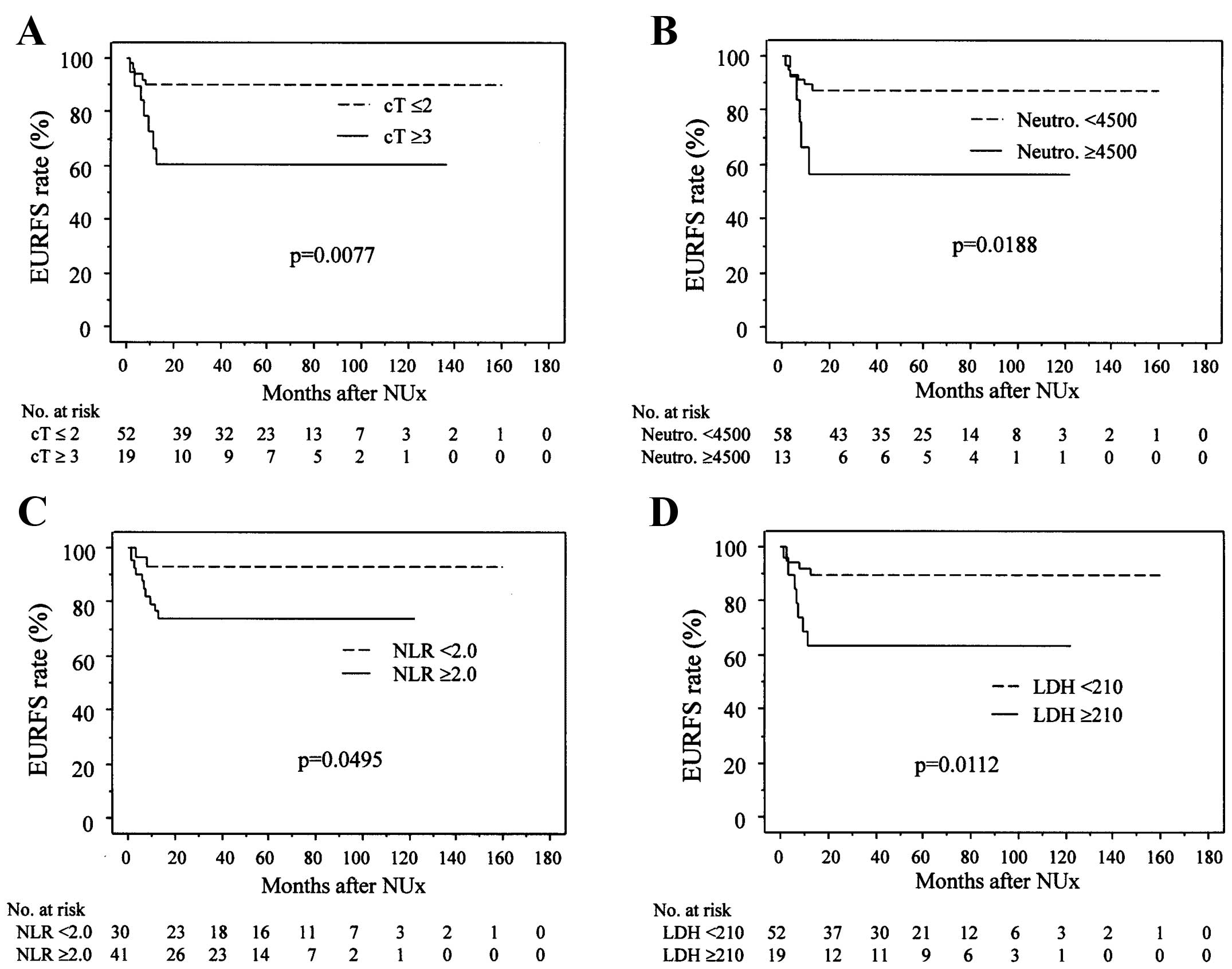
(Fig. 1). The EURFS rates (Fig. 2) were significantly lower in patients
with cT ≥3 than in those with cT ≤2 (Fig.
2A), patients with neutrophil counts ≥4,500/µl than in those
with neutrophil counts <4,500/µl (Fig.
2B), patients with NLR ≥2.0 than in those with NLR <2.0
(Fig. 2C), patients with LDH ≥210
IU/l than in those with LDH <210 IU/l (Fig. 2D), and patients with WBC counts
≥7600/µl than in those with WBC counts <7600/µl (P=0.0243; data
not shown).
The Cox proportional hazards model was used to
evaluate preoperative predictors of EURFS. Univariate analysis
showed cT ≥3, WBC count ≥7600/µl, neutrophils ≥4,500/µl, and LDH
≥210 IU/l significantly associate with EURFS. Multivariate analysis
showed that cT ≥3 (HR=3.759) and LDH ≥210 (HR=3.521) were
significant predictors of EURFS, but WBC counts ≥7600/µl and
neutrophil counts ≥4,500/µl were not (Table II).
 | Table II.Preoperative factors predicting
extra-urothelial recurrence in N0M0 patients with renal pelvic
cancer. |
Table II.
Preoperative factors predicting
extra-urothelial recurrence in N0M0 patients with renal pelvic
cancer.
|
| Univariate |
| Multivariate |
|---|
|
|
|
|
|
|---|
| Variables | P-value | P-value | Hazard ratio | Relative risk ratio
95% CI |
|---|
| Age ≥65 y/o | 0.2844 |
|
|
|
| Gender | 0.7859 |
|
|
|
| Tumor side | 0.7167 |
|
|
|
| Past history of BT
and/or concomitant BT | 0.2455 |
|
|
|
| Symptomatic | 0.3664 |
|
|
|
| Concomitent ureteral
lesion (radiological) | 0.6902 |
|
|
|
| Presence of
hydrocalyx | 0.4242 |
|
|
|
| Clinical T stage
≥3 | 0.0143 | 0.0244 | 3.759 | 1.188–11.905 |
| Maximal tumor size ≥3
cm | 0.6033 |
|
|
|
| Positive urine
cytology | 0.1418 |
|
|
|
| WBC ≥7600/µl | 0.0375 |
|
|
|
| Neutrophil
≥4,500/µl | 0.0281 |
|
|
|
| CRP ≥0.3 mg/dl | 0.4225 |
|
|
|
| NLR ≥2.0 | 0.07 |
|
|
|
| LDH ≥210 IU/l | 0.019 | 0.0322 | 3.521 | 1.112–11.111 |
| eGFR <60
ml/min/1.73 m2 | 0.9113 |
|
|
|
The 71 patients were stratified into three groups
according to the number of risk factors (n=0, 1, or 2). The 3-year
EURFS rates of the three groups were 94.5, 76.3 and 33.3%,
respectively (Fig. 3A). We found a
significant difference in EURFS rates between the low-risk and
intermediate-risk groups (P=0.0353) and the difference was close to
significant between the intermediate-risk and high-risk groups
(P=0.069). When the postoperative factors in each group were
further evaluated, the high-risk group was associated with higher
pT stages and higher percentages of LVI (Table III).
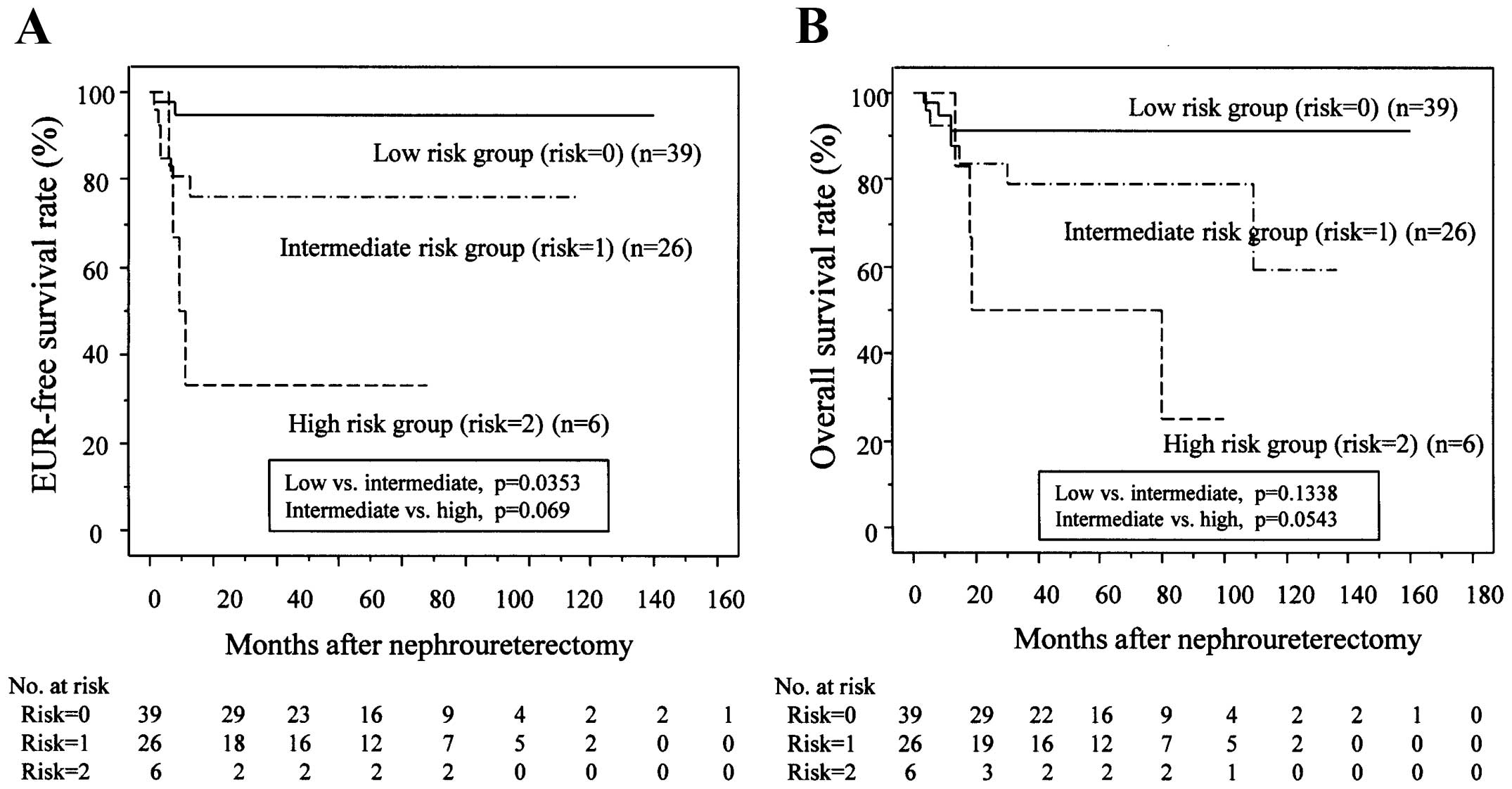 | Figure 3.Risk stratification using two
predictive factors for EUR. (A) Risk stratification according to
the number of risk factors. Patients stratified into low-,
intermediate-, and high-risk groups (0, 1, and 2 risk factors,
respectively), had 1- and 3-year EURFS rates of 94.5 and 94.5%,
80.6 and 76.3%, and 33.3 and 33.3%, respectively. Kaplan-Meier
EURFS curves show a significant difference between the low- and
intermediate-risk groups (P=0.0353) and closely significant
differences between the intermediate- and high-risk groups
(P=0.059). (B) OS rates in each risk group. Patients stratified
into low-, intermediate-, and high-risk groups, had 1- and 3-year
OS rates of 94.6 and 91.5%, 92.3 and 79.1%, and 100 and 50%,
respectively. Patients in the low-risk group lived longer than
those in the intermediate-risk group (P=0.1338), and patients in
the intermediate-risk group lived longer than those in the
high-risk group (P=0.0543). |
 | Table III.Distribution on postoperative
prognostic factors and extraurothelial recurrence in each risk
group. |
Table III.
Distribution on postoperative
prognostic factors and extraurothelial recurrence in each risk
group.
| Variables | Low-risk
(n=39) | Intermediate-risk
(n=26) | High-risk(n=6) | P-value |
|---|
| Pathological T (pT)
stage (pTis/a/1/2/3/4) | 2/12/7/3/15/0 | 0/5/1/2/18/0 | 0/0/0/1/4/1 | 0.0150a |
| pT stage <2 vs.
≥2 | 21/18 | 6/20 | 0/6 | 0.0058a |
| Grade (grade
1/2/3) | 1/15/23 | 1/6/19 | 0/0/6 | 0.2936a |
| LVI (−/+) | 32/7 | 17/9 | 2/4 | 0.0311a |
| Adjuvant
chemotherapy (−/+) | 36/3 | 20/6 | 3/3 | 0.0208a |
| Lymph node sampling
(−/+) | 32/7 | 22/4 | 4/2b | 0.5173 |
| Extraurothelial
recurrence (−/+) | 37/2c | 20/6d | 2/4e | 0.0005a |
Discussion
The present study focused on RPC and evaluated
preoperative factors to identify predictors of EUR. Multivariate
analysis revealed that cT stage ≥3 and preoperative LDH level were
independent predictors of EUR. The present study also stratified
the patients into three groups according to the number of risk
factors. In our previous study evaluating UTC, we identified cT ≥3,
tumor length, positive urine cytology, and eGFR <60 as
independent predictors for EUR (11).
Only cT stage was an independent predictor for both UTC and RPC.
Also, in our previous study, univariate analysis revealed that cT
stage, tumor length along the ureter, maximal tumor diameter,
positive urine cytology, NLR ≥3.0, and eGFR ≤60 ml/min/1.73
m2 were significant risk factors for UTC (11). Notably, even significant factors
identified by univariate analysis considerably differed between
patients with UTC and those with RPC. Because preoperative
predictors for EUR differed (with the exception of cT stage between
UTC and RPC), it may be more advantageous to determine preoperative
predictors for EUR in patients with UTC and those with RPC,
separately.
Previously, a number of authors attempted to
identify preoperative predictors of UTUC recurrence (3–5). Hashimoto
et al (5) identified that cT
stage and neutrophil count of ≥4,000/µl were independent predictors
of recurrence (5). However, the
present study separately evaluated patients with UTC and those with
RPC, which was a novel approach. Another three studies reported
that ureteroscopic grade and tumor architecture determined by
ureteroscopy were independent predictors of muscle-invasive or
non-organ-confined disease (2–4). Although
factors which can be determined by ureteroscopic examination such
as ureteroscopic grade and tumor architecture appear to be strong
predictors for EUR, the current study aimed to identify predictors
for EUR by using simple preoperative factors that can be determined
without ureteroscopic examination.
The results of the present study demonstrated that
cT stage was an independent predictor for EUR. It is difficult to
determine pathological T stage by radiological examination. By
radiography, microscopic T3 tumors do not show obvious extension
towards renal parenchyma or peripelvic fat tissue, and are likely
to be categorized as cT≤2. In the present study, a total of 17 of
19 tumors which were categorized as cT≥3 were diagnosed as pT3 or
more (89.5%, 16 patients had pT3 tumors and one had pT4). By
contrast, 21 of the 51 tumors categorized as cT≤2 were diagnosed as
pT3 (41.2%), suggesting there was difficulty in determining the pT
stages by radiological examinations. Furthermore, pT3 tumors
categorized as cT≤2 appeared to have a better EURFS rate than pT3
tumors that were categorized as cT≥3 (2-year-EURFS rate: 85.2 vs.
59.6%, P=0.1381). This may be one of the reasons for cT stage being
a strong predictor for EUR.
Preoperative LDH level is a novel preoperative
predictor for EUR in renal pelvic cancer patients. Additionally,
LDH level is reported to be an important prognostic factor in
patients with metastatic renal cell carcinoma (14). LDH level likely reflects the quantity
of tumor cells in the body. Therefore, elevated LDH levels may
reflect the presence of latent metastases in patients with
radiological N0M0 RPC. Preoperative LDH levels appeared to be
higher in RPC patients with EUR (n=12) compared with those who did
not recur (n=59; 199 vs. 181 IU/L, P=0.0591 by Mann-Whitney U
test). When 12 patients who had EUR were reviewed, LDH once
decreased postoperatively in all 12 patients. When EURs were
detected, only 3 patients had LDH levels ≥210 IU/l. However, in 9
of the 12 patients (75%) maximal LDH levels after EUR were more
than 210 IU/l (215–5370). Although postoperative LDH levels
appeared not to correlate with systemic quantity of tumor cells in
all patients, postoperative LDH levels increased in the majority of
the 12 patients as their disease was progressed.
In previous reports evaluating prognostic factors
after RNU, inflammatory indices, such as CRP (15,16),
neutrophil count (5) and NLR
(17), were independent prognostic
factors. Saito et al (15)
reported that preoperative CRP level, pT stage, and lymph node
involvement were significant prognostic factors for
disease-specific and recurrence-free survival. A previous
multi-institutional study revealed that elevated preoperative NLR
was an independent predictor for disease recurrence (17). However, these inflammatory indices
were not independent factors in the present study or our previous
study evaluating UTC (11). A
possible reason to explain the differences between the findings of
our two studies and previous studies (15,17) may be
that we evaluated RPC and UTC separately. Another possible reason
may be that we evaluated only N0M0 UTUC patients.
In addition, the present study did not evaluate
tumor markers, such as cancer antigen 19-9, carcinoembryonic
antigen, or squamous cell carcinoma antigen, in the present study.
These factors were evaluated in our previous study of UTC (11). However, these markers were examined
for only 60–70% of patients with RPC. When these markers were
analyzed by univariate analyses, none were significant (data not
shown).
In our previous study of UTC, eGFR was an
independent predictor for EUR (11).
However, eGFR was not an independent predictor for EUR in the
current study. In case of RPC, the whole dilation of renal pelvis
and renal calyces in the unilateral kidney can occur only when a
tumor is located near the ureteropelvic junction or when the renal
pelvis contains a large tumor. If the tumor is located near the
renal calyx, the whole dilation of renal pelvis and renal calyces
is not likely to occur even if the tumor is invasive. Because
tumors located within limited renal calyces that invade the renal
parenchyma usually decreases renal function partially, the
impairment of renal function is probably minimal compared to that
in patients with tumors that cause the whole dilation of renal
pelvis and renal calyces. By contrast, invasive UTC can easily
cause the whole dilation of renal pelvis and renal calyces, and
eGFR can decrease markedly. In our previous study (11), the median eGFR of 70 patients with UTC
was 58.9 ml/min/1.73 m2 and in the present study this
was 64.8 ml/min/1.73 m2 (UTC vs. RPC, P=0.0722 by the
Mann-Whitney U test). Moreover, although 56 of 70 patients with UTC
exhibited varied degrees of hydronephrosis, 26 of 71 patients with
RPC had selected hydrocalyx or hydronephrosis. The presence of
hydronephrosis is related to tumor invasiveness in UTC (18), thus eGFR might be an independent
predictor for EUR in patients with UTC in our previous study
(11). By contrast, partial RPC
invasion of the renal parenchyma (pT3 disease) is likely to
slightly decrease eGFR. In the present study, eGFR was not a
significant factor to predict EUR even by univariate analysis.
In addition to EUR prediction, our risk
stratification using preoperative factors also appeared to predict
patient survival (Fig. 3B), which was
nicely correlated with pT stage and the presence of LVI (Table III). As shown in Fig. 3, low-risk patients were at a
relatively low risk for EUR (3-year EURFS=94.5%) and may be
candidates for RNU without LND. In this study, there were 39
(54.9%) low-risk patients and LND may be potentially avoided in
more than half of N0M0 patients with RPC, according to the proposed
risk classification. By contrast, high-risk patients experience
rapid recurrence within a short period after RNU. 50% of the
high-risk patients who developed recurrence had distant metastases
without LNM at the initial radiologically confirmed recurrence.
Therefore, neoadjuvant chemotherapy should be considered for
high-risk patients and following RNU with ELND may improve survival
of some high-risk patients. In two multicenter retrospective
studies, neoadjuvant chemotherapy followed by RNU was suggested to
improve the prognosis of UTUC patients with LNM (19,20).
Furthermore, a recent meta-analysis suggested that cisplatin-based
neoadjuvant chemotherapy might prolong survival of UTUC patients
(21).
The present study has several limitations that
should be addressed. First, it was a nonrandomized, retrospective,
single-center study; therefore, external validation and further
prospective studies with larger numbers of patients are required.
Second, some patients underwent LND, the extent of which was not
standardized. Analysis of more uniform populations, such as
patients who did not undergo LND, presents a better method to
determine EUR. However, 58 (81.7%) of 71 patients did not undergo
LND, limited LND (removal of LN around renal hilus) was performed
in 11 patients (15.5%), and ELND was performed for only two
patients (these patients did not have LNM pathologically). Of the
39 low-risk patients, only 7 (17.9%) underwent LND and none had
pathological LNM. Therefore, LND appeared to be able to be omitted
in the majority of low-risk patients. Third, 12 patients with
pathologically confirmed muscle-invasive RPC received postoperative
chemotherapy. However, 3 (25%) of 12 patients who underwent
postoperative chemotherapy developed recurrence, as compared to
only 9 (15.5%) of 59 patients who did not. There was no apparent
benefit of postoperative chemotherapy on EUR in our series.
Moreover, only 3 (7.7%) patients in the low-risk group received
postoperative chemotherapy (Table
III) and the effect of chemotherapy on recurrence in the
low-risk patients appeared to be minimal. By contrast, 23.1% of the
intermediate-risk patients and 50% of the high-risk patients
received postoperative chemotherapy. Fourth, EURFS rate was rather
better compared to some previous reports (2,3). A small
number of RPC patients in a single institute might influence on the
oncological outcome in the present study. Furthermore, adjuvant
chemotherapy may somewhat influence patient outcome. Although the
present study had several limitations, it was possible to establish
a risk classification for EUR in RPC patients using preoperative
factors that could be easily determined.
In conclusion, clinical T stage ≥3 and LDH ≥210 IU/l
were independent preoperative predictors of EUR in patients with
N0M0 RPC treated by RNU. Risk stratification using these
preoperative factors may be useful to select candidates for
neoadjuvant chemotherapy and for RNU without LND. However, further
studies with larger numbers of patients and external validation are
required.
Acknowledgements
The authors thank Professor Jun Nakashima, Tokyo
Medical University, for his helpful comments and valuable
discussion.
References
|
1
|
Saito K, Kawakami S, Fujii Y, Sakura M,
Masuda H and Kihara K: Lymphovascular invasion is independently
associated with poor prognosis in patients with localized upper
urinary tract urothelial carcinoma treated surgically. J Urol.
178:2291–2296; discussion 2296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kikuchi E, Margulis V, Karakiewicz PI,
Roscigno M, Mikami S, Lotan Y, Remzi M, Bolenz C, Langner C, Weizer
A, et al: Lymphovascular invasion predicts clinical outcomes in
patients with node-negative upper tract urothelial carcinoma. J
Clin Oncol. 27:612–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Margulis V, Shariat SF, Matin SF, Kamat
AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD and Wood CG:
Upper Tract Urothelial Carcinoma Collaboration. The Upper Tract
Urothelial Carcinoma Collaboration: Outcomes of radical
nephroureterectomy: A series from the upper tract urothelial
carcinoma collaboration. Cancer. 115:1224–1233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zigeuner R, Shariat SF, Margulis V,
Karakiewicz PI, Roscigno M, Weizer A, Kikuchi E, Remzi M, Raman JD,
Bolenz C, et al: Tumour necrosis is an indicator of aggressive
biology in patients with urothelial carcinoma of the upper urinary
tract. Eur Urol. 57:575–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hashimoto T, Ohno Y, Nakashima J, Gondo T,
Ohori M and Tachibana M: Clinical significance of preoperative
peripheral blood neutrophil count in patients with non-metastatic
upper urinary tract carcinoma. World J Urol. 31:953–958. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brien JC, Shariat SF, Herman MP, Ng CK,
Scherr DS, Scoll B, Uzzo RG, Wille M, Eggener SE, Terrell JD, et
al: Preoperative hydronephrosis, ureteroscopic biopsy grade and
urinary cytology can improve prediction of advanced upper tract
urothelial carcinoma. J Urol. 184:69–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Margulis V, Youssef RF, Karakiewicz PI,
Lotan Y, Wood CG, Zigeuner R, Kikuchi E, Weizer A, Raman JD, Remzi
M, et al: Preoperative multivariable prognostic model for
prediction of nonorgan confined urothelial carcinoma of the upper
urinary tract. J Urol. 184:453–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Favaretto RL, Shariat SF, Savage C, Godoy
G, Chade DC, Kaag M, Bochner BH, Coleman J and Dalbagni G:
Combining imaging and ureteroscopy variables in a preoperative
multivariable model for prediction of muscle-invasive and non-organ
confined disease in patients with upper tract urothelial carcinoma.
BJU Int. 109:77–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park S, Hong B, Kim CS and Ahn H: The
impact of tumor location on prognosis of transitional cell
carcinoma of the upper urinary tract. J Urol. 171:621–625. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akdogan B, Dogan HS, Eskicorapci SY, Sahin
A, Erkan I and Ozen H: Prognostic significance of bladder tumor
history and tumor location in upper tract transitional cell
carcinoma. J Urol. 176:48–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ito K, Kuroda K, Asakuma J, Hamada S,
Tachi K, Tasaki S, Sato A, Horiguchi A, Seguchi K and Asano T:
Preoperative risk factors for extraurothelial recurrence in
patients with ureteral cancer treated with radical
nephroureterectomy. J Urol. 191:1685–1692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sobin LH, Gospodarowicz M and Wittekind C:
Kidney. TNM classification of malignant tumors (7th). (New York,
NY). Wiley-Blackwell. 258–p261. 2009.
|
|
13
|
Ohno Y, Nakashima J, Ohori M, Hatano T and
Tachibana M: Pretreatment neutrophil-to-lymphocyte ratio as an
independent predictor of recurrence in patients with nonmetastatic
renal cell carcinoma. J Urol. 184:873–878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motzer RJ, Mazumdar M, Bacik J, Berg W,
Amsterdam A and Ferrara J: Survival and prognostic stratification
of 670 patients with advanced renal cell carcinoma. J Clin Oncol.
17:2530–2540. 1999.PubMed/NCBI
|
|
15
|
Saito K, Kawakami S, Ohtsuka Y, Fujii Y,
Masuda H, Kumagai J, Kobayashi T, Kageyama Y and Kihara K: The
impact of preoperative serum C-reactive protein on the prognosis of
patients with upper urinary tract urothelial carcinoma treated
surgically. BJU Int. 100:269–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka N, Kikuchi E, Shirotake S, Kanao K,
Matsumoto K, Kobayashi H, Miyazaki Y, Ide H, Obata J, Hoshino K, et
al: The predictive value of C-reactive protein for prognosis in
patients with upper tract urothelial carcinoma treated with radical
nephroureterectomy: A multi-institutional study. Eur Urol.
65:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka N, Kikuchi E, Kanao K, Matsumoto K,
Shirotake S, Miyazaki Y, Kobayashi H, Kaneko G, Hagiwara M, Ide H,
et al: A multi-institutional validation of the prognostic value of
the neutrophil-to-lymphocyte ratio for upper tract urothelial
carcinoma treated with radical nephroureterectomy. Ann Surg Oncol.
21:4041–4048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ito Y, Kikuchi E, Tanaka N, Miyajima A,
Mikami S, Jinzaki M and Oya M: Preoperative hydronephrosis grade
independently predicts worse pathological outcomes in patients
undergoing nephroureterectomy for upper tract urothelial carcinoma.
J Urol. 185:1621–1626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Youssef RF, Shariat SF, Lotan Y, Wood CG,
Sagalowsky AI, Zigeuner R, Kikuchi E, Weizer A, Raman JD, Remzi M,
et al: Upper urinary tract urothelial carcinoma with loco-regional
nodal metastases: Insights from the upper tract urothelial
carcinoma collaboration. bju int. 108:1286–1291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kitamura H, Igarashi M, Tanaka T, Shindo
T, Masumori N, Tamakawa M, Kawaai Y and Tsukamoto T: A role for
preoperative systemic chemotherapy in node-positive upper tract
urothelial carcinoma treated with radical nephroureterectomy. Jpn J
Clin Oncol. 42:1192–1196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leow JJ, Martin-Doyle W, Fay AP, Choueiri
TK, Chang SL and Bellmunt J: A systematic review and meta-analysis
of adjuvant and neoadjuvant chemotherapy for upper tract urothelial
carcinoma. Eur Urol. 66:529–541. 2014. View Article : Google Scholar : PubMed/NCBI
|