Introduction
Gastric carcinoma, a common malignant tumor of the
digestive tract, is the fourth more common cause of morbidity and
the second cause of mortality among all malignant tumors worldwide,
and is particularly common in Latin America, Eastern Europe and
East Asian countries (1,2). The radical therapy of gastric carcinoma
mainly relies on surgical resection; however, local recurrence and
distant metastasis may still occur, even in patients who have
undergone complete surgical resection (3–5). Among
patients with failed surgical intervention, ~90% experienced local
recurrence, which led to death in ~80% of these patients,
particularly those with serosal invasion or lymphatic metastasis
(6,7).
The prognosis with surgery alone is poor for patients with locally
advanced gastric carcinoma, while the 5-year survival rate is only
~8–34% (8). In order to improve the
survival rate of patients with gastric carcinoma and reduce the
recurrence rate, adjuvant therapy following gastrectomy has become
a consensus (9). However,
chemotherapy alone after surgery did not confer an obvious survival
benefit to such patients in the majority of studies (9,10).
Since the results of the INT-0116 study (11) were published in 2001, adjuvant
concurrent chemoradiotherapy (CRT) after surgery has been commonly
used by oncologists for the treatment of gastric carcinoma;
therefore, certain European and American guidelines have included
adjuvant CRT and recommend it as the standard treatment following
surgery. Among selected patients, the proportion of those receiving
limited lymph node dissection (D0 or D1) is ≤90%, whereas the
proportion of those receiving extended D2 lymph node dissection
(D2) is only ~10% (11). However, in
some Asian countries, patients with gastric carcinoma are more
likely to receive D2 radical surgery, which limits the significance
of this study for Asian patients. Thus, Kim et al compared
patients who had received adjuvant concurrent CRT following D2
radical surgery for gastric carcinoma with those who had received
surgery alone, and demonstrated that adjuvant concurrent CRT
following D2 radical surgery may improve the survival rate and
reduce the recurrence rate (12). The
ARTIST trial further compared patients who had received concurrent
CRT following D2 surgery for gastric carcinoma with those who had
received chemotherapy alone, and demonstrated that the overall
3-year disease-free survival rate was marginally higher compared
with that of the group with concurrent CRT, although the difference
was not statistically significant (13).
Intensity-modulated radiation therapy (IMRT) is a
more advanced radiotherapy technique, which is able to concentrate
high doses of radiation on the target area, while better protecting
the adjacent normal tissue and has a dose advantage compared with
conventional radiotherapy and three-dimensional conformal radiation
therapy (3DCRT) following surgery for gastric carcinoma (14,15).
To evaluate the efficacy of IMRT combined with
concurrent chemotherapy following D2 radical surgery for gastric
carcinoma and investigate the clinical value of its combination
with different chemotherapies, we conducted a study including 102
patients who had received IMRT combined with concurrent
chemotherapy with intravenous infusion of fluorouracil (F-CRT) or
oral capecitabine (C-CRT) following D2 radical surgery for gastric
carcinoma.
Patients and methods
Patient eligibility
A total of 102 patients treated with D2 radical
surgery for gastric carcinoma were enrolled from January, 2008 to
March, 2012. The characteristics of the patients are shown in
Table I. The criteria for inclusion
were as follows: Pathologically proven diagnosis of gastric
adenocarcinoma; radical surgical resection (R0) of the tumor ≤4
weeks prior; D2 lymph node dissection; age ≥18 and ≤70 years; World
Health Organization performance status score ≤2; TNM stage T3, T4
or N+ (IB-III) according to the American Joint Committee on Cancer
(AJCC), 7th edition (16); normal
cardiac, hepatic and renal function; normal bone marrow function
(neutrophil count ≥1,500/µ1, blood platelet 100,000/µl, hemoglobin
level ≥10 g/dl); no malignant tumor at other sites; no intolerance
of CRT due to various systemic diseases; no residual tumor or
positive surgical margins after surgery; no TNM stage T1-2N0; no
distant metastasis (M1); and no D0/D1 lymph node dissection. The
present study was approved by the Ethics Committee of Anhui
Provincial Hospital (Hefei, China) and all participating patients
provided written informed consent.
 | Table I.Clinicopathological characteristics
of the 102 patients. |
Table I.
Clinicopathological characteristics
of the 102 patients.
|
Characteristics | Total number
(n=102) | F-CRT, n (%)
(n=45) | C-CRT, n (%)
(n=57) |
|---|
| Gender |
|
|
|
|
Male | 69 | 31 (68.9) | 38 (66.7) |
|
Female | 33 | 14 (31.1) | 19 (33.3) |
| Age, years |
|
|
|
|
≤55 | 56 | 24 (53.3) | 32 (56.1) |
|
>55 | 46 | 21 (46.7) | 25 (43.9) |
| Tumor site |
|
|
|
|
Esophagogastric junction | 22 | 9 (20.0) | 13 (22.8) |
| Gastric
body | 24 | 11 (24.4) | 13 (22.8) |
| Gastric
antrum | 56 | 25 (55.6) | 31 (54.4) |
| Pathological
type |
|
|
|
|
Canalicular and papillary
adenocarcinoma | 62 | 27 (60.0) | 35 (61.4) |
| Poorly
differentiated adenocarcinoma | 21 | 10 (22.2) | 11 (19.3) |
|
Others | 19 | 8 (17.8) | 11 (19.3) |
| Lymph node
metastasis |
|
|
|
|
Yes | 66 | 30 (66.7) | 36 (63.2) |
| No | 36 | 15 (33.3) | 19 (36.8) |
| TNM stage |
|
|
|
| IB | 11 | 5 (11.1) | 6 (10.5) |
| II | 38 | 17 (37.8) | 21 (36.8) |
|
III | 53 | 23 (51.1) | 30 (52.7) |
| Vascular cancer
embolus or neural invasion |
|
|
|
|
Positive | 25 | 11 (24.4) | 14 (24.6) |
|
Negative | 77 | 34 (75.6) | 43 (75.4) |
Radiotherapy
Radiotherapy was initiated within 4 weeks after
surgery. All the patients were required to fast for at least 4 h,
and were scanned after orally taking 20 ml compound diatrizoate
meglumine solution and 500–800 ml water to highlight the gastric
stump and part of the intestinal loop structure. The patients were
maintained at the supine position by a restraining device to
undergo computed tomography (CT) enhancement scanning, which
required a 5-mm stratum depth. The scanning range included the
section between the fourth thoracic vertebra and the fourth lumbar
vertebra, and the images were transferred to a treatment planning
system after restructuring as 2.5 mm per stratum. First, the
clinical target volume (CTV), which includes the preoperative tumor
bed, anastomotic stoma, duodenal stump and high-risk lymphatic
drainage area, was delineated on the restructured CT images by
combining contrast agent development, postoperative silver clips
and preoperative imaging data (17,18).
Planning target volume (PTV) included CTV taking into consideration
organ excursion, with the enlarged irradiation area caused by
repeatability error of the patient's position during positioning
and treatment, as well as taking into account a change of target
positions and target volumes to ensure the irradiation dose of CTV.
Accordingly, PTV usually included a 7-10-mm margin around CTV, with
a 10-mm craniocaudal margin. The organs at risk included the spinal
cord, liver, heart, kidney and small intestine.
The number of the beams and incidence angle were
adjusted according to the ray path and the association of the tumor
target volume with surrounding normal tissue, while the dose of
each beam was weighted with the limits of the organs at risk
calculated. The treatment plan was formulated through automatic
optimization by a computer reverse planning system. Isocenter
irradiation was conducted using 5 or 7 intensity-modulated
irradiation fields, which were coplanar and non-through. The
5-field IMRT beam orientation was usually at the following gantry
angle: 0, 72, 144, 216 and 288°; the 7-field IMRT beam orientation
was usually at the following gantry angle: 0, 51, 103, 154, 206,
257 and 309°. Based on the patient's target shape and the dose to
target volume and normal tissue, adjustments were made until the
plan requirements were met. The prescription dose of 95% PTV was 45
Gy in 25 fractions (1.8 Gy/fr). The doses to each organ at risk
were as follows: Maximum dose to the spinal cord ≤45 Gy; liver V30
<30%; mean dose to each kidney <18 Gy, V15 ≤50%; small
intestine V50 ≤10%; heart dose V40 ≤30%, V30 ≤40%; whole-lung dose
V20 ≤25%, V5 ≤60%.
Chemotherapy
For comparison, during concurrent CRT the patients
were separated into two groups: 45 patients received intravenous
infusion of 400 mg/m2/day 5-fluorouracil and 20
mg/m2/day tetrahydrofolic acid (THFA) 4 days before and
3 days after radiotherapy; and 57 patients received 825
mg/m2 oral capecitabine twice each day (morning and
evening) during radiotherapy, and four cycles of XELOX chemotherapy
(capecitabine 1,000 mg/m2 on days 1–14 + oxaliplatin 130
mg/m2 on day 1, repeated every 3 weeks) 4 weeks after
the end of radiotherapy.
Evaluation of toxicity
The Common Terminology Criteria for Adverse Events
v3.0 (19) of the National Cancer
Institute were used for the evaluation of toxicity.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (Armonk, NY, USA). The Chi-square test was used for the
comparison of rates and the Student's t-test was used for the
comparison of means. The survival rate was calculated by
non-parametric Kaplan-Meier test, while the log-rank test was used
to compare survival curve distribution between the two groups and
to determine whether the difference was statistically significant.
The multivariate Cox regression model was used to perform
multivariate analysis of prognosis. P<0.05 was considered to
indicate statistically significant differences.
Results
Patients
A total of 102 patients who met the aforementioned
criteria were enrolled in this study. The characteristics of these
patients are listed in Table I. There
were no significant differences in gender distribution, age, tumor
site, pathological type, TNM stage, or the extent of neurovascular
invasion between the F-CRT and C-CRT groups. Overall, 98 patients
completed the concurrent CRT; 4 patients only completed
radiotherapy but not chemotherapy, due to the toxic side effects,
including 3 patients from the F-CRT group who only completed the
first 4-day concurrent chemotherapy without the subsequent 3-day
chemotherapy, and 1 patient from the C-CRT group who received
concurrent capecitabine for only 2 weeks. A total of 6 patients did
not complete the subsequent 4-cycle XELOX chemotherapy, of whom 4
patients were from the F-CRT group (2 patients only received two
cycles of chemotherapy, 1 received only one cycle, and 1 patient
did not receive any subsequent chemotherapy) and 2 patients were
from the C-CRT group (1 received only two cycles of chemotherapy,
and 1 did not receive any subsequent chemotherapy).
Safety evaluation
The individual toxic side effects in the F-CRT and
C-CRT groups during the entire course of treatment are shown in
Table II. Overall, the incidence of
grade 3 hematological toxicities (leukopenia, anemia and
thrombocytopenia) was 23.5%; grade 3 gastrointestinal reactions
(nausea, vomiting and diarrhea) 13.7%; grade 1–2 hepatic and renal
function impairment 9.8%; and grade ≥3 hepatic and renal function
impairment 0%. The most common grade 1–2 hematological toxicity was
anemia (76.5%), and the most common grade 3 toxicity was leukopenia
(21.6%). Grade 1–2 gastrointestinal reactions included nausea
(71.6%), vomiting (34.3%) and diarrhea (26.5%), and grade 3
gastrointestinal reactions included nausea (10.8%), vomiting (3%)
and diarrhea (1%). During the entire course of treatment, the
incidence of grade 3 hematological toxicities (leukopenia, anemia
and thrombocytopenia) in the F-CRT and C-CRT groups was 28.9 and
19.3%, respectively, and the incidence of grade 3 gastrointestinal
reactions (nausea, vomiting and diarrhea) was 17.8 and 10.5%,
respectively. Grade 3 hematological toxicities and gastrointestinal
reactions were more common in the F-CRT group, but the difference
was not statistically significant. The incidence of grade 1–2
hepatic and renal function impairment in the F-CRT and C-CRT groups
was 11.1 and 8.8%, respectively, but the difference was again not
statistically significant. The incidence of grade 1–2 hand-foot
syndrome was 35.6 and 40.4%, respectively, with 1 patient in each
group experiencing grade 3 hand-foot syndrome, without
statistically significant differences between the two groups
(Table II).
 | Table II.Toxicities in the F-CRT and C-CRT
groups during the entire course of treatment. |
Table II.
Toxicities in the F-CRT and C-CRT
groups during the entire course of treatment.
|
| F-CRT (n=45) | C-CRT (n=57) |
|---|
|
|
|
|
|---|
| Toxicities | Grade 1–2 n
(%) | Grade 3–4 n
(%) | Grade 1–2 n
(%) | Grade 3–4 n
(%) |
|---|
| Nausea | 34 (75.6) | 6 (13.3) | 39 (68.4) | 5 (8.8) |
| Vomiting | 17 (37.8) | 2 (4.4) | 18 (31.2) | 1 (1.8) |
| Diarrhea | 12 (26.7) | 1 (2.2) | 15 (26.3) | 0 (0.0) |
| Leukopenia | 28 (62.2) | 11 (24.4) | 34 (59.6) | 10 (17.5) |
| Anemia | 35 (77.8) | 2 (4.4) | 43 (75.4) | 2 (3.6) |
|
Thrombocytopenia | 13 (28.9) | 1 (2.2) | 17 (29.8) | 0 (0.0) |
| HFS | 16 (35.6) | 1 (2.2) | 23 (40.4) | 1 (1.8) |
| ALT | 4 (8.9) | 0 (0.0) | 5 (8.8) | 0 (0.0) |
| GFR | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
As shown in Table
III, during the concurrent CRT, the incidence of grade 1–2
hematological toxicities in the F-CRT group was significantly
higher compared with that in the C-CRT group (73.3 and 50.9%,
respectively; P=0.021). The incidence of grade 1–2 gastrointestinal
reactions in the F-CRT group was also significantly higher compared
with that in the C-CRT group (77.8 and 57.9%, respectively;
P=0.034). However, during the concurrent CRT, no statistically
significant difference was observed in grade 3–4 hematological
toxicities and gastrointestinal reactions between the F-CRT and the
C-CRT groups.
 | Table III.Toxicities in the F-CRT and C-CRT
groups during the concurrent radiochemotherapy. |
Table III.
Toxicities in the F-CRT and C-CRT
groups during the concurrent radiochemotherapy.
|
| F-CRT (n=45) | C-CRT (n=57) |
|---|
|
|
|
|
|---|
| Toxicities | Grade 1–2 n
(%) | Grade 3–4 n
(%) | Grade 1–2 n
(%) | Grade 3–4 n
(%) |
|---|
|
Gastrointestinal | 35 (77.8) | 1 (2.2) | 33 (57.9) | 0 (0.0) |
| Hematological | 33 (73.3) | 1 (2.2) | 29 (50.9) | 1 (1.8) |
Survival analysis
The median follow-up time in the overall population
was 40 months (range, 18–61.2 months), the follow-up rate was 98%,
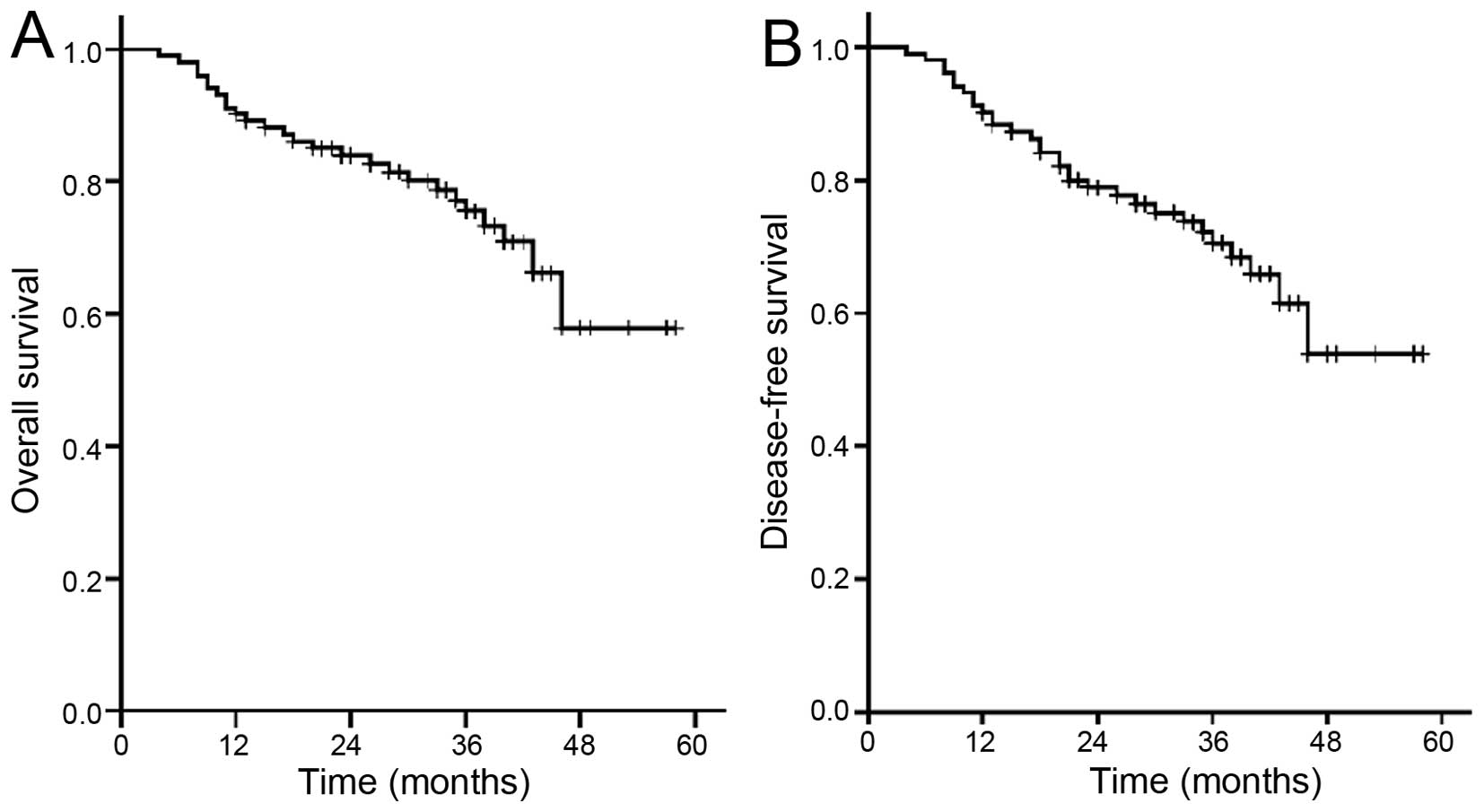
and loss to follow-up was considered as death. The 3-year survival
rate was 75.5% and the disease-free survival rate was 70.5%
(Fig. 1), while the survival rate was
84.3% in a local group.
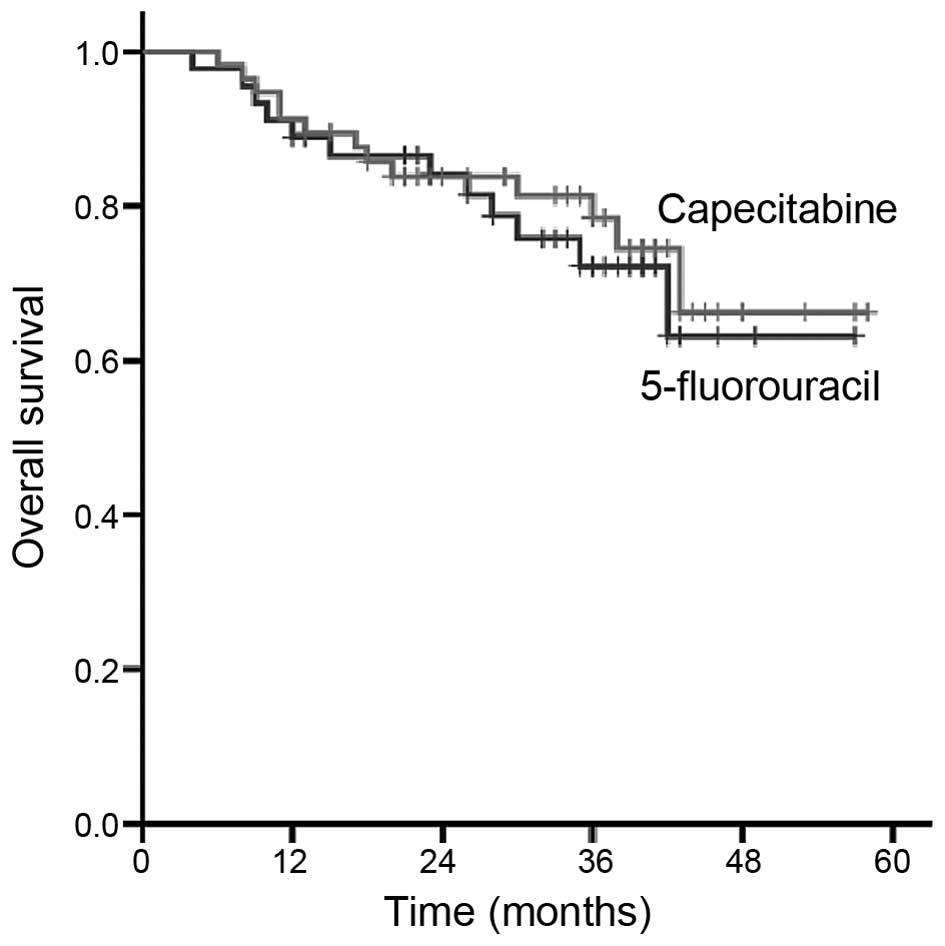
As shown in Fig. 2,
the total 3-year survival rate of the F-CRT and C-CRT groups was
72.2 and 78.5%, respectively (P>0.05), and the 3-year
disease-free survival rate was 67.7 and 72.8%, respectively
(P>0.05). No statistically significant differences were
observed.
Analysis of prognostic factors
Lymph node metastasis, TNM stage, vascular cancer
embolus and neural invasion are usually the factors that affect
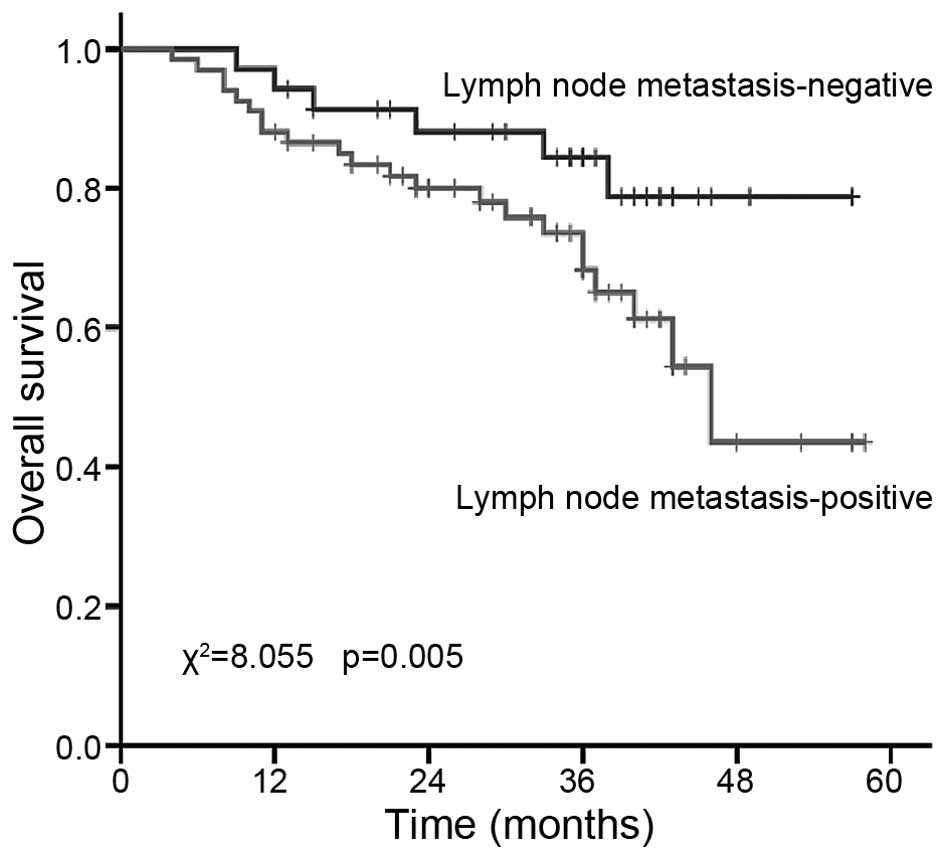
survival. In our study, as shown in Table IV, the 3-year survival rate of
patients with and without lymph node metastasis was 63.8 vs. 84.4%,
respectively (P=0.005; Fig. 3). The
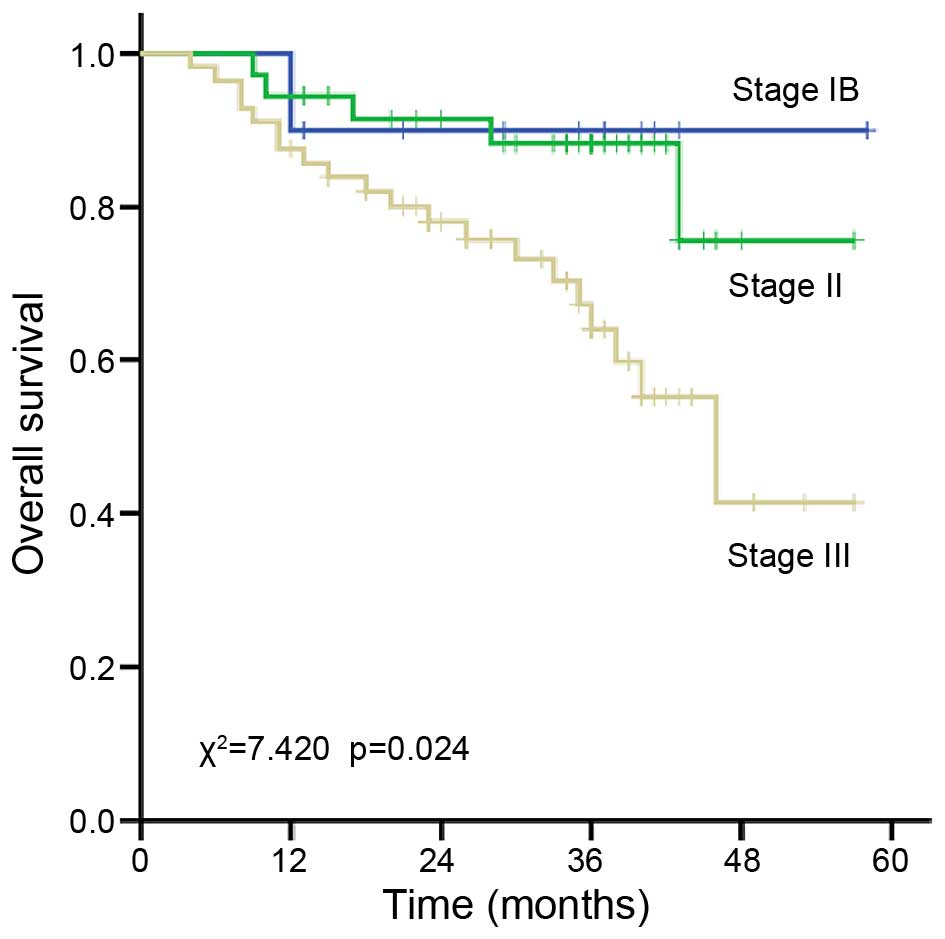
3-year survival rate of patients with stage IB, II and III disease
was 90.9, 86.8 and 64.2%, respectively (P=0.024; Fig. 4). The 3-year survival rate of patients
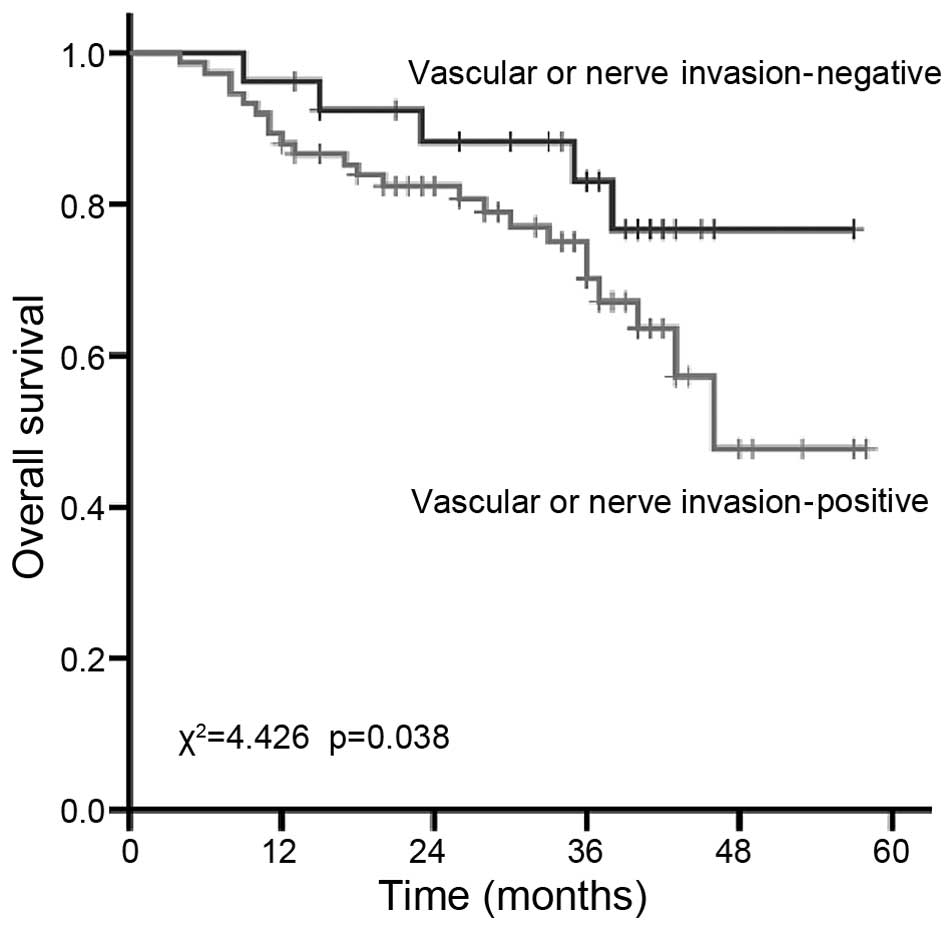
with positive and negative neurovascular invasion was 70.2 and
82.4%, respectively (P=0.038; Fig.
5). In accordance with previous studies (11,12), lymph
node metastasis, TNM stage and vascular cancer embolus or neural
invasion are statistically significant prognostic factors, while
factors such as gender, age, tumor site, pathological type and
different chemotherapies were not significantly associated with
prognosis.
 | Table IV.Prognostic univariate analysis in the
overall population. |
Table IV.
Prognostic univariate analysis in the
overall population.
| Variables | Number | 3-year survival
rate | χ2 | P-value |
|---|
| Gender |
|
|
|
|
|
Male | 69 | 76.8 | 2.613 | 0.116 |
|
Female | 33 | 72.7 |
|
|
| Age, years |
|
|
|
|
|
≤55 | 56 | 73.2 | 1.436 | 0.217 |
|
>55 | 46 | 78.3 |
|
|
| Tumor site |
|
|
|
|
|
Esophagogastric junction | 22 | 68.2 | 5.129 | 0.078 |
| Gastric
body | 24 | 75.0 |
|
|
| Gastric
antrum | 56 | 78.6 |
|
|
| Pathological
type |
|
|
|
|
|
Canalicular and papillary
adenocarcinoma | 62 | 79.0 | 5.682 | 0.062 |
| Poorly
differentiated | 21 | 71.4 |
|
|
|
adenocarcinoma | 19 | 68.4 |
|
|
|
Others |
|
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
Yes | 66 | 63.8 | 8.055 | 0.005 |
| No | 36 | 84.4 |
|
|
| TNM stage |
|
|
|
|
| IB | 11 | 90.9 | 7.420 | 0.024 |
| II | 38 | 86.8 |
|
|
|
III | 53 | 64.2 |
|
|
| Vascular cancer
embolus or neural invasion |
|
|
|
|
|
Positive | 25 | 70.2 | 4.426 | 0.038 |
|
Negative | 77 | 82.4 |
|
|
| Chemotherapy |
|
|
|
|
|
5-FU+CF | 45 | 72.2 | 3.204 | 0.085 |
|
Capecitabine | 57 | 78.5 |
|
|
To determine the independent prognostic factors,
multivariate Cox regression analysis was used. As shown in Table V, lymph node metastasis and TNM stage
were independent prognostic factors, while vascular and/or neural
invasion was not identified as an independent prognostic
factor.
 | Table V.Multivariate Cox regression analysis
of prognosis after surgery for gastric cancer. |
Table V.
Multivariate Cox regression analysis
of prognosis after surgery for gastric cancer.
| Variables | Regression
coefficient (B) | Standard error | Statistics (Wald)
value | P-value | 95% confidence
interval |
|---|
| TNM stage | 0.304 | 0.121 | 5.136 | 0.032 | 1.084–1.843 |
| Lymph node
metastasis | 0.362 | 0.146 | 6.523 | 0.010 | 1.097–1.965 |
| Vascular/nerve
invasion | 0.148 | 0.092 | 2.385 | 0.138 | 0.984–1.764 |
Discussion
The majority of previous studies have compared
adjuvant concurrent CRT after surgery with surgery alone in
patients with gastric carcinoma undergoing D0/D1 surgery (11,20,21), while
only a limited number of studies compared CRT after D2 radical
surgery with surgery alone (12). As
D2 resection (surgery with an enlarged range of lymph node
dissection) is common in Asia, particularly in Eastern Asia, this
study was focused on such patients in order to estimate the effects
of concurrent CRT following D2 resection in gastric carcinoma.
The clinical SWOG 9008/INT-0116 study (11) was a milestone study designed to
observe the effects of surgery and postoperative concurrent CRT in
patients with resectable gastric and gastroesophageal junction
carcinoma. The study included two groups, the R0 resection with
concurrent CRT group (281 patients) and the surgery alone group
(275 patients). All the patients had stage IB-IV (M0) gastric or
gastroesophageal junction carcinoma, of whom 68% had stage T3 or T4
lesions, and 85% had lymph node metastasis (11). In the concurrent CRT group, the local
recurrence rate was significantly reduced compared with that in the
surgery alone group (19 vs. 29%, respectively), the median survival
was significantly prolonged (36 vs. 27 months, respectively), the
3-year disease-free rate was significantly improved (48 vs. 31%,
respectively), the survival rate was significantly improved (50 vs.
41%, respectively; P=0.005) and the incidence of grade 3–4 adverse
reactions was 41 and 32%, respectively (11). Moreover, concurrent CRT after surgery
was shown to benefit patients with stage IB-IV (M0) gastric cancer
without increasing chronic toxicity in a follow-up study (20). The INT-0116 study established the use
of concurrent CRT as standard postoperative adjuvant treatment of
gastric carcinoma in the US and several European countries
(21). However, a major disadvantage
of that study was that only 10% of the selected patients received
D2 resection, while 36% of the patients received D1 resection, and
over half of the patients (54%) received <D0 resection.
Kim et al compared the outcome of gastric
carcinoma patients with or without CRT following D2 radical surgery
(12), of whom 554 patients had CRT
after D2 radical surgery, while 446 patients had D2 radical surgery
alone. A significant increase in the median survival time and
median relapse-free survival time were observed in the concurrent
CRT group compared with the surgery alone group. The 5-year
survival rate (57.1 vs. 51.0%, P=0.0198) and relapse-free rate
(54.5 vs. 4.9%, P=0.0161) of the concurrent CRT group were
significantly higher compared with those in the surgery alone
group, and the mortality risk was reduced by 20% (12). These data indicated that concurrent
CRT following D2 gastric cancer surgery may improve the survival
rate and reduce the recurrence rate.
In the ARTIST study (13), which aimed to compare the results of
the gastric cancer patients who had received concurrent CRT with
those of patients who had received chemotherapy alone, 228 patients
were randomized into the 6-cycle adjuvant chemotherapy XP group
(capecitabine plus cisplatin) and 230 patients were randomized to
receive 2-cycle concurrent XP chemotherapy, sequential concurrent
CRT (total dose of 45 Gy in 25 fractions + capecitabine), and
sequential 2-cycle XP chemotherapy. After a median follow-up of
53.2 months, no significant differences were found in 3-year
disease-free survival rate (78.2% for CRT vs. 74.2% for
chemotherapy; P=0.0862), but only marginal differences were found
in the 3-year disease-free survival rate in patients with positive
lymph nodes (77.5 vs. 72.3%, respectively; P=0.0365) (13).
In our study, the 3-year survival rate in patients
who received concurrent CRT following gastric cancer surgery was
75.5%, and the disease-free survival rate was 70.5%, while the
local control rate was 84.3%. However, a limitation of our study
was that we only investigated concurrent CRT after surgery, without
setting up control groups to compare with surgery alone and
postoperative chemotherapy alone. However, the 3-year disease-free
survival rate was higher compared with that reported in the
INT-0116 study, which may be explained by the fact that all our
patients had received D2 radical surgery, suggesting a better
prognosis with chemotherapy after D2 surgery rather than D0/D1
surgery in terms of disease-free survival. When compared to the
ARTIST study, our study demonstrated a lower 3-year disease-free
survival rate, possibly because all our patients were at advanced
TNM stage, which is also the cause for the outcome differences with
treatments between European patients and patients from Japan and
Korea.
Since the adjuvant concurrent CRT was limited by the
tolerance dose of major organs, such as the gastrointestinal tract,
liver and kidney, the conventional radiotherapy usually led to the
intolerable radiation reactions in the gastrointestinal tract,
liver and other organs at risk. Compared with conventional
radiotherapy and 3DCRT, IMRT had the advantage of three-dimensional
conformity of high doses to the target volume, increasing the total
dose delivered (14,15). Milano et al (14) also reported that the IMRT reduced the
liver V30 (63.6 vs. 18.9%, P=0.010) and right kidney V20 (20.9 vs.
11.6%, P=0.027) compared with 2/3-field 3DCRT. Boda-Heggemann et
al (22) observed that the V30 of
the left and right kidneys was 26.8 vs. 19.5%, respectively
(P=0.0015) when investigating 27 patients who received concurrent
CRT with 3DCRT. A study conducted by Stanford University (23) also indicated that IMRT led to a
reduction of the liver V30 from 28 to 16.1% compared with 3DCRT.
Moreover, IMRT also improved the postoperative gastric cancer
treatment. Badakhshi et al (24) investigated 25 cases of patients who
received concurrent CRT with IMRT after gastric cancer surgery, and
observed that the incidences of grade 3 nausea and diarrhea during
treatment were 4 and 8%, respectively, and the incidences of grade
3 decrease in hemoglobin level, leukocyte and platelet counts were
12, 25 and 4%, respectively.
Both the INT-0116 study and ARTIST trial used
front-back parallel opposed-field radiotherapy (11). In our study, we used 5/7-field IMRT,
with an incidence of grade 3 hematological toxicities during
treatment of 23.5%; grade 3 gastrointestinal reactions of 13.7%;
grade 1–2 liver and kidney function impairment of 9.8%; and grade 3
liver and kidney function impairment of 0%; these findings were
similar to the results reported by Badakhshi et al (24). In addition, we observed that grade 3
hematological toxicities and gastrointestinal reactions mostly
occurred due to the cumulative effect of subsequent adjuvant
chemotherapy, and the toxic effects were usually grade 1–2 during
concurrent CRT. Moreover, all the patients were able to tolerate
concurrent CRT after symptomatic treatment.
The concurrent chemotherapy used by the INT-0116
study was 5-fluorouracil + leucovorin, and the efficacy and safety
of oral capecitabine as concurrent CRT following gastric cancer
surgery had also been demonstrated (25–27). The
CLASSIC trial (28) demonstrated the
effectiveness and good tolerance of capecitabine plus oxaliplatin
as adjuvant therapy after gastric cancer surgery. To determine the
differences between 5-fluorouracil and capecitabine, we compared
the F-CRT and C-CRT groups following concurrent CRT, with 45
patients receiving concurrent chemotherapy for the first 4 and last
3 days of radiotherapy with 400 mg/m2/day fluorouracil
and 20 mg/m2/day THFA, and 57 patients receiving 825
mg/m2/day capecitabine twice daily (in the morning and
evening) during the radiotherapy. The 3-year overall survival rate
of the F-CRT and C-CRT groups was 72.2 and 78.5%, respectively
(P>0.05); and the 3-year disease-free survival rate was 67.7 and
72.8%, respectively (P>0.05); the differences were not
statistically significant. During the entire therapy course, the
grade 1–2 hematological toxicities and grade 1–2 gastrointestinal
reactions were similar between the F-CRT and C-CRT groups; the
incidence of grade 3 hematological toxicities was 28.9 and 19.3%,
respectively; the incidence of grade 3 gastrointestinal reactions
was 17.8 and 10.5%, respectively; there was no statistically
significant difference. However, the incidences of grade 1–2
hematological toxicities in the F-CRT and C-CRT groups were 73.3
vs. 50.9%, respectively (χ2=5.320; P=0.021) and the
incidences of grade 1–2 gastrointestinal reactions 77.8 vs. 57.9%,
respectively (χ2=4.474; P=0.034) during concurrent CRT.
Thus, concurrent chemotherapy with capecitabine reduced grade 1–2
hematological toxicities and grade 1–2 gastrointestinal reactions
in the C-CRT group, and was able to help the patients tolerate and
complete the radiotherapy.
As regards prognostic factors following gastric
cancer surgery, most scholars consider the status of lymph node
metastasis to be an important independent factor affecting the
prognosis of gastric cancer (29,30). Other
studies demonstrated that the prognosis of the patients was
associated with the absolute number of positive lymph nodes when
the lymph node sampling was sufficient (16 required by TNM, AJCC
7th edition); however, the metastatic lymph node ratio and log odds
of positive lymph nodes may be used to better assess prognosis when
lymph node sampling was inadequate (31,32). In
addition to the status of lymph node metastasis, the factors
affecting the prognosis of gastric cancer also included TNM stage,
depth of tumor infiltration, vascular invasion and nerve invasion
(33,34). Histological classification (34) and tumor size (35,36) may
also be used to predict the prognosis of patients with advanced
gastric carcinoma. We also evaluated the prognostic factors of
patients following gastric cancer surgery, with the single-factor
analysis showing that lymph node metastasis, TNM stage and
intravascular cancer emboli or perineural invasion were the factors
affecting patient survival, while the other factors, including
gender, age, tumor site, pathology and different chemotherapies,
were not significantly correlated with prognosis. The multivariate
Cox regression analysis indicated that lymph node metastasis and
TNM stage were independent prognostic factors for the patients
(Table V).
In conclusion, our study analyzed 102 cases of
gastric carcinoma patients receiving CRT following D2 surgery, with
similar overall survival rates observed compared with previous
reports, while the toxic side effects were reduced by using IMRT.
No significant difference in survival rates was observed between
oral capecitabine or intravenous infusion of fluorouracil. However,
concurrent chemotherapy with capecitabine was associated with fewer
hematological toxicities and gastrointestinal reactions compared
with intravenous infusion of fluorouracil; thus, the patients were
able to better tolerate and complete the radiotherapy.
Acknowledgements
The authors would like to thank the colleagues at
the Department of Radiation Oncology of Anhui Provincial Hospital
for the helpful discussions.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Landry J, Tepper JE, Wood WC, Moulton EO,
Koerner F and Sullinger J: Patterns of failure following curative
resection of gastric carcinoma. Int J Radiat Oncol Biol Phys.
19:1357–1362. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim DH, Kim DY, Kang MK, Kim YI, Kang WK,
Park CK, Kim S, Noh JH, Joh JW, Choi SH, et al: Patterns of failure
in gastric carcinoma after D2 gastrectomy and chemoradiotherapy: A
radiation oncologist's view. Br J Cancer. 91:11–17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wisbeck WM, Becher EM and Russell AH:
Adenocarcinoma of the stomach: Autopsy observations with
therapeutic implications for the radiation oncologist. Radiother
Oncol. 7:13–18. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gunderson LL: Gastric carcer-patterns of
relapse after surgical resection. Semin Radiat Oncol. 12:150–161.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hundahl SA, Philips JL and Menck HR: The
National Cancer Data Base Report on poor survival of U.S. gastric
carcinoma patients treated with gastrectomy: Fifth edition American
Joint Committee on Cancer Staging, proximal disease and the
‘different disease’ hypothesis. Cancer. 88:921–932. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kulig J, Kolodziejczyk P, Sierzega M,
Bobrzynski L, Jedrys J, Popiela T, Dadan J, Drews M, Jeziorski A,
Krawczyk M, et al: Adjuvant chemotherapy with etoposide, adriamycin
and cisplatin compared with surgery alone in the treatment of
gastric cancer: A phase III randomized, multicenter, clinical
trial. Oncology. 78:54–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Costanzo F, Gasperoni S, Manzione L,
Bisagni G, Labianca R, Bravi S, Cortesi E, Carlini P, Bracci R,
Tomao S, et al: Adjuvant chemotherapy in completely resected
gastric cancer: A randomized phase III trial conducted by GOIRC. J
Natl Cancer Inst. 100:388–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Macdonald JS, Smalley SR, Benedetti J,
Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA,
Gunderson LL, Jessup JM and Martenson JA: Chemoradiotherapy after
surgery compared with surgery alone for adenocarcinoma of the
stomach or gastroesophageal junction. N Engl J Med. 345:725–730.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim S, Lim DH, Lee J, Kang WK, MacDonald
JS, Park CH, Park SH, Lee SH, Kim K, Park JO, et al: An
observational study suggesting clinical benefit for adjuvant
postoperative chemoradiation in a population of over 500 cases
after gastric resection with D2 nodal dissection for adenocarcinoma
of the stomach. Int J Radiat Oncol Biol Phys. 63:1279–1285. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee J, Limdo H, Kim S, Park SH, Park JO,
Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, et al: Phase III trial
comparing capecitabine plus cisplatin vs. capecitabine plus
cisplatin with concurrent capecitabine radiotherapy in completely
resected gastric cancer with D2 lymph node dissection: The ARTIST
trial. J Clin Oncol. 30:268–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Milano MT, Garofalo MC, Chmura SJ, Farrey
K, Rash C, Heimann R and Jani AB: Intensity-modulated radiation
therapy in the treatment of gastric cancer: Early clinical outcome
and dosimetric comparison with conventional techniques. Br J
Radiol. 79:497–503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ringash J, Perkins G, Brierley J, Lockwood
G, Islam M, Catton P, Cummings B, Kim J, Wong R and Dawson L: IMRT
for adjuvant radiation in gastric cancer: A preferred plan? Int J
Radiat Oncol Biol Phys. 63:732–738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer (UICC) TNM Classification of
Malignant Tumours (7th). Wiley-Liss. New York: 73–76. 2010.
|
|
17
|
Smalley SR, Gunderson L, Tepper J,
Martenson JA Jr, Minsky B, Willett C and Rich T: Gastric surgical
adjuvant radiotherapy consensus report: Rationale and treatment
implementation. Int J Radiat Oncol Biol Phys. 52:283–293. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tepper JE and Gunderson LL: Radiation
treatment parameters in the adjuvant postoperative therapy of
gastric cancer. Semin Radiat Oncol. 12:187–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oblak I, Velenik V, Anderluh F and Strojan
P: Results of adjuvant radiochemotherapy for gastric adenocarcinoma
in Slovenia. EurJ Surg Oncol. 33:982–987. 2007. View Article : Google Scholar
|
|
21
|
Macdonald JS, Benedetti J, Smalley S, et
al: Chemoradiation of resected gastric cancer: A 10-year follow-up
of the phase III trial INT0116 (SWOG 9008). J Clin Oncol.
27:4515–4520. 2009.PubMed/NCBI
|
|
22
|
Boda-Heggemann J, Hofheinz RD, Weiss C,
Mennemeyer P, Mai SK, Hermes P, Wertz H, Post S, Massner B, Hieber
U, et al: Combined adjuvant radiochemotherapy with IMRT/XELOX
improves outcome with low renal toxicity in gastric cancer. Int J
Radiat Oncol Biol Phys. 75:1187–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minn AY, Hsu A, La T, Kunz P, Fisher GA,
Ford JM, Norton JA, Visser B, Goodman KA, Koong AC and Chang DT:
Comparison of intensity-modulated radiotherapy and 3-dimensional
conformal radiotherapy as adjuvant therapy for gastric cancer.
Cancer. 116:3943–3952. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Badakhshi H, Gruen A, Graf R, Boehmer D
and Budach V: Image-guided intensity-modulated radiotherapy for
patients with locally advanced gastric cancer: A clinical
feasibility study. Gastric Cancer. 17:537–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaishampayan UN, Ben-Josef E, Philip PA,
Vaitkevicius VK, Du W, Levin KJ and Shields AF: A
single-institution experience with concurrent capecitabine and
radiation therapy in gastrointestinal malignancies. Int J Radiat
Oncol Biol Phys. 53:675–679. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osti MF, Agolli L, Bracci S, Monaco F,
Tubin S, Minniti G, De Sanctis V and Enrici RM: Adjuvant
chemoradiation with 5-fluorouracil or capecitabine in patients with
gastric cancer after D2 nodal dissection. Anticancer Res.
32:1397–1402. 2012.PubMed/NCBI
|
|
27
|
Hofheinz RD, Wenz F, Lukan N, Mai S, Kripp
M, Staiger W, Schwarzbach M, Willeke F, Möhler M, Post S and
Hochhaus A: Oxaliplatin and capecitabine-based chemoradiotherapy
for gastric cancer-an extended phase I MARGIT and AIO trial. Int J
Radiat Oncol Biol Phys. 73:142–147. 2012. View Article : Google Scholar
|
|
28
|
Bang YJ, Kim YW, Yang HK, Chung HC, Park
YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al: Adjuvant
capecitabine and oxaliplatin for gastric cancer after D2
gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled
trial. Lancet. 379:315–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim DY, Seo KW, Joo JK, Park YK, Ryu SY,
Kim HR, Kim YJ and Kim SK: Prognostic factors in patients with
node-negative gastric carcinoma: A comparison with node-positive
gastric carcinoma. World J Gastroenterol. 12:1182–1186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saito H, Kuroda H, Matsunaga T, Fukuda K,
Tatebe S, Tsujitani S and Ikeguchi M: Prognostic indicators in
node-negative advanced gastric cancer patients. J Surg Oncol.
101:622–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lemmens VE, Dassen AE, van der Wurff AA,
Coebergh JW and Bosscha K: Lymph node examination among patients
with gastric cancer: Variation between departments of pathology and
prognostic impact of lymph node ratio. Eur J Surg Oncol.
37:488–496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim CY and Yang DH: Adjustment of N stages
of gastric cancer by the ratio between the metastatic and examined
lymph nodes. Ann Surg Oncol. 16:1868–1874. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deng J, Liang H, Sun D, Zhang R, Zhan H
and Wang X: Prognosis of gastric cancer patients with node-negative
metastasis following curative resection: Outcomes of the survival
and recurrence. Can J Gastroenterol. 22:835–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baiocchi GL, Tiberio GA, Minicozzi AM,
Morgagni P, Marrelli D, Bruno L, Rosa F, Marchet A, Coniglio A,
Saragoni L, et al: A multicentric Western analysis of prognostic
factors in advanced, node-negative gastric cancer patients. Ann
Surg. 252:70–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du C, Zhou Y, Huang K, Zhao G, Fu H and
Shi Y: Defining a high-risk subgroup of pathological T2N0 gastric
cancer by prognostic risk stratification for adjuvant therapy. J
Gastrointest Surg. 15:2153–2158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiu MZ, Wang ZQ, Luo HY, Zhang DS, Zhou
ZW, Li YH, Jiang WQ and Xu RH: Prognostic analysis in node-negative
gastric cancer patients in China. Tumor Biol. 32:489–492. 2011.
View Article : Google Scholar
|