Introduction
Bladder carcinoma is the most common malignancy of
the urinary tract and the ninth most common malignancy worldwide
(1). In Europe it represents the
fifth most common cancer type affecting predominantly men
>65-years-old, usually smokers (2). Urothelial transitional cell carcinoma
(TCC) accounts for ~90% of all bladder cancer types.
In locally advanced or metastatic disease,
platinum-based combination chemotherapy regimens are the standard
of care as the first-line treatment (3). The combination of methotrexate,
vinblastine, doxorubicin and cisplatin (MVAC) was the first regimen
providing disease control and overall survival (OS) benefit
(4), however, it is associated with
several toxic effects, particularly when administered in a
high-dose-intensity schema (5). A
phase III trial demonstrated that compared with MVAC, the
combination of gemcitabine and cisplatin (GC) resulted in similar
objective response (49 vs. 36%) and similar OS (14 vs. 15 months)
(6) with a more favorable toxicity
profile (7). The triple combination
of paclitaxel and GC (PGC) is another option for patients with
metastatic urothelial carcinoma. Compared with GC, PGC is
associated with a significant improvement in the OS among patients
with primary bladder cancer (median, 16 vs. 12 months; hazard
ratio, 0.80; 95% confidence intervals, 0.66–0.97), however, with
increased incidence of serious grade III and IV toxicities
(8). For patients with impaired renal
function, carboplatin-based regimens can be used as the first-line
for metastatic TCC, however, its efficacy remains to be evaluated
with larger phase III trials (9).
Non-platinum regimens, particularly those combining gemcitabine
with either paclitaxel or docetaxel have also shown promising
results in several phase II studies (10,11).
Unfortunately, the majority of the patients with
advanced or metastatic bladder cancer fail first-line chemotherapy
in <1 year. A large proportion of these patients are not fit for
second-line chemotherapy due to poor performance status, impaired
renal function, advanced age and comorbidities. For those patients
who are able to receive further treatment, no second-line regimen
has been established. Chemotherapeutic agents, including vinflunine
(12), pemetrexed (13), paclitaxel (14), docetaxel (15), gemcitabine (16) and ifosfamide (17), are used either as single agent
regimens offering a response rate of a maximum of 20% or in
combination providing improved response rates without necessarily
improved OS, or with substantial cost in terms of toxicity
(18–23).
Single agent paclitaxel is active in urothelial
cancer. A phase II trial demonstrated a response rate of 42% in
chemotherapy naïve patients with advanced TCC (24). In first-line treatment, the response
rates ranged between 35 and 65%, when combined with platinum agents
(25). The experience with single
agent paclitaxel following the failure of a platinum based regimen
is limited and based upon small retrospective, or phase II
studies.
The present study aimed to assess the response and
toxicity rates of single agent paclitaxel in patients with
metastatic urothelial cancer having progressed following a
first-line cisplatin-based chemotherapy.
Patients and methods
The present study retrospectively evaluated 42
patients with metastatic urothelial bladder carcinoma treated with
first-line cisplatin-based combination regimens and second-line
paclitaxel monotherapy between January 2004 and January 2014 at the
Jules Bordet Institute (Brussels, Belgium). The present study was
approved by the Jules Bordet Institute Ethics Review Committee on
the 18th December 2014. The histological diagnosis and staging of
metastatic urothelial bladder carcinoma were based on the World
Health Organization Classification and the TNM staging system,
respectively. The eligibility criteria were histologically
confirmed urothelial bladder cancer and metastatic stage treated by
first-line cisplatin-based combination regimens and second-line
single agent paclitaxel chemotherapy. Prior to initiating
chemotherapy, each patient underwent physical examination, blood
examinations, chest radiography, and thorax, abdomen and pelvis
computed tomography. Brain and bone imaging were performed in the
case of complaints. Pooled prospectively collected data were
retrospectively analyzed. The optimal clinical response and maximum
tumor shrinkage, according to the Response Evaluation Criteria In
Solid Tumors (version 1.1), were considered as the tumor response.
Complete response (CR) was defined as the disappearance of all
target lesions, whereas partial response (PR) was a decrease in the
sum of the target lesion diameters by at least 30% compared with
the baseline. A progressive disease (PD) was considered if an
increase of at least 20% occurred in the sum of the target lesion
diameters compared with the smallest sum occurring during the
study, whereas stable disease (SD) was an insufficient shrinkage or
expansion to qualify as PR or PD. Progression free survival (PFS)
was calculated from the treatment initiation of the second-line
therapy until PD. The OS was recorded from the paclitaxel treatment
initiation until mortality, or was censored on the date of the
final follow-up. Kaplan-Meier survival curves were created and
compared using the log-rank test. All categorical variables were
analyzed using Fisher's exact test. The Cox proportional hazards
model, with stepwise regression, was applied to determine the
prognostic factors for PFS at second-line treatment and OS
following the start of second-line therapy, and to estimate the
hazard ratios and 95% confidence intervals. P<0.05 was
considered to indicate a statistically significant difference for
both one-sided and two-sided tests. All statistical analyses were
performed using SAS 9.4 software (University of Massachussets,
Amherst, MA, USA).
Results
Patient characteristics
The patient characteristics are shown in Table I. A total of 42 patients with
metastatic urothelial bladder cancer (15 women and 27 men)
received, in the period of the present study at the Jules Bordet
Institute, platinum-based combination regimen as a first-line
therapy and paclitaxel single agent treatment at progression, and
were included in the present analysis. The median age at diagnosis
was 61-years-old. Two thirds of the cohort presented with lung
metastases, 43% with other visceral involvements, notably pleural
and peritoneal metastases, 29% with bone metastases, whereas liver
and central nervous system involvement were more rare (Table I). All patients were treated with
cisplatin-based regimens: 27 patients as a first-line chemotherapy
regimen for metastatic disease, 12 patients in the post-operative
setting and 3 patients in the pre-operative setting. A median
progression duration of 5 months following the initial diagnosis
was noted during which a median number of 4.6 cycles of
cisplatin-based chemotherapy was administered on a 3 weekly basis.
No CRs were observed. Following three cycles of chemotherapy,
almost half of the patients achieved SD and only 20% exhibited PR.
In total, 62% of the patients progressed and stopped the treatment,
whereas in 16.7% of patients, the treatment was discontinued due to
toxicity, predominantly cisplatin-associated renal impairment.
Following the failure of the standard approach by cisplatin-based
systemic chemotherapy, all patients received treatment with
paclitaxel. At the initiation of this treatment, two thirds of the
patients presented with lymph node involvement, >50% with lung
metastases, 42% with bone metastases and >50% with other
metastatic sites, notably pleural and peritoneal involvement,
whereas only 1 patient exhibited metastatic cerebral lesions (Data
not shown). From the biological point of view, it was noted that
the median neutrophil to lymphocyte ratio at the initiation of
paclitaxel was 7.8, almost double in comparison with the identical
ratio at the initiation of cisplatin-based chemotherapy.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | No. of patients |
|---|
| Gender |
|
|
Female | 15 |
| Male | 27 |
| Median age (range),
years | 61 (51–78) |
| Treatment for
cisplatin-based regimen |
|
|
Pre-operative | 3 |
|
Post-operative | 12 |
|
Metastatic | 27 |
| Metastatic sites at
the initiation of second-line therapy |
|
| Lymph
node | 31 |
| Lung | 24 |
|
Liver | 13 |
| CNS | 1 |
| Bone | 23 |
| Biological parameters
at the initiation of second-line therapy |
|
|
Hemoglobin (gr/dl) | 10.7 |
| Platelets
(/mm3) | 311682 |
|
Neutrophil/lymphocyte
ratio | 7.814 |
| Albumin
(gr/l) | 3.594 |
| Lactate
dehydrogenase (IU/l) | 537 |
|
Creatinine (mg/dl) | 1.35 |
| Alkaline
phosphatase (ALP) IU/l | 277 |
|
C-reactive protein
(mg/dl) | 39.27 |
Treatment efficacy of second-line
single agent paclitaxel following first-line cisplatin-based
combination regimens
This regimen consisted of weekly intravenous
administration of paclitaxel (80 mg/m2) for 3
consecutive weeks, followed by 1 week without treatment. The median
duration of this regimen was 3 months. The median number of
paclitaxel administrations was seven, which corresponded to the
administration of 2.42 cycles. During the observation period, no
patients exhibited CR, while 4, 15 and 21 patients met the criteria
for PR, SD and PD, respectively (Table
II). Therefore, the overall response rate and disease control
rate were 9.5 and 45.2%, respectively. It was noted that 26.2% of
patients exhibited no favorable outcome and presented with
progressive disease following the first cycle of paclitaxel. The
median PFS of second-line chemotherapy was 3 months and the median
OS following the start of second-line therapy was 6.4 months
(Table II). The PFS following
second-line therapy was shorter compared with that following
first-line therapy (log rank, P<0.05). The OS of patients since
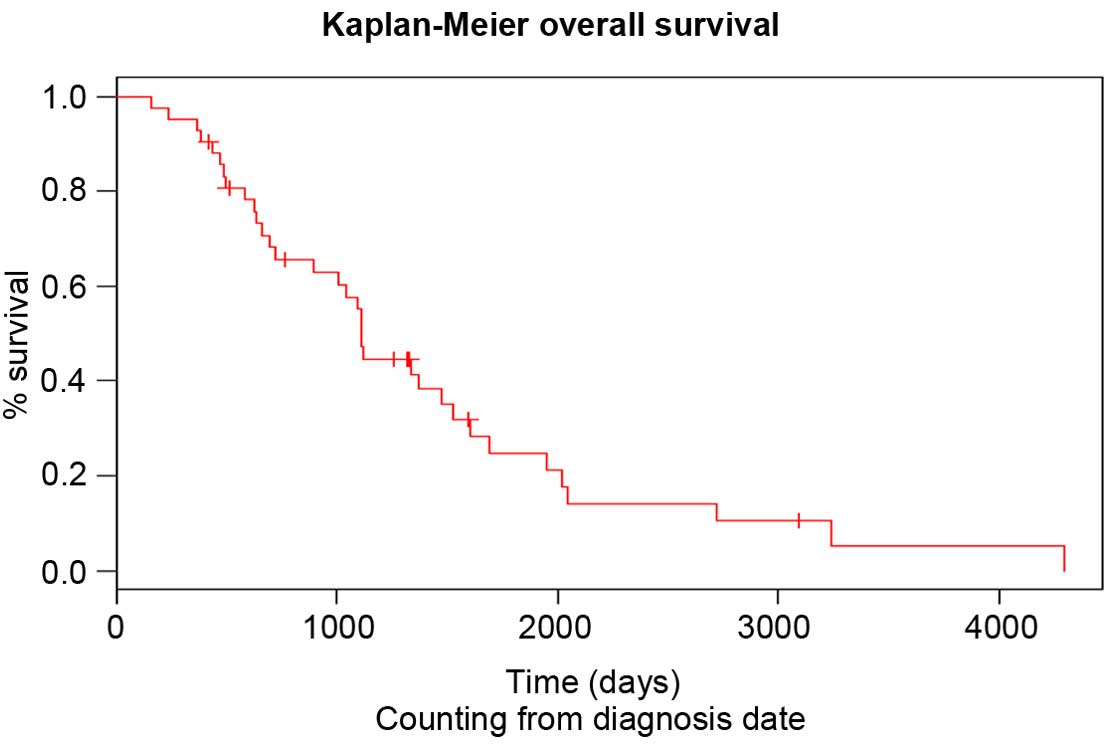
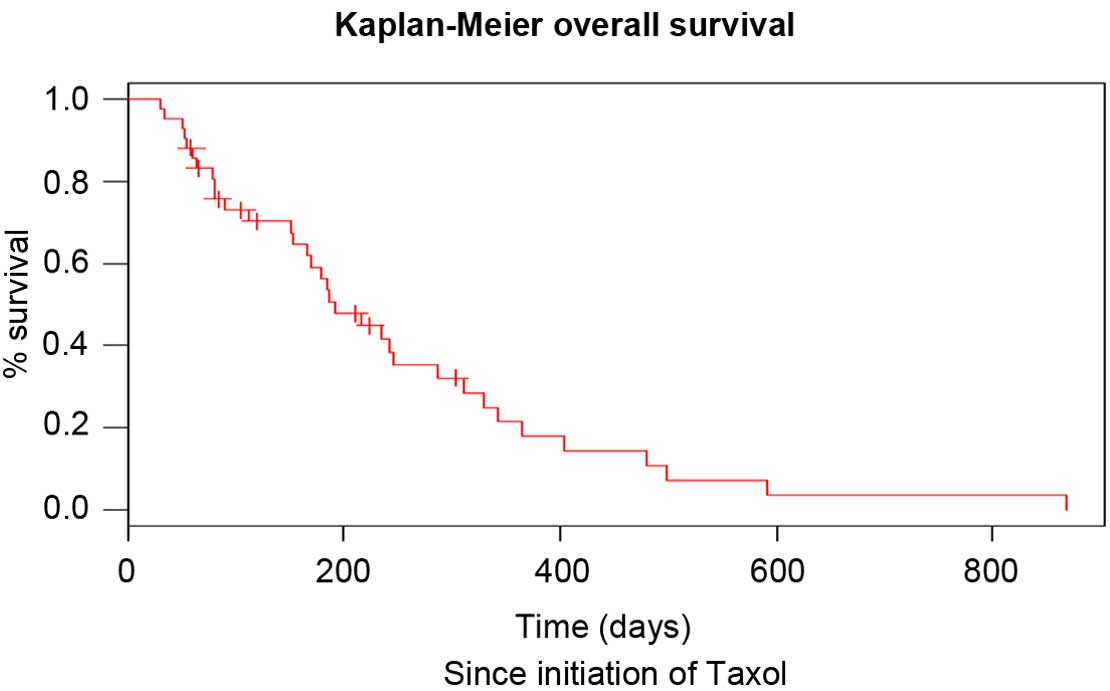
diagnosis and since initiation of paclitaxel treatment is shown in
Figs. 1 and 2, respectively. The neutrophil to lymphocyte
(N/l) ratio at diagnosis was not statistically correlated with the
OS of the patients (P=0.96). At the initiation of paclitaxel, the
cut-off value for this ratio was 4.62 and was also not prognostic
for the OS (P=0.34). In addition, no statistically significant
difference was observed between male and female patients
(P=0.722).
 | Table II.Response to first-line platinum based
regimens and second-line paclitaxel chemotherapy. |
Table II.
Response to first-line platinum based
regimens and second-line paclitaxel chemotherapy.
| Response | No. patients (%)
first-line cisplatin | No. of patients (%)
second line paclitaxel |
|---|
| Complete
response | 0 (0) | 0 (0) |
| Partial response | 9 (21.4) | 4 (9.5) |
| Stable disease | 20 (47.6) | 15 (35.7) |
| Progressive
disease | 11 (26.2) | 21 (50.0) |
| Not available | 2 (4.8) | 2 (4.8) |
| Response rate
(%) | 21.4 | 7.1 |
| Disease control
rate | 69.1 | 45.2 |
| Median time to
progression | 5 months | 3 months |
| Discontinuation due
to progression | 26 (61.9) | 33 (78.5) |
| Discontinuation due
to toxicities | 7 (16.7) | 1 (2.4) |
Treatment toxicity of second-line
single agent paclitaxel following first-line cisplatin-based
combination regimens
Weekly paclitaxel was well-tolerated. The only grade
III/IV treatment-associated toxicities encountered were anemia and
fatigue for <10% of the population (Table III). Pulmonary embolism was detected
in 19% of patients and was associated with the advanced stage of
the disease compared with the treatment itself. Pain was present in
38% of patients. It was also associated with bone metastases and
controlled by radiotherapy and oral analgesic treatment.
 | Table III.Grade III/IV toxicities for patients
with metastatic urothelial cancer treated with weekly
paclitaxel. |
Table III.
Grade III/IV toxicities for patients
with metastatic urothelial cancer treated with weekly
paclitaxel.
| Grade III/IV
toxicity | No. of patients
(%) |
|---|
| Anemia | 3 (7) |
| Fatigue | 1 (2) |
| Pulmonary
embolism | 8 (19) |
| Pain due to bone
metastases requiring RTa | 16 (38) |
Discussion
Recurrence following first-line therapy is
associated with a very poor prognosis. In this stage, management
remains controversial due to the absence of randomized trials
comparing second-line therapy to the optimum supportive care.
Clearly, efficient well-tolerated agents suitable for palliative
therapy are required. Multiple single agents have been investigated
in small phase II trials, however, response rates were grim and no
second-line therapy has been clearly established. Several studies
have suggested that single agent paclitaxel may be active in
urothelial cancer and examined its use in the second-line setting
(Table IV). The first data published came from small phase II
studies evaluating the response to a 3-weekly schedule of single
agent paclitaxel in patients relapsing following the first-line
therapy. The authors reported low efficacy with only one patient
responding. Severe toxicity was non-negligible and two early
mortalities occurred (26).
Afterwards, it was demonstrated that weekly paclitaxel shows
improved tolerance and is less toxic compared with the standard
3-weekly schedule, and therefore may provide a good second-line
palliative option (27,28). It is noteworthy to mention that weekly
administration schedules of paclitaxel are also proven to be
effective and well-tolerated in breast cancer, ovarian cancer, lung
cancer and other solid tumor types (29,30). The
first study, which assessed the efficacy and toxicity of this
schedule in patients with advanced urothelial cancer progressing
following first-line therapy revealed a modest overall response
rate, however a good safety profile (14). Results from a French multicenter,
Groupe d'Etude des Tumeurs Uro-Génitales, phase II trial confirmed
the limited objective overall response rate (31). However, the authors demonstrated
disease control and clinical benefits in 47 and 24% of cases,
respectively. In the present study, the efficacy of second-line
paclitaxel as a single agent following first-line cisplatin-based
combination regimens was also demonstrated. The disease control
rate, median time to progression and median time to mortality were
comparable to the previous two studies (14,31). The
limited overall response rate observed with paclitaxel may be
partially explained by the development of resistance involving a
multidrug resistance phenotype (32).
The treatment was also well-tolerated with <10% of grade III/IV
treatment-associated toxicities and no mortality-associated
toxicities were reported. The most common severe toxicities were
fatigue and anemia.
The importance of the N/l ratio as a prognostic
factor for survival was previously elaborated in the medical
literature. Cho et al (33)
demonstrated that patients with ovarian cancer presenting a
relative lymphopenia at diagnosis, therefore a higher N/l ratio,
exhibited worse disease outcome, probably due to a poorer
lymphocyte-mediated immune response to malignancy (33). The identical observation was reported
for several other malignancies, including gastric cancer (34), hepatic cancer (35) and non-small cell lung cancer (36). Gondo et al (37) were the first to report that the
pre-treatment N/l ratio is an independent prognostic factor for the
survival of patients with bladder cancer, treated with radical
cystectomy (37). To the best of our
knowledge, no study has demonstrated the predictive value of this
ratio at the initiation of paclitaxel for patients with metastatic
urothelial cancer. In the present study, this ratio was not
statistically correlated with the OS of these patients.
In conclusion, weekly paclitaxel treatment is a
well-tolerated regimen for patients with advanced urothelial cancer
who fail first-line cisplatin-based chemotherapy. Despite the fact
that it offers a relatively low objective response rate, weekly
paclitaxel is likely to provide non-negligible disease control and
therefore should be considered a legitimate option as a second-line
chemotherapeutic regimen for frail patients with advanced disease.
For fit patients, clinical trials may be considered as the optimal
option of second-line treatment for metastatic disease. If the
above mentioned results are confirmed in a prospective randomized
trial, weekly paclitaxel treatment (80 mg/m2) may be
considered a legitimate treatment option, particularly for frail
patients with advanced or metastatic urothelial cancer who failed
platinum-based chemotherapy.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pelucchi C, Bosetti C, Negri E, Malvezzi M
and La Vecchia C: Mechanisms of disease: The epidemiology of
bladder cancer. Nat Clin Pract Urol. 3:327–340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oosterlinck W, Lobel B, Jakse G, Malmström
PU, Stöckle M and Sternberg C: Guidelines on bladder cancer. Eur
Urol. 41:105–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loehrer PJ Sr, Einhorn LH, Elson PJ,
Crawford ED, Kuebler P, Tannock I, Raghavan D, Stuart-Harris R,
Sarosdy MF, Lowe BA, et al: A randomized comparison of cisplatin
alone or in combination with methotrexate, vinblastine and
doxorubicin in patients with metastatic urothelial carcinoma: A
cooperative group study. J Clin Oncol. 10:1066–1073.
1992.PubMed/NCBI
|
|
5
|
Sternberg CN, de Mulder PH, Schornagel JH,
Théodore C, Fossa SD, van Oosterom AT, Witjes F, Spina M, van
Groeningen CJ, de Balincourt C, et al: Randomized phase III trial
of high-dose-intensity methotrexate, vinblastine, doxorubicin and
cisplatin (MVAC) chemotherapy and recombinant human granulocyte
colony-stimulating factor versus classic MVAC in advanced
urothelial tract tumors: European organization for research and
treatment of cancer protocol no. 30924. J Clin Oncol. 19:2638–2646.
2001.PubMed/NCBI
|
|
6
|
Von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077.
2000.PubMed/NCBI
|
|
7
|
Von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bellmunt J, von der Maase H, Mead GM,
Skoneczna I, De Santis M, Daugaard G, Boehle A, Chevreau C,
Paz-Ares L, Laufman LR, et al: Randomized phase III study comparing
paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in
patients with locally advanced or metastatic urothelial cancer
without prior systemic therapy: EORTC intergroup study 30987. J
Clin Oncol. 30:1107–1113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dogliotti L, Cartenì G, Siena S, Bertetto
O, Martoni A, Bono A, Amadori D, Onat H and Marini L: Gemcitabine
plus cisplatin versus gemcitabine plus carboplatin as first-line
chemotherapy in advanced transitional cell carcinoma of the
urothelium: Results of a randomized phase 2 trial. Eur Urol.
52:134–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meluch AA, Greco FA, Burris HA III,
O'Rourke T, Ortega G, Steis RG, Morrissey LH, Johnson V and
Hainsworth JD: Paclitaxel and gemcitabine chemotherapy for advanced
transitional-cell carcinoma of the urothelial tract: A phase II
trial of the Minnie pearl cancer research network. J Clin Oncol.
19:3018–3024. 2001.PubMed/NCBI
|
|
11
|
Gitlitz BJ, Baker C, Chapman Y, Allen HJ,
Bosserman LD, Patel R, Sanchez JD, Shapiro RM and Figlin RA: A
phase II study of gemcitabine and docetaxel therapy in patients
with advanced urothelial carcinoma. Cancer. 98:1863–1869. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bellmunt J, Théodore C, Demkov T, Komyakov
B, Sengelov L, Daugaard G, Caty A, Carles J, Jagiello-Gruszfeld A,
Karyakin O, et al: Phase III trial of vinflunine plus best
supportive care compared with best supportive care alone after a
platinum-containing regimen in patients with advanced transitional
cell carcinoma of the urothelial tract. J Clin Oncol. 27:4454–4461.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sweeney CJ, Roth BJ, Kabbinavar FF, Vaughn
DJ, Arning M, Curiel RE, Obasaju CK, Wang Y, Nicol SJ and Kaufman
DS: Phase II study of pemetrexed for second-line treatment of
transitional cell cancer of the urothelium. J Clin Oncol.
24:3451–3457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vaughn DJ, Broome CM, Hussain M, Gutheil
JC and Markowitz AB: Phase II trial of weekly paclitaxel in
patients with previously treated advanced urothelial cancer. J Clin
Oncol. 20:937–940. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCaffrey JA, Hilton S, Mazumdar M, Sadan
S, Kelly WK, Scher HI and Bajorin DF: Phase II trial of docetaxel
in patients with advanced or metastatic transitional-cell
carcinoma. J Clin Oncol. 15:1853–1857. 1997.PubMed/NCBI
|
|
16
|
Lorusso V, Pollera CF, Antimi M, Luporini
G, Gridelli C, Frassineti GL, Oliva C, Pacini M and De Lena M: A
phase II study of gemcitabine in patients with transitional cell
carcinoma of the urinary tract previously treated with platinum.
Italian co-operative group on bladder cancer. Eur J Cancer.
34:1208–1212. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pronzato P, Vigani A, Pensa F, Vanoli M,
Tani F and Vaira F: Second line chemotherapy with ifosfamide as
outpatient treatment for advanced bladder cancer. Am J Clin Oncol.
20:519–521. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bellmunt J, Cos J, Clèries R, Pérez M,
Ribas A, Eres N, Murio JE, Margarit C and Baselga J: Feasibility
trial of methotrexate-paclitaxel as a second-line therapy in
advanced urothelial cancer. Cancer Invest. 20:673–685. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krege S, Rembrink V, Börgermann C, Otto T
and Rübben H: Docetaxel and ifosfamide as second-line treatment for
patients with advanced or metastatic urothelial cancer after
failure of platinum chemotherapy: A phase 2 study. J Urol.
165:67–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin CC, Hsu CH, Huang CY, Keng HY, Tsai
YC, Huang KH, Cheng AL and Pu YS: Gemcitabine and ifosfamide as a
second-line treatment for cisplatin-refractory metastatic
urothelial carcinoma: A phase II study. Anticancer Drugs.
18:487–491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soga N, Onishi T, Arima K and Sugimura Y:
Paclitaxel Carboplatin chemotherapy as a second-line chemotherapy
for advanced platinum resistant urothelial cancer in Japanese
cases. Int J Urol. 14:828–832. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suyama T, Ueda T, Fukasawa S, Imamura Y,
Nakamura K, Miyasaka K, Sazuka T, Egoshi K, Nihei N, Hamano M, et
al: Combination of gemcitabine and paclitaxel as second-line
chemotherapy for advanced urothelial carcinoma. Jpn J Clin Oncol.
39:244–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Albers P, Park SI, Niegisch G, Fechner G,
Steiner U, Lehmann J, Heimbach D, Heidenreich A, Fimmers R and
Siener R: AUO Bladder Cancer Group: Randomized phase III trial of
2nd line gemcitabine and paclitaxel chemotherapy in patients with
advanced bladder cancer: short-term versus prolonged treatment
[German Association of Urological Oncology (AUO) trial AB 20/99].
Ann Oncol. 22:288–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bajorin DF: Paclitaxel in the treatment of
advanced urothelial cancer. Oncology (Williston Park). 14:43–52;
discussion 58. 2000.PubMed/NCBI
|
|
25
|
Raghavan D: Progress in the chemotherapy
of metastatic cancer of the urinary tract. Cancer. 97(Suppl 8):
S2050–S2055. 2003. View Article : Google Scholar
|
|
26
|
Papamichael D, Gallagher CJ, Oliver RT,
Johnson PW and Waxman J: Phase ii study of paclitaxel in pretreated
patients with locally advanced/metastatic cancer of the bladder and
ureter. Br J Cancer. 75:606–607. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marchetti P, Urien S, Cappellini GA,
Ronzino G and Ficorella C: Weekly administration of paclitaxel:
Theoretical and clinical basis. Crit Rev Oncol Hematol. 44(Suppl):
S3–S13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Markman M: Management of toxicities
associated with the administration of taxanes. Expert Opin Drug
Saf. 2:141–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perez EA, et al: A large phase II study of
paclitaxel administered as a weekly one hour infusion in patients
with metastatic breast cancer. Proc Am Soc Clin Onco.
18:126a1999.
|
|
30
|
Fennely D, Aghajanian C, Shapiro F,
O'Flaherty C, McKenzie M, O'Connor C, Tong W, Norton L and Spriggs
D: Phase I and pharmacologic study of paclitaxel administered
weekly in patients with relapsed ovarian cancer. J Clin Oncol.
15:187–192. 1997.PubMed/NCBI
|
|
31
|
Joly F, Houédé N, Noal S, Chevreau C,
Priou F, Chinet-Charrot P, Rolland F, Fléchon A, Henry-Amar M and
Culine S: Do patients with advanced urothelial carcinoma benefit
from weekly paclitaxel chemotherapy? A GEUG phase II study. Clin
Genitourin Cancer. 7:E28–E33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raghavan D and Huben R: Management of
bladder cancer. Curr Probl Cancer. 19:1–64. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim
YT and Lee K: Pre-treatment neutrophil to lymphocyte ratio is
elevated in epithelial ovarian cancer and predicts survival after
treatment. Cancer Immunol Immunother. 58:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamanaka T, Matsumoto S, Teramukai S,
Ishiwata R, Nagai Y and Fukushima M: The baseline ratio of
neutrophils to lymphocytes is associated with patient prognosis in
advanced gastric cancer. Oncology. 73:215–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gomez D, Farid S, Malik HZ, Young AL,
Toogood GJ, Lodge JP and Prasad KR: Preoperative
neutrophil-to-lymphocyte ratio as a prognostic predictor after
curative resection for hepatocellular carcinoma. World J Surg.
32:1757–1762. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sarraf KM, Belcher E, Raevsky E, Nicholson
AG, Goldstraw P and Lim E: Neutrophil/lymphocyte ratio and its
association with survival after complete resection in non-small
cell lung cancer. J Thorac Cardiovasc Surg. 137:425–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gondo T, Nakashima J, Ohno Y, Choichiro O,
Horiguchi Y, Namiki K, Yoshioka K, Ohori M, Hatano T and Tachibana
M: Prognostic value of neutrophil-to-lymphocyte ratio and
establishment of novel pre-operative risk stratification model in
bladder cancer patients treated with radical cystectomy. Urology.
79:1085–1091. 2012. View Article : Google Scholar : PubMed/NCBI
|