Introduction
Uterine cervical cancer is among the most lethal
malignancies affecting women worldwide. For early invasive cervical
cancer, radical hysterectomy plays a pivotal role in patient
outcome, and it is widely accepted that it should be expedited.
However, patients must occasionally suffer long wait times due to
patient overload of skilled oncologists and qualified facilities.
For patients with life-threatening cancers, these progressively
longer wait times prior to initial surgery represent a serious
problem. The effect of delays between the onset of symptoms and the
initial treatment is highly controversial and, unfortunately,
cannot be investigated in randomized controlled trials due to major
ethical issues.
In breast cancer, delays of 3–6 months prior to
initial surgical treatment are associated with poorer survival
(1). Recently, Elit et al
(2) retrospectively analyzed 9,417
patients with uterine cancer regarding the association between
their wait time for surgery and their prognosis, and found that
patients with wait times of >12 weeks had worse survival
compared with those with wait times of <12 weeks. Thus, the
evidence that delays should be kept to a minimum is compelling.
Generally, neoadjuvant chemotherapy (NAC) aims to
reduce the size or extent of the cancer prior to a more radical
treatment, making later procedures easier and more likely to
succeed. In addition, NAC may attack micrometastatic disease. NAC
regimens for cervical cancer treatment have been developed,
although to date there has been little clear evidence of any
survival benefits for any of them. NAC has been performed to reduce
cervical tumor bulk prior to radical hysterectomy (3). The drugs used for NAC are generally
administered systemically in multiple cycles. However, Japanese
researchers have been developing a special method for
intra-arterial neoadjuvant chemotherapy (IANAC) delivered directly
to the tumor, with the aim to expose the tumor to higher drug
concentrations than would systemic intravenous delivery, while
decreasing the systemic side effects (3). Thus, the idea that IANAC administered
during the wait time prior to a delayed surgery may inhibit tumor
growth and remove micrometastatic disease with minimum side
effects, may be highly appealing to gynecological oncologists.
Indeed, when informed of such potential benefits, patients often
prefer an early start to this therapy, when faced with long wait
times to initial surgery.
In this study, we retrospectively analyzed 12 cases
of operable stage IB1-IIB cervical cancer who were treated with
single-dose IANAC during their wait for subsequent radical
hysterectomy, in order to better understand the efficacy and safety
of IANAC for this purpose.
Patients and methods
Patients
We retrospectively reviewed the medical records of
patients with uterine cervical cancer who received single-dose
IANAC prior to radical hysterectomy at the Osaka University
Hospital (Osaka, Japan). From January, 2002 to December, 2012, 12
patients with International Federation of Gynecology and Obstetrics
(FIGO) stage IB1-IIB cervical cancer were treated with single-dose
IANAC with cisplatin, followed by intravenous paclitaxel, prior to
radical hysterectomy. Written informed consent for the treatment
was obtained from all the patients. Due to backups, the patients
were expected to wait for >2 months until their surgery, and
each expressed a desire for an earlier start of some form of
therapy. A total of 57 patients who were surgically treated without
prior NAC were used as a control group. These patients comprised
the primary surgery alone (PS) group, and were matched to the IANAC
group for age, histology and tumor stage. The clinicopathological
characteristics of the 69 patients are summarized in Table I. Age, tumor size, histology and FIGO
stage were intentionally similar between the IANAC and PS
groups.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | IANAC (n=12) | PS (n=57) | P-value |
|---|
| Age ± SD, years | 53±7 | 49±10 | 0.21a |
| Tumor size ± SD,
mm | 46±6 | 44±11 | 0.60a |
| Histology, n (%) |
|
|
|
| SCC | 8 (66.6) | 39 (68.4) | 0.90b |
|
Non-SCC | 4 (33.3) | 18 (31.5) |
|
| FIGO stage, n
(%) |
|
|
|
| IB1 | 1 (8.3) | 4 (7.0) | 0.87b |
| IB2 | 1 (8.3) | 4 (7.0) | 0.87 |
| IIA | 1 (8.3) | 4 (7.0) | 0.87 |
| IIB | 9 (75.0) | 45 (78.9) | 0.76 |
Intra-arterial NAC
For the IANAC group, a paclitaxel plus cisplatin
regimen (TP regimen) was applied at 4 weeks prior to subsequent
radical hysterectomy. Prior to IANAC, angiography was performed to
detect the tumor feeding vessels by the Seldinger technique. A
microcatheter was selectively inserted into each of the uterine or
hypogastric arteries. Via the microcatheter, cisplatin was injected
over 2 h, to a final dosage of 75 mg/m2. Intravenous
hydration with normal saline/5% dextrose was initiated 3 h prior to
IANAC and was continued afterwards to maintain urine volume. After
IANAC, but on the same day, paclitaxel (175 mg/m2) was
administered intravenously for 3 h. Prior to surgery, the clinical
response of the tumor to IANAC was evaluated by magnetic resonance
imaging (MRI) in 11 of the 12 patients; the remaining case was not
evaluated due to the patient's refusal. A Piver type III radical
hysterectomy was performed in all the patients in both groups.
Operative outcome and prognosis
The operative results and prognosis were compared
between the two groups. Tumor size, measured by MRI at the initial
diagnostic procedure and immediately prior to surgery, was used to
evaluate tumor response to NAC, and the response was classified
according to the Response Evaluation Criteria in Solid Tumors,
version 1.1 (4). Complete response
(CR) was defined as disappearance of the tumor; partial response
(PR) as a ≥30% decrease of the longest diameter (LD) of the tumor;
progressive disease (PD) as a ≥20% increase of the LD; and stable
disease (SD) as a change of less than the PR or PD limits.
Statistical analysis
Statistical analyses were performed using MedCalc
for Windows, version 11.3.3.0 (MedCalc Software, Mariakerke,
Belgium). Disease-free survival (DFS) was defined as the time (in
months) from the date of completion of initial therapy to the date
of a radiologically confirmed recurrence. Overall survival (OS) was
calculated from the date of completion of initial therapy to the
date of death. A multivariate Cox proportional hazards analysis
with selected variables was used to determine the significantly
important factors for recurrence. The Kaplan-Meier statistical
method was used for the calculation of DFS and OS. Statistical
significance was analyzed by the log-rank test. We considered the
results to be statistically significant when the P-value was
<0.05.
Results
Comparison of surgical outcomes
between IANAC and PS
The results of the comparison of surgical outcomes
between the IANAC and PS groups are presented in Table II. The mean wait times ± standard
deviation from diagnosis to surgery were 57±11 and 40±15 days for
the IANAC and PS groups, respectively, indicating significantly
longer wait times for the IANAC group. There were no significant
differences in terms of operative time, blood loss, number of lymph
nodes removed, or transfusion rate. As regards early complications,
there was a tendency for surgical site infection to occur more
frequently in IANAC (25.0%) than in PS (17.5%), whereas urinary
disorders tended to occur less frequently in IANAC (8.3%) than in
PS (26.3%). Ileus and urinary tract injury were not observed in
IANAC; by contrast, in the PS group, ileus and urinary tract injury
occurred in 12.2 and 3.5% of the patients, respectively.
 | Table II.Comparison of surgical outcome between
IANAC and PS cervical cancer groups. |
Table II.
Comparison of surgical outcome between
IANAC and PS cervical cancer groups.
| Variables | IANAC (n=12) | PS (n=57) | P-value |
|---|
| Time to surgery ± SD,
days | 57±11 | 40±15 | 0.001a |
| Operative time ± SD,
min | 433±132 | 391±96 | 0.20a |
| Blood loss ± SD,
ml | 1362±666 | 1041±786 | 0.19a |
| Lymph nodes removed ±
SD, n | 27±12 | 25±12 | 0.71a |
| Transfusion, n
(%) | 8 (66.6) | 39 (68.4) | 0.90b |
| Early complications,
n (%) |
|
|
|
| Surgical
site infection | 3 (25.0) | 10 (17.5) | 0.54b |
| Urinary
disorder | 1 (8.3) | 15 (26.3) | 0.17 |
|
Ileus | 0 (0.0) | 7 (12.2) | 0.20 |
| Urinary
tract injury | 0 (0.0) | 2 (3.5) | 0.51 |
| Adjuvant therapy |
|
|
|
| CCRT | 9 (75.0) | 43 (75.4) | 0.97b |
| RT | 1 (8.3) | 9 (15.7) | 0.50 |
|
Chemotherapy | 2 (16.6) | 3 (5.2) | 0.16 |
| Median follow-up,
months | 41 | 54 |
|
Adjuvant therapy was performed in patients with deep
stromal invasion, lymphovascular invasion, or positive lymph nodes.
Concurrent chemotherapy and radiotherapy was applied in 9/12 (75%)
IANAC cases and in 43/57 (75%) cases in the PS group.
In the IANAC group, 11/12 patients were evaluated
with MRI immediately prior to surgery for the tumor response to
IANAC. The overall clinical response rate was 54.5% (6/11), which
included a CR in 1 case (9.0%) and a PR in 5 cases (45.5%). The
remaining 5 patients (45.5%) had SD; no patients with PD were
observed. IANAC-related toxicity was well tolerated; only 1 patient
developed grade 3 neutropenia.
During the postoperative follow-up (median, 41
months) of IANAC, disease recurrence was observed in 5/12 cases
(41.6%). In the PS group, the rate was 22/57 cases (38.5%) (median,
54 months). There was no significant difference in DFS between the
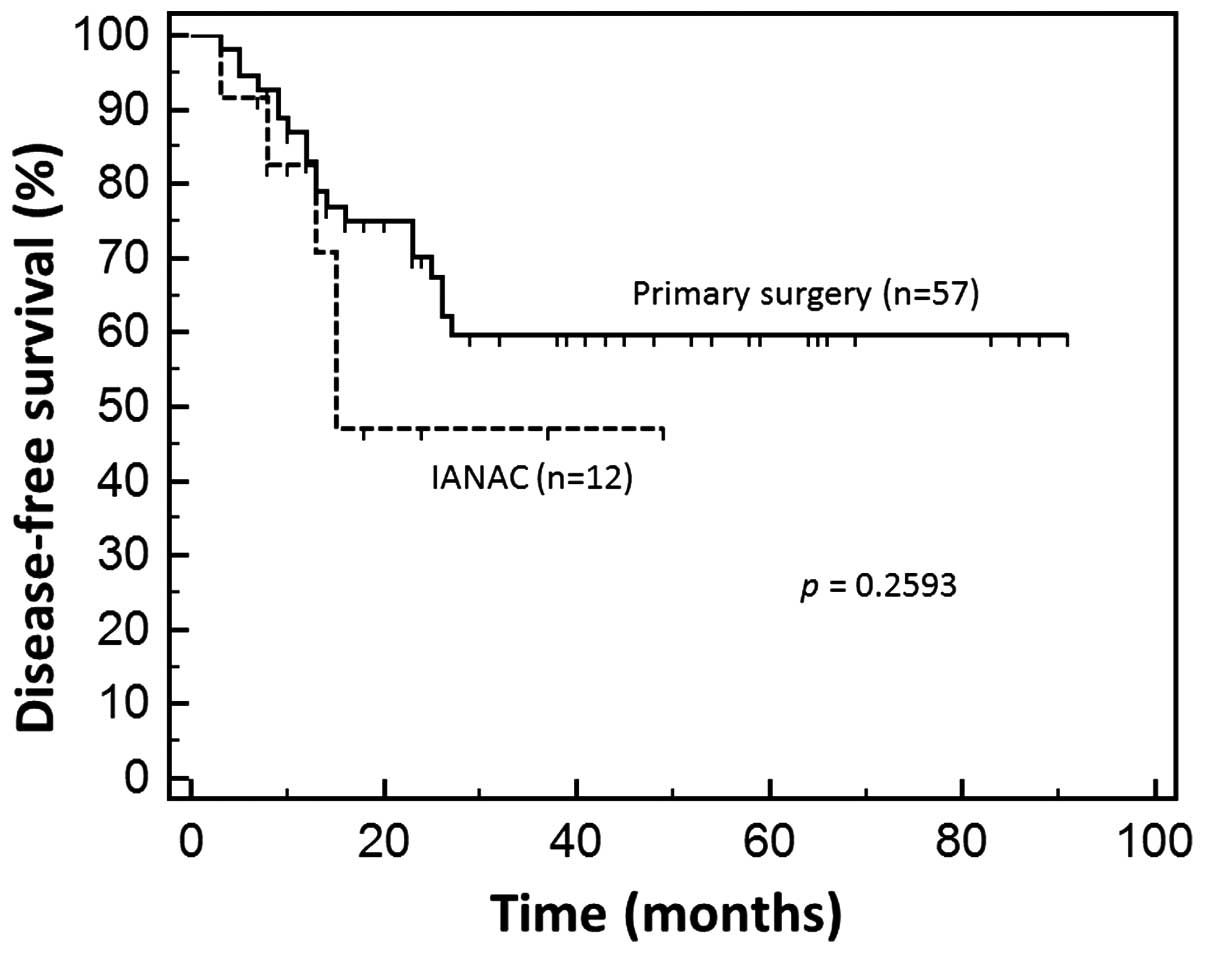
IANAC and PS groups (Fig. 1). In
addition, there was a difference in the 3-year survival rate
between the IANAC and PS groups (91.6 and 71.9%, respectively);
however, this difference was not statistically significant
(P=0.1399).
Comparison of recurrence sites between
the IANAC and PS groups
As shown in Table
III, local recurrence was observed in 1/12 cases (8.3%) in the
IANAC group and in 7/57 cases (12.2%) in the PS group, with no
significant difference. Among these local recurrences, 1 IANAC and
2 PS cases were rescued by surgery or interstitial radiotherapy,
with no further evidence of tumor to date. Recurrences in the
pelvic lymph nodes were observed only in the PS group (3 cases,
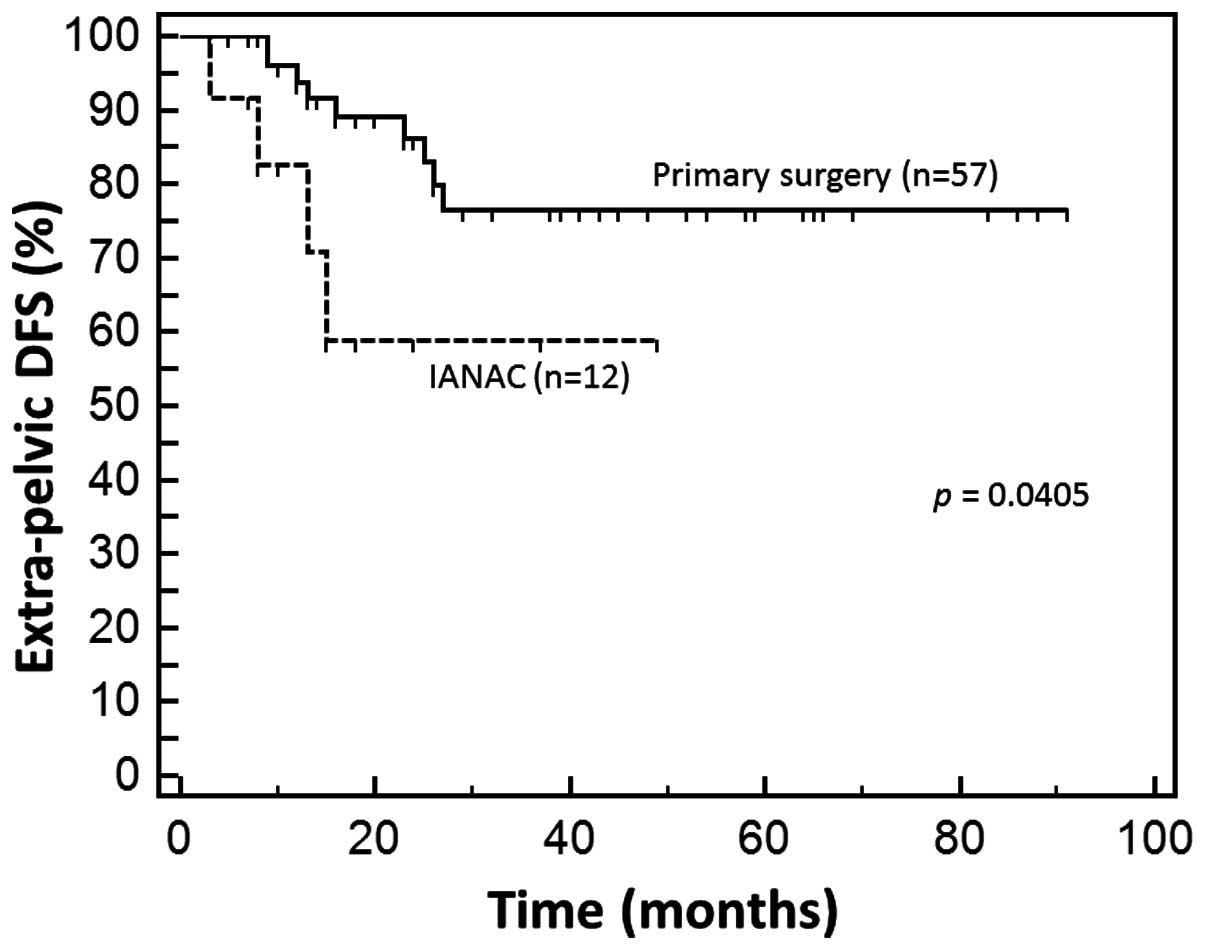
5.2%). However, extrapelvic recurrence was observed more frequently
in IANAC than in PS, with a statistically significant difference
(P=0.0405; Fig. 2). Recurrences in
the para-aortic lymph nodes (PAN) were observed in 2 IANAC cases
(16.6%), which was significantly higher compared with the PS group
(1.7%; P=0.021). Both IANAC cases with PAN recurrence were rescued
by radiotherapy. Distal recurrence (except PAN) was observed in 2
IANAC (16.6%) and 11 PS (19.3%) cases.
 | Table III.Comparison of recurrence sites between
the IANAC and PS groups. |
Table III.
Comparison of recurrence sites between
the IANAC and PS groups.
| Sites of recurrence,
n (%) | IANAC (n=12) | PS (n=57) | P-value |
|---|
| Local recurrence | 1 (8.3) | 7 (12.2) | 0.67a |
| Pelvic lymph
nodes | 0 (0.0) | 3 (5.2) | 0.41a |
| PAN | 2 (16.6) | 1 (1.7) | 0.02a |
| Distal recurrence
(except PAN) | 2 (16.6) | 11 (19.3) | 0.83a |
| Total | 5 (41.6) | 22 (38.5) | 0.54a |
Multivariate Cox proportional hazards
analysis for DFS
The results of the multivariate analysis for DFS are
shown in Table IV. The type of tumor
histology (squamous vs. non-squamous) was determined to be an
independent prognostic factor for DFS (hazard ratio = 0.35, 95%
confidence interval: 0.1415–0.8967, P=0.0292). However, age (≥50
vs. <50 years), wait time until surgery (≥45 vs. <45 days),
initial tumor size (≥5 vs. <5 cm), and lymph node metastasis
status (positive vs. negative) did not affect DFS in our
series.
 | Table IV.Multivariate Cox proportional hazards
analysis for DFS in cervical cancer. |
Table IV.
Multivariate Cox proportional hazards
analysis for DFS in cervical cancer.
| Variables | HR | 95% CI of HR | P-value |
|---|
| Age, years |
|
| 0.76 |
| ≥50 | 0.86 | 0.3471–2.1719 |
|
|
<50 | 1 |
|
|
| Wait time,
days |
|
| 0.88 |
|
≥45 | 0.93 | 0.3567–2.4326 |
|
|
<45 | 1 |
|
|
| Tumor size, cm |
|
| 0.58 |
| ≥5 | 0.79 | 0.3508–1.8003 |
|
|
<5 | 1 |
|
|
| IANAC |
|
| 0.4 |
|
Yes | 1.61 | 0.5317–4.9045 |
|
| No | 1 |
|
|
| Histology |
|
| 0.0292 |
|
SCC | 0.35 | 0.1415–0.8967 |
|
|
Non-SCC | 1 |
|
|
| Lymph node
metastasis |
|
| 0.15 |
|
Positive | 2.01 | 0.7719–5.2549 |
|
|
Negative | 1 |
|
|
Discussion
Our study demonstrated that single-dose IANAC,
delivered while waiting for radical hysterectomy, was not
associated with an objective benefit for patients with stage IB-IIB
cervical cancer. In our study, there were no significant
differences in DFS or 3-year survival rates between the IANAC and
PS groups. A multivariate analysis revealed that the wait time
until surgery (≥45 vs. <45 days) or the use or of IANAC did not
affect DFS. Only the type of histology (squamous vs. non-squamous)
was found to be an independent prognostic factor for DFS.
Surprisingly, we observed distal recurrences more frequently in the
IANAC rather than in the PS control group, with a statistically
significant difference. This may indicate that IANAC may promote
new spreading of tumor cells, rather than the hoped for effect of
eradicating pre-existing micrometastases.
Previous studies, usually performed with 3 cycles of
platinum and taxanes, have shown the benefits of IANAC for
controlling bulky cervical cancer. IANAC was effective at reducing
tumor bulk, with reduced toxicity (relative to intravenous
treatments), with safety and improved prognosis (3,5–9).
In ovarian cancer, >3 cycles of NAC are required
to efficiently control the primary as well as the distant tumors.
In early-stage ovarian cancer, 6 cycles of adjuvant carboplatin and
paclitaxel significantly reduced recurrence, compared with 3
cycles). This result suggested that, to vanquish micrometastases of
hardier cancer cells, repeated administrations of chemotherapy are
required. Therefore, if IANAC use is inevitable, multiple cycles of
administration is clearly preferable to a single dose, as was
attempted in this study.
Due to the high ratio of patients with technically
difficult surgical needs to the low numbers of fully skilled
gynecologic oncologists and hospitals with advanced facilities,
wait times for cancer surgeries have increased, causing serious
problems for the patients. These longer wait times for surgery may
cause the patient anxiety, thereby negatively affecting prognosis.
For patients to cope with longer wait times, concurrent
chemoradiotherapy (CCRT) may be the most reasonable option. The
evidence for the efficacy of CCRT in cervical cancer is well
established; therefore, in order to start therapy early, some
patients have been treated with CCRT instead of surgery. However,
some patients prefer to undergo surgery instead of CCRT, due to the
long-lasting side effects of radiation. In such cases, multiple
cycles of IANAC, followed by radical hysterectomy, may be a viable
option. The limitations of our study were the relatively small
number of IANAC cases, the retrospective design that may cause
bias, and the relatively short follow-up period.
In conclusion, although single-dose IANAC may help
reduce patient anxiety and permit earlier surgery (compared with
multiple cycles of IANAC), its survival benefit is equivocal and it
should not be performed lightly. Furthermore, it is recommended
that single-cycle IANAC should not be performed without careful
consideration. It is likely that multiple-cycle IANAC will perform
better than a single dose for controlling distant micrometastases,
as higher response rates and better prognosis were previously
reported using ≥3 cycles.
Acknowledgements
We would like to thank Dr G.S. Buzard for his
helpful comments and editing.
Glossary
Abbreviations
Abbreviations:
|
CCRT
|
concurrent chemoradiotherapy
|
|
CR
|
complete response
|
|
DFS
|
disease-free survival
|
|
IANAC
|
intra-arterial neoadjuvant
chemotherapy
|
|
LD
|
longest diameter
|
|
MRI
|
magnetic resonance imaging
|
|
NAC
|
neoadjuvant chemotherapy
|
|
OS
|
overall survival
|
|
PAN
|
para-aortic lymph nodes
|
|
PD
|
progressive disease
|
|
PR
|
partial response
|
|
PS
|
primary surgery alone
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
SD
|
stable disease
|
|
TP
|
paclitaxel plus cisplatin
|
References
|
1
|
Richards MA, Westcombe AM, Love SB,
Littlejohns P and Ramirez AJ: Influence of delay on survival in
patients with breast cancer: A systematic review. Lancet.
353:1119–1126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elit LM, O'Leary EM, Pond GR and Seow HY:
Impact of wait times on survival for women with uterine cancer. J
Clin Oncol. 32:27–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugiyama T, Nishida T, Hasuo Y, Fujiyoshi
K and Yakushiji M: Neoadjuvant intraarterial chemotherapy followed
by radical hysterectomy and/or radiotherapy for locally advanced
cervical cancer. Gynecol Oncol. 69:130–136. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D and
Verweij J: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamakawa Y, Fujimura M, Hidaka T, Hori S
and Saito S: Neoadjuvant intraarterial infusion chemotherapy in
patients with stage IB2-IIIB cervical cancer. Gynecol Oncol.
77:264–270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aoki Y, Sato T, Watanabe M, Sasaki M,
Tsuneki I and Tanaka K: Neoadjuvant chemotherapy using low-dose
consecutive intraarterial infusions of cisplatin combined with
5-fluorouracil for locally advanced cervical adenocarcinoma.
Gynecol Oncol. 81:496–499. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Motoyama S, Hamana S, Ku Y,
Laoag-Fernandez JB, Deguchi M, Yoshida S, Tominaga M, Iwasaki T,
Ohara N and Maruo T: Neoadjuvant high-dose intraarterial infusion
chemotherapy under percutaneous pelvic perfusion with
extracorporeal chemofiltration in patients with stages IIIa-IVa
cervical cancer. Gynecol Oncol. 95:576–582. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Terai Y, Kanemura M, Sasaki H, Tsunetoh S,
Tanaka Y, Yamashita Y, Yamamoto K, Narabayashi I and Ohmichi M:
Long-term follow-up of neoadjuvant intraarterial chemotherapy using
an original four-lumen double-balloon (4L-DB) catheter for locally
advanced uterine cervical cancer. Int J Clin Oncol. 14:56–62. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaku S, Takahashi K, Murakami Y, Wakinoue
S, Nakagawa T, Shimizu Y, Kita N, Noda Y and Murakami T:
Neoadjuvant intraarterial chemotherapy for stage IIB-IIIB cervical
cancer in Japanese women. Exp Ther Med. 1:651–655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bell J, Brady MF, Young RC, Lage J, Walker
JL, Look KY, Rose GS and Spirtos NM: Gynecologic Oncology Group:
Randomized phase III trial of three versus six cycles of adjuvant
carboplatin and paclitaxel in early stage epithelial ovarian
carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol.
102:432–439. 2006. View Article : Google Scholar : PubMed/NCBI
|