Introduction
Treatment for malignant gliomas typically requires a
combined approach that includes surgery, radiotherapy and
chemotherapy. Radiotherapy is an important adjuvant treatment for
malignant gliomas. Intensity-modulated radiotherapy (IMRT) has been
demonstrated to be superior to three-dimensional conformal
radiotherapy (3D-CRT) in patients with malignant gliomas. MacDonald
et al (1) compared the
dosimetric distribution of non-coplanar IMRT in malignant gliomas
with that of 3D-CRT, and identified that non-coplanar IMRT improved
the target coverage and reduced the radiation dose to the brain,
brainstem and optic chiasm. Lorentini et al (2) performed a dosimetric comparison between
IMRT and 3D-CRT in glioblastoma. IMRT appears to be a superior
radiation technique compared with 3D-CRT when multiple overlaps
exist between the planning target volume (PTV) and organs at risk
(OARs). IMRT allows for improved target coverage while maintaining
equivalent OARs, sparing and reducing normal brain irradiation.
Intensity-modulated arc radiotherapy (IMAT) represents the latest
evolution of cancer treatment technology, setting novel benchmarks
for speed, precision and patient comfort. IMAT, which at Varian
Medical Systems, Inc. (Palo Alto, CA, USA) is termed RapidArc, is
similar to Elekta's (Stockholm, Sweden) Elekta Synergy®
volumetric-modulated arc therapy (VMAT) and Philips' (Amsterdam,
The Netherlands) Pinnacle3 SmartArc treatment planning
solution. RapidArc uses a unique algorithm that provides
unprecedented treatment delivery control. As a result, treatment
plans that excel in covering target goals, while sparing critical
structures, can be developed with performance speeds faster than
ever before. Clinicians are able to develop treatments that take
one-half to one-eighth the time of conventional IMRT treatments:
Only 2 min in a number of cases. IMAT treatment may also result in
less radiation leakage and scatter, so that peripheral tissues
receive a lower overall dose. IMAT was used to evaluate the effect
on dosage distributions in OARs and normal brain tissue compared
with IMRT and 3D-CRT in high-grade gliomas, which were
predominantly located in the frontal and temporal lobes of the
cerebral hemisphere (3,4). In order to compare the dosimetric
parameters of IMRT with those of RapidArc with single arc (RA1) and
dual arc (RA2) in malignant gliomas involving the parietal lobe, in
the present study IMRT, RA1 and RA2 treatment plans were developed
for each of 10 patients with malignant glioma.
Materials and methods
Patient selection and delineation of
the PTV and OARs
A total of 10 patients (five men and five women)
with malignant glioma involving the parietal lobe were enrolled in
the present study. The study was approved by the Medical Ethics
Committee of Xiangya Hospital of Central South University and all
participants gave written content. All the participants had been
surgically treated, and their condition was confirmed by
pathological diagnosis. Their ages ranged from 16 to 59 years (mean
age, 45.8 years). According to the World Health Organization (WHO)
2007 classification of tumors of the central nervous system (CNS),
there were five cases of grade III and five cases of grade IV
(5). Temozolomide was used in all
patients as adjuvant chemotherapy to surgery and radiotherapy,
referring to Stupp's method (6).
Patients with malignant glioma received concomitant chemotherapy
consisting of daily temozolomide (75 mg/m2/day) with
IMRT or RapidArc and adjuvant chemotherapy consisting of up to six
cycles of maintenance temozolomide (150–200mg/m2/day on
days 1–5 repeated every 28 days). The clinical data of the 10
patients with malignant glioma are shown in Table I.
 | Table I.Clinical characteristics of the 10
patients with malignant glioma. |
Table I.
Clinical characteristics of the 10
patients with malignant glioma.
| No. | Gender | Age (years) | Side | Location | Size
(cm2) | Extent of
surgery | Pathological
grade |
|---|
| 1 | F | 44 | Right | Parietooccipital
lobe | 4.1×5.1 | GTR | III |
| 2 | M | 58 | Right | Parietooccipital
lobe | 2.0×2.0 | PR | IV |
| 3 | F | 26 | Right | Parietofrontal
lobe | 3.0×4.0 | GTR | IV |
| 4 | M | 45 | Left | Parietofrontal
lobe | 4.5×4.0 | PR | III |
| 5 | M | 40 | Right | Parietotemporal
lobe | 8.0×6.5 | GTR | III |
| 6 | M | 16 | Left | Parietal lobe | 2.6×2.1 | PR | III |
| 7 | F | 59 | Left | Parietotemporal and
frontal lobe | 5.0×7.0 | PR | IV |
| 8 | F | 56 | Left | Parietotemporal,
and occipital lobe | 4.0×4.5 | GTR | IV |
| 9 | F | 58 | Right | Parieto frontal
lobe | 4.0×5.0 | PR | III |
| 10 | M | 56 | Left | Parietotemporal
lobe | 5.0×4.0 | PR | IV |
Patients were scanned with simulated computed
tomography (CT) using a Somatom Definition AS CT scanner (Siemens
AG, Munich, Germany) at a 3-mm slice thickness, with T1-weighted
magnetic resonance imaging (MRI) using a Magnetom Sonata 1.5T MRI
scanner (Siemens AG), with contrast being performed in the meantime
and registered with CT. The gross tumor volume tumor bed (GTVtb)
was contoured as the residual tumor and postoperative tumor bed
according to the operative record, preoperative MRI and
postoperative MRI within 3 days following the surgery; the GTVtb
with 0.5 cm margins was identified as the planning (P)GTVtb. The
clinical target volume 1 (CTV1) was outlined as the GTVtb with
1.5–2.0 cm margins, and the CTV2 was delineated as the GTVtb with
2–2.5 cm margins; CTV1 and CTV2 were based on the pathological
grades of gliomas and limitation of dose to OARs. Dose limitation
to OARs was undertaken with reference to the Radiation Therapy
Oncology Group 0825 protocol. (7).
CTV1 and CTV2 were expanded with 0.5 cm margins, resulting in the
PTV1 and PTV2, respectively. OARs included the brainstem, bilateral
lenses, bilateral optic nerve, bilateral hippocampus, optic chiasm,
pituitary gland and normal brain tissue [which meant the whole
brain minus PTV2, or B-P)]. The brainstem, bilateral lens and optic
chiasm with 0.3 cm margins were created as the brainstem planning
risk volume (PRV), the bilateral lens PRV and the optical chiasm
PRV, respectively.
Prescribed doses, plan objective and
OAR constraints
PTVs were divided into various subPTVs, including
the PGTVtb, PTV1 and PTV2, as described above, which delivered
various prescribed doses of radiation. PGTVtb received 64.2 Gy in
30 fractions (2.14 Gy per fraction), whereas PTV1 received 60 Gy in
30 fractions (2 Gy per fraction); and PTV2 received 54 Gy in 30
fractions (1.8 Gy per fraction) using the simultaneous integrated
boost technique. Measured as a percentage, 95% of the PTV received
95% of the prescribed dose; the volume of PTV that received ≥110%
of the prescribed dose was <20%; the volume of PTV that received
≤93% of the prescribed dose was <3%; and areas outside of the
PTV were not allowed to receive >110% of the prescribed dose.
The maximum dose (Dmax) to the brainstem was limited to 54 Gy; Dmax
to the lens was limited to 9 Gy; and Dmax to the optical nerve,
optical chiasm and pituitary gland were limited to 50 Gy.
Planning techniques
The IMRT, RA1 and RA2 treatment plans were designed
by using the identical CT data fused with regular MRI T1-weighted
images contrasted for every patient on the Varian Eclipse™
treatment planning system (version 8.6.05; Varian Medical Systems,
Inc.) with 6 MV photon beams from a Varian Trilogy, respectively.
The prescription and planning objectives used for the three
treatment plans were identical.
IMRT was computed with a fixed gantry, with the
couch angle set to 0° and the collimator set at 10°; the type of
multileaf collimator (MLC) was the Varian Millennium 120 leaf MLC
(Varian Medical Systems, Inc.). MLC leaf sequences were generated
using the dynamic sliding window IMRT delivery (8,9). Plans
were individually optimized by using seven co-planar fields
selecting for the best geometry for each patient. A fixed dose rate
(DR) of 600 monitor units (MUs)/min was selected for IMRT.
RA1 used a single-arc rotation intensity-modulated
technology, consisting of a single 360° rotation (clockwise) with
the couch angle set to 0° and the collimator set to 10°. The arc
starts with a gantry angle of 181°, and stops at a gantry angle of
179°. RA2 used a dual-arc rotation intensity-modulated technique,
consisting of two co-planar arcs of 360° optimized simultaneously
to be delivered with opposite rotation (clockwise and
counter-clockwise). For the RA2 plans, the couch was set to 0° for
the two arcs, whereas the collimator rotation was set to the
identical angle as in the RA1 plans for the first arc and to 325°
for the second arc. The first arc (clockwise) started with a gantry
angle of 181°, and stopped at a gantry angle of 179°. The second
arc (counter-clockwise) started with a gantry angle of 179° and
stopped at a gantry angle of 181°. Plans for RA1 and RA2 were
optimized by selecting a maximum DR of 600 MU/min.
The anisotropic analytical algorithm (AAA) was used
for IMRT, RA1 and RA2 (10–12). The dose calculation grid was set to
0.125 cm (13).
Plan quality evaluation, dose
distribution and parameter comparison
Dose-volume histograms (DVHs) of IMRT, RA1 and RA2
were generated with use of the Eclipse™ Treatment system (Varian
Medical Systems, Inc.). Comparisons of dosimetric parameters and
plan quality were performed among IMRT, RA1 and RA2, and the
conformal index (CI) was calculated according to the method
described by van't Riet et al (14): CI =
TVRI2/TVxVRI, where
TVRI is the target volume covered by the reference
isodose, TV is the target volume and VRI is the volume
of the reference isodose; higher values of CI represented an
improved PTV conformality. The homogeneity index (HI) refers to the
formula described by Wu et al (15): HI =
(D2%-D98%)/Dp, where Dp
is the prescription dose, Dnear-max (D2%) is the dose/2%
volume of PTV received, and Dnear-min (D98%) is the
dose/98% volume of PTV received; lower values of HI represented an
improved PTV homogeneity. median dose (D50%) was the dose/50%
volume of PTV. D2%, D50%, V5, V10, V15, V20, V25, V30, V35, V40,
V45 and V50 of B-P were compared among IMRT, RA1 and RA2; Vn refers
to the volume of the B-P receiving at least nGy.
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used to perform the statistical analysis. Statistical tests of
differences between dosimetric parameters of IMRT, RA1 and RA2 were
evaluated using a two-sided Wilcoxon matched-pair signed-rank test
(each pair in the test consisting of the patient-specific
dosimetric parameters for IMRT, RA1 and RA2). P<0.05 was
considered to indicate a statistically significant difference.
Results
In the present study, with respect to the D2% to
OARs, the D2% values to the left lens, right lens and left optic
nerve in RA1 were significantly less compared with those in IMRT
(P<0.05), respectively (Table
II). D2% to the right lens and right optic nerve in RA2 were
significantly less compared with those in IMRT (P<0.05). D2% to
the optic chiasma in RA2 was significantly less compared with that
in RA1 (P<0.05). With respect to the D50% to OARs, the D50% to
the right lens and right optic nerve in RA1 and RA2 were
significantly less compared with those in IMRT (P<0.05). D50% to
the brainstem in RA2 was significantly less compared with that in
RA1 (P<0.05); in addition, V45 and V50 of B-P from RA1 were less
compared with those from IMRT, with statistically significant
differences (P<0.05). V30-V50 of B-P in RA2 were significantly
less compared with those in IMRT (P<0.05), respectively. Without
prospectively optimizing to spare the hippocampus, D2% and D50% to
the right and left hippocampi did not yield any significant
differences among IMRT, RA1 and RA2, which indicated that the
hippocampus is not affected by different radiotherapy techniques
that feature no effort to spare it (Table II).
 | Table II.Dosimetric parameters of IMRT, RA1
and RA2. |
Table II.
Dosimetric parameters of IMRT, RA1
and RA2.
| Parameter | IMRT, mean ±
SD | P for IMRT vs.
RA1 | RA1, mean ± SD | P for IMRT vs.
RA2 | RA2, mean ± SD | P for RA1 vs.
RA2 |
|---|
| OARs (Gy) |
|
|
Brainstem D2% | 45.9±15.3 | 0.96 | 45.5±16.0 | 0.80 | 45.4±15.7 | 0.80 |
|
D50% | 20.2±16.8 | 0.24 | 20.7±16.9 | 0.88 | 19.7±16.1 | 0.01c |
| Lens
RD2% | 3.0±1.1 | 0.01a | 2.6±0.99 | 0.01b | 2.6±1.1 | 0.88 |
|
D50% | 2.3±1.0 | 0.01a | 2.00±0.8 | 0.01b | 2.0±0.9 | 0.96 |
| Lens
LD2% | 3.0±1.1 | 0.04a | 2.6±0.9 | 0.11 | 2. 9±1.2 | 0.29 |
|
D50% | 2.4±0.9 | 0.06 | 2.1±0.9 | 0.14 | 2.2±0.9 | 0.20 |
| Optic
nerve R D2% | 6.8±5.1 | 0.11 | 5.9±3.4 | 0.01b | 5.9±3.9 | 0.58 |
|
D50% | 5.1±3.7 | 0.01a | 4.3±3.0 | 0.01b | 4.5±3.5 | 0.20 |
| Optic
nerve L D2% | 6.6±2.9 | 0.01a | 6.2±2.6 | 0.07 | 6.2±2.6 | 0.72 |
|
D50% | 4.8±2.4 | 0.17 | 4.1±1. 8 | 0.58 | 4.4±1.9 | 0.14 |
| Optic
chiasma D2% | 21.4±14.5 | 0.45 | 21.8±15.1 | 0.96 | 21.1±14.9 | 0.01c |
|
D50% | 17.5±12.3 | 0.88 | 16.8±12.7 | 0.96 | 16.8±12.4 | 0.80 |
|
Pituitary D2% | 15.4±10.5 | 0.24 | 14.3±9.4 | 0.58 | 14.0±8.8 | 0.45 |
|
D50% | 13.0±9.0 | 0.33 | 12.0±8.3 | 0.20 | 11.8±7.8 | 0.45 |
|
Hippocampus R D2% | 53.89±8.20 | 0.77 | 53.88±8.48 | 0.99 | 53.88±8.49 | 0.41 |
|
D50% | 37.95±18.04 | 0.19 | 39.49±17.36 | 0.21 | 38.99±17.36 | 0.33 |
|
Hippocampus L D2% | 49.05±17.08 | 0.64 | 48.59±18.33 | 0.39 | 44.75±22.81 | 0.41 |
|
D50% | 38.82±20.62 | 0.32 | 37.65±21.47 | 0.22 | 37.72±20.88 | 0.90 |
| B-P (%) |
|
| V5 | 84.6±18.1 | 0.22 | 84.1±18.6 | 0.08 | 84.0±18.3 | 0.80 |
|
V10 | 75.8±17.5 | 0.06 | 77.1±17.9 | 0.11 | 77.2±17.6 | 0.37 |
|
V15 | 62.8±14.6 | 0.11 | 64.0±15.8 | 0.06 | 65.4±14.1 | 0.20 |
|
V20 | 53.1±12.6 | 0.17 | 55.2±14.7 | 0.17 | 53.8±13.1 | 0.26 |
|
V25 | 44.3±12.4 | 0.88 | 44.8±13.8 | 0.07 | 43.3±12.6 | 0.11 |
|
V30 | 36.1±11.5 | 0.37 | 35.7±12.5 | 0.01b | 34.3±11.4 | 0.06 |
|
V35 | 27.6±9.8 | 0.37 | 27.0±10.2 | 0.03b | 25.8±9.5 | 0.08 |
|
V40 | 20.1±7.9 | 0.11 | 19.0±7.7 | 0.03b | 18.3±7.3 | 0.09 |
|
V45 | 13.9±6.3 | 0.01a | 12.2±6.1 | 0.01b | 13.0±6.0 | 0.44 |
|
V50 | 8.4±5.6 | 0.01a | 6.6±5.4 | 0.01b | 6.6±5.5 | 0.73 |
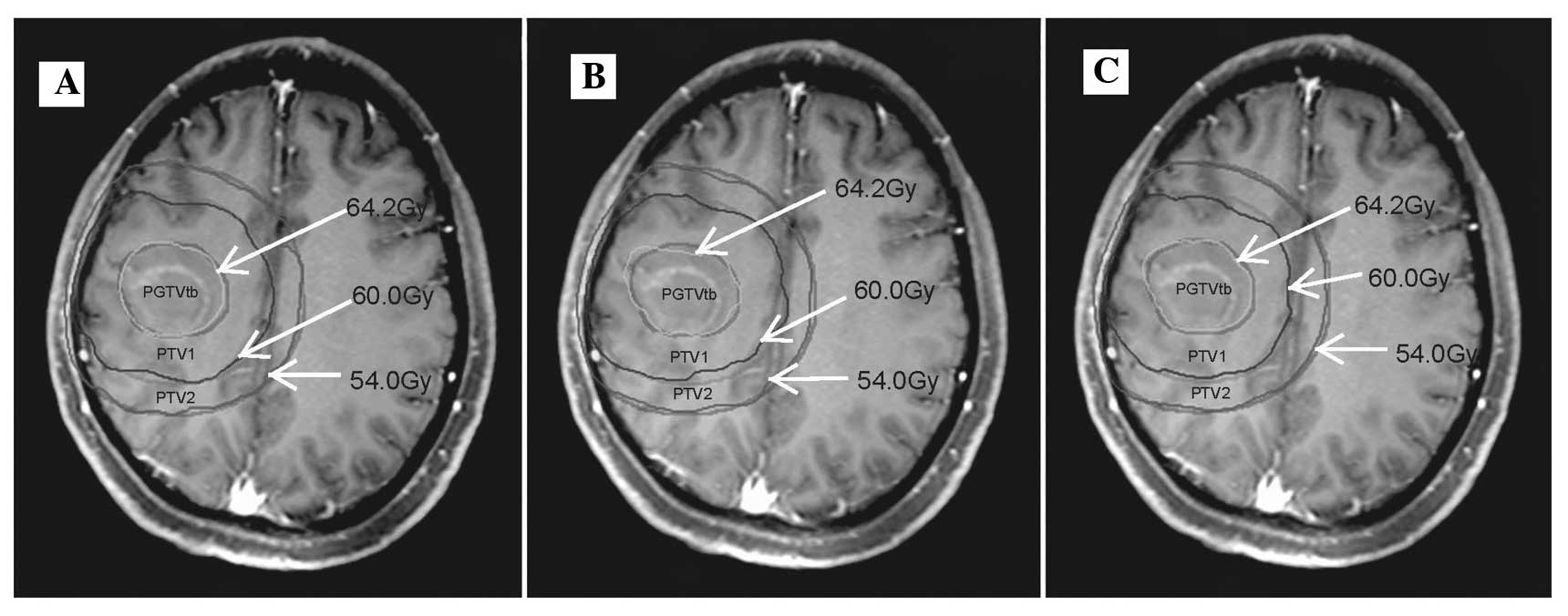
The dose distributions of one representative patient
generated by IMRT, RA1 and RA2 are shown in Fig. 1. D2% and D50% of OARs, with
significant differences (P<0.05) are shown in Fig. 2. The mean DVHs for the OARs of all the
patients treated with different radiotherapy techniques are shown
in Fig. 3. In terms of CI, HI of
subPTV and MUs per fraction, all CI and HI values of subPTV in RA1
were less compared with those in IMRT (P<0.05); by contrast, all
CI and HI values of subPTV in RA2 were similar to those in IMRT,
and they were not significantly different (P>0.05) (Table III). Therefore, this suggests that,
although RA did not improve the coverage and homogeneity of the
target volume with sparing OARs, RA markedly reduced the MUs per
fraction compared with IMRT (P<0.05), and no significant
differences in MUs per fraction were identified between RA1 and
RA2. RA1 and RA2 significantly decreased the treatment times
compared with those of IMRT; the treatment time of RA1 was lower
compared with that of RA2, with a significant difference noted
(P<0.05). Data for the parameters CI, HI of PTV, MUs per
fraction and treatment times in IMRT, RA1, and RA2 are shown in
Table III.
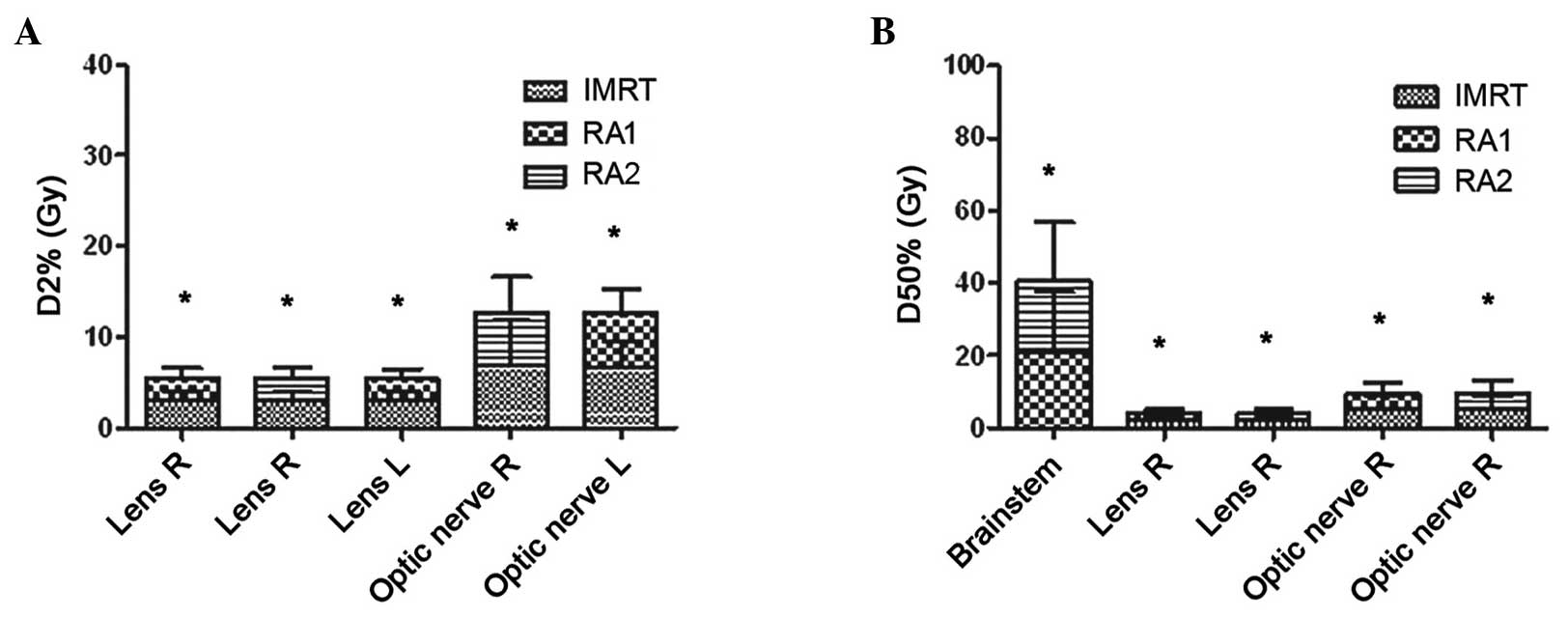 | Figure 2.(A) D2% and (B) D50% of OARs.
*P<0.05 with significant difference. D2% of OARs: Lens R, RA1
vs. IMRT and RA2 vs. IMRT; Lens L, RA1 vs. IMRT; Optic nerve R, RA2
vs. IMRT; Optic nerve L, RA1 vs. IMRT. D50% of OARs: Brainstem, RA1
vs. RA2; Lens R, RA1 vs. IMRT and RA2 vs. IMRT; Optic nerve R, RA1
vs. IMRT and RA2 vs. IMRT. OARs, organs at risk; L, left; R, right;
IMRT, intensity modulated radiotherapy; RA1, RapidArc with single
arc; RA2, RapidArc with dual arc; D2%, near-maximum dose; D50%,
median dose. |
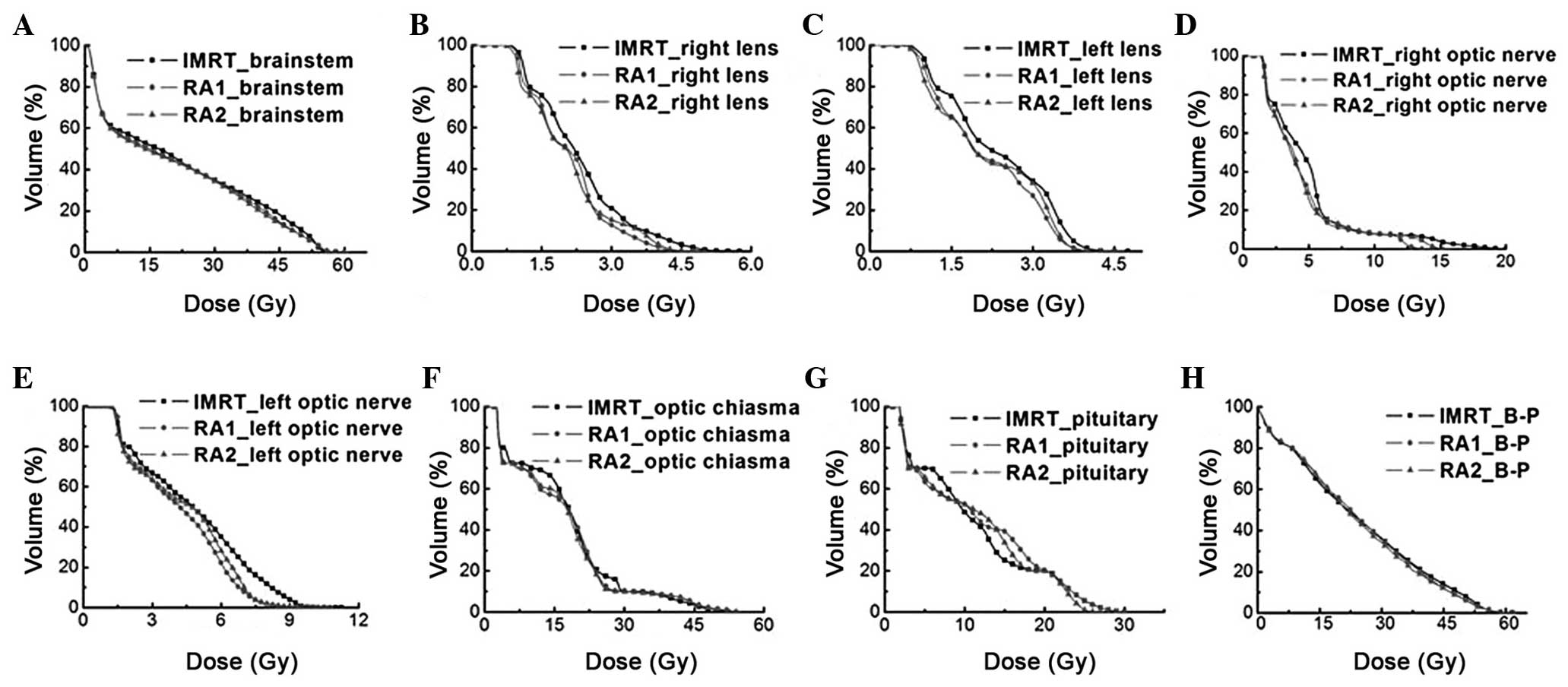 | Figure 3.Mean dose-volume histogram of patients
with IMRT, RA1, and RA2, showing results for the (A) brainstem, (B)
right lens, (C) left lens, (D) right optic nerve, (E) left optic
nerve, (F) optic chiasma, (G) pituitary and (H) B-P. IMRT,
intensity modulated radiotherapy; RA1, RapidArc with single arc;
RA2, RapidArc with dual arc; B-P, whole brain minus planned target
volume 2. |
 | Table III.CI, HI, MU per fraction and treatment
time for PGTVtb, PTV1, and PTV2 of IMRT, RA1, and RA2. |
Table III.
CI, HI, MU per fraction and treatment
time for PGTVtb, PTV1, and PTV2 of IMRT, RA1, and RA2.
| Parameter | IMRT Mean ± SD | P for IMRT vs.
RA1 | RA1 Mean ± SD | P for IMRT vs.
RA2 | RA2 Mean ± SD | P for RA1 vs.
RA2 |
|---|
| CI |
|
|
PGTVtb | 0.79±0.04 | 0.01a | 0.77±0.49 | 0.96 | 0.80±0.05 | 0.01c |
|
PTV1 | 0.88±0.01 | 0.01a | 0.85±0.02 | 0.39 | 0.87±0.02 | 0.01c |
|
PTV2 | 0.87±0.02 | 0.03a | 0.84±0.01 | 0.05 | 0.88±0.02 | 0.01c |
| HI |
|
|
PGTVtb | 0.04±0.00 | 0.03a | 0.05±0.01 | 0.10 | 0.04±0.01 | 0.02c |
|
PTV1 | 0.11±0.00 | 0.01a | 0.12±0.01 | 0.17 | 0.11±0.01 | 0.02c |
|
PTV2 | 0.23±0.01 | 0.01a | 0.24±0.02 | 0.09 | 0.23±0.01 | 0.01c |
| MU per
fraction | 630.30±98.68 | 0.01a | 363.30±40.97 | 0.01b | 356.60±37.30 | 0.45 |
| Treatment time | 302.00±25.30 |
<0.01a | 73.10±7.71 |
<0.01b | 186.50±15.83 |
<0.01c |
Discussion
IMAT (RapidArc; Varian Medical Systems, Inc.) has
been increasingly used for numerous types of tumors from different
anatomical sites, including those in the CNS. Shaffer et al
(3) compared the treatment plans in
10 cases with frontal and temporal high-grade gliomas between VMAT
with single arc and IMRT. PTV coverage, conformality and
homogeneity were shown to be equivalent in VMAT and IMRT. VMAT
significantly reduced the maximum and mean retinal, lens and
contralateral optic nerve doses compared with IMRT (P<0.05),
whereas the brainstem, chiasm and ipsilateral optic nerve doses
were similar. VMAT significantly reduced the mean MUs and treatment
time compared with IMRT. The results of the present study are
similar to those of Shaffer et al (3) on the whole; however, the CI and HI in
RA1 were inferior to those in IMRT. One explanation may be that the
different location of the gliomas led to different results. Wagner
et al (16) analyzed 11 cases
of malignant gliomas, and identified that PTV coverage was higher
for IMRT (94.7%) compared with that for RA1 (90.5%) and 3D-CRT
(81.2%). The inhomogeneity was higher for 3D-CRT (8.2 Gy) compared
with for RA1 (8.0 Gy), and lowest for IMRT (6.8 Gy). V5% of healthy
tissue, equivalent to a low-dose area, was lowest for 3D-CRT and
highest for RA1. All OARs received a slightly lower dose by RA1
compared with IMRT or 3D-CRT. The number of MUs was 1.8 times lower
for RA1 (321.1±58.8) compared with IMRT (587.8±196.2), and 1.4
times higher compared with 3D-CRT (224.0±12.6). These results were
similar to those in the present study in terms of coverage and
homogeneity of PTV, however, the present study has shown that RA1
reduced the high dose volume in B-P, but compromised on sparing
coverage and homogeneity of PTV. In contrast with the results of
the present study, Munck Af Rosenschöld et al (17) reported an RA technique that tended to
have a more conform target coverage compared with IMRT (not
significant) in malignant gliomas. Panet-Raymond et al
(4) demonstrated that significant
differences were observed in CIs, with improved CIs noted in VMAT
plans (IMRT, 0.88 and non-coplanar IMRT, 0.89 vs. VMAT, 0.917 and
non-coplanar, VMAT 0.923; P<0.05), whereas HIs were similar
across the techniques evaluated (HI, 0.99 for all techniques) in
fronto-temporal lobe high-grade glioma. It is hypothesized that the
location of lesions and differences in the treatment plan
strategies due to using co-planar or non-coplanar radiation
techniques resulted in the different results of the dosimetric
parameters in the above-mentioned studies.
The associations between the number of arcs with
RapidArc and the optimal dose distribution and complexity of target
volume have been studied previously (18). RapidArc plans have been extended to
use more than one arc. In several cases, the use of two arcs rather
than one has resulted in improved dose distributions (19). Verbakel et al (20) reported that, compared with IMRT, RA1
reduced target volume coverage and homogeneity, and RA2 improved
the dosimetric distribution in target volume with lower doses to
OARs. Similar results were made by Vanetti et al (21), who concluded that RA1 and RA2
exhibited certain improvements in sparing OARs and healthy tissue.
Target coverage and homogeneity results improved with RA2 plans
compared with those of RA1 and IMRT in head-and-neck cancer
patients. Clivio et al (22)
analyzed 10 patients with anal canal cancer who were treated with
RA1, RA2 or IMRT. All techniques resulted in similar target
coverage, and in terms of sparing OARs, RA2 was superior to RA1 and
IMRT. The present study has shown that RA1 was inferior to RA2 in
terms of coverage of PTV and in sparing OARs, and that normal brain
tissue received low-dose irradiation of malignant gliomas involving
the parietal lobe. The results reported for previous studies were
similar to those obtained in the present study.
A body of amassed evidence has indicated that
radiation can induce cancer in the human. Radiation-induced
neoplasms following fractionated radiation therapy in the CNS have
been well documented, and it is considered that the risk of
developing a radiation induced tumor is ~1–3% (23–25). Three
cases of radiation-induced neoplasms have been reported following
radiosurgery (26–28). The risk of a radiation-associated
brain tumor in survivors of childhood cancer is positively
associated with a young age at time of radiation (<6 years),
higher radiation doses (>30 Gy), and concomitant treatment with
antimetabolites (particularly in patients with thiopurine
methyltransferase deficiency) (29–31).
Information regarding radiation dose-response associations and
subsequent tumors of the CNS is sparse. Neglia et al
(29) identified statistically
significant radiation dose-response associations for gliomas and
meningiomas in childhood cancer survivors, and the relative risks
at a specified dose were higher for meningiomas than for gliomas.
IMRT has the potential to increase the number of radiation-induced
second cancers (32,33). There are two reasons why the IMRT may
result in an increase in second malignancies compared with
conventional radiotherapy. First, the change from IMRT involves the
use of more fields, and, as a consequence, a bigger volume of
normal tissue is exposed to lower doses. Secondly, delivery of a
specified dose to the isocenter from a modulated field, delivered
by IMRT, will require the accelerator to be energized for longer
(thus more monitor units are required) compared with delivering the
identical dose from an unmodulated field (34). There are estimates in the literature
that the number of MUs in an IMRT plan is two to three times higher
compared with a conventional radiotherapy plan, with an increase in
the incidence of radiation-induced secondary malignancies from
1–1.75% for patients who survive for 10 years or more (34,35). The
present study has demonstrated that RA1 and RA2 markedly reduced
the MUs per fraction, and the median and high dose volume of the
healthy brain compared with those in IMRT; therefore, RA1 and RA2
are likely to decrease the incidence of radiation-induced second
cancer in the healthy brain.
Late sequelae of radiotherapy, which appear from 6
months to a number of years following treatment, are usually
irreversible and progressive. They are considered to be due to
white matter damage from vascular injury, demyelination and
necrosis. The pathophysiology of radiation-induced neurocognitive
damage is complex, and involves intercellular and intracellular
interactions between vasculature and parenchymal cells,
particularly oligodendrocytes, which are important for myelination
(36). Corn et al (37) performed a phase I/II randomized trial
to analyze the association between white matter changes and serial
imaging scans (i.e. MRI and CT scans) that are associated with
bis-chlorethyl nitrosourea and hyper-fractionated cranial
irradiation. They observed grade 3 or worse changes in 8.3, 20.0
and 36.5% of patients in the low-, intermediate- and high-dose
groups, respectively. For a toxicity of grade 3 or worse, a
chi-squared test revealed P-values of 0.04 (low vs. intermediate
dose), 0.09 (intermediate vs. high dose), and 0.0005 (low vs. high
dose). The present study indicated that V45-V50 in RA1, and V35-V50
in RA2, of B-P were significantly less compared with those in IMRT;
therefore, RA1 and RA2 may be decrease white matter damage and
lessen the sequelae of brain irradiation.
Radiation damage to cells is not always lethal. It
is well documented that sublethal damage caused by radiation may be
repaired within hours following irradiation. Sublethal damage
repair occurs not only in normal tissues, but also in tumors, and
takes place not only between fractions, but also during
irradiation. Therefore, the treatment time of each fraction affects
the level of cell survival. As the treatment time is extended, the
biological effect of a specified dose is generally reduced. The
effect of prolonged delivery times of IMRT treatments on tumor
control has been studied by Wang et al (38). When the identical prescribed doses are
delivered with more MUs in IMRT, the clinical results may be worse
when compared with the outcomes in RapidArc with fewer MUs. Long
treatment time resulted in a reduction of local control rate. On
the other hand, prolonged beam delivery time of IMRT compared with
RapidArc may worsen the accuracy of treatment, due to increased
intrafractional patient motion; in addition, patient throughput is
reduced, with economical consequences. The present study has shown
that RA1 and RA2 significantly decreased MUs per fraction and the
treatment time compared with IMRT in gliomas involving the parietal
lobe, and the treatment time of radiotherapy was subsequently
reduced, which led to a decrease in sublethal damage repair.
Although statistically significant differences were
observed in the dosimetric parameters of specific OARs among IMRT
and the RA1 and RA2 plans, the difference between the dosimetric
parameters is small, and so it is not clear whether RA1 and RA2 are
able to reduce radiation-induced cancer and late sequelae of
radiotherapy, including brain radionecrosis and cognition
impairment. Teoh et al (39)
considered that the distinction of dosage parameters of OARs and
normal tissue between VMAT and fixed-field IMRT is less clear. The
data suggest that, for most tumor sites, VMAT and fixed-field IMRT
do produce largely equivalent target volume coverage, dose
conformity and homogeneity. The absolute difference in dosimetric
parameters reported as being statistically significant in certain
of the planning studies is comparatively small, and may not be
clinically significant. In the future, a prospective study will be
undertaken to clarify the effect of RA1 and RA2 on the rate of
radiation-induced cancer and late sequelae of radiotherapy compared
with those of IMRT. The subsequent selection of RapidArc will
depend on its availability, the size, location and morphology of
the brain tumor, and economic conditions.
Acknowledgements
The present study was supported by the Hunan
Province Development and Reform Committee Science Research Fund
(nos. 2010–1060 and 2014-463), the Hunan Province Science and
Technology Program (no. 2011SK3223), the Hunan Province Science and
Technology Program (no. 2011FJ4184), the Hunan Provincial Natural
Science Foundation of China (no. 2012JJ5043), and the
Neuro-oncology research project, Chinese Society of Neuro-oncology
(no. CNSO-2014-MSD14).
References
|
1
|
MacDonald SM, Ahmad S, Kachris S, Vogds
BJ, DeRouen M, Gittleman AE, DeWyngaert K and Vlachaki MT:
Intensity modulated radiation therapy versus three-dimensional
conformal radiation therapy for the treatment of high grade glioma:
A dosimetric comparison. J Appl Clin Med Phys. 8:47–60.
2007.PubMed/NCBI
|
|
2
|
Lorentini S, Amelio D, Giri MG, Fellin F,
Meliado G, Rizzotti A, Amichetti M and Schwarz M: IMRT or 3D-CRT in
glioblastoma? A dosimetric criterion for patient selection. Technol
Cancer Res Treat. 12:411–420. 2013.PubMed/NCBI
|
|
3
|
Shaffer R, Nichol AM, Vollans E, Fong M,
Nakano S, Moiseenko V, Schmuland M, Ma R, McKenzie M and Otto K: A
comparison of volumetric modulated arc therapy and conventional
intensity-modulated radiotherapy for frontal and temporal
high-grade gliomas. Int J Radiat Oncol Biol Phys. 76:1177–1184.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Panet-Raymond V, Ansbacher W, Zavgorodni
S, Bendorffe B, Nichol A, Truong PT, Beckham W and Vlachaki M:
Coplanar versus noncoplanar intensity-modulated radiation therapy
(IMRT) and volumetric-modulated arc therapy (VMAT) treatment
planning for fronto-temporal high-grade glioma. J Appl Clin Med
Phys. 13:38262012.PubMed/NCBI
|
|
5
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stupp R, Mason WP, Van Den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ali AN, Ogunleye T, Hardy CW, Shu HK,
Curran WJ and Crocker IR: Improved hippocampal dose with reduced
margin radiotherapy for glioblastoma multiforme. Radiat Oncol.
9:202014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chui CS, LoSasso T and Spirou S: Dose
calculation for photon beams with intensity modulation generated by
dynamic jaw or multileaf collimations. Med Phys. 21:1237–1244.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spirou SV and Chui C-S: A gradient inverse
planning algorithm with dose-volume constraints. Med Phys.
25:321–333. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bragg CM, Wingate K and Conway J: Clinical
implications of the anisotropic analytical algorithm for IMRT
treatment planning and verification. Radiother Oncol. 86:276–284.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knöös T, Wieslander E, Cozzi L, Brink C,
Fogliata A, Albers D, Nyström H and Lassen S: Comparison of dose
calculation algorithms for treatment planning in external photon
beam therapy for clinical situations. Phys Med Biol. 51:5785–5807.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ulmer W, Pyyry J and Kaissl W: A 3D photon
superposition/convolution algorithm and its foundation on results
of Monte Carlo calculations. Phys Med Biol. 50:1767–1790. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cozzi L, Dinshaw KA, Shrivastava SK,
Mahantshetty U, Engineer R, Deshpande DD, Jamema SV, Vanetti E,
Clivio A, Nicolini G and Fogliata A: A treatment planning study
comparing volumetric arc modulation with RapidArc and fixed field
IMRT for cervix uteri radiotherapy. Radiother Oncol. 89:180–191.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van't Riet A, Mak AC, Moerland MA, Elders
LH and van der Zee W: A conformation number to quantify the degree
of conformality in brachytherapy and external beam irradiation:
Application to the prostate. Int J Radiat Oncol Biol Phys.
37:731–736. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu Q, Mohan R, Morris M, Lauve A and
Schmidt-Ullrich R: Simultaneous integrated boost
intensity-modulated radiotherapy for locally advanced head-and-neck
squamous cell carcinomas. I: Dosimetric results. Int J Radiat Oncol
Biol Phys. 56:573–585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wagner D, Christiansen H, Wolff H and
Vorwerk H: Radiotherapy of malignant gliomas: Comparison of
volumetric single arc technique (RapidArc), dynamic
intensity-modulated technique and 3D conformal technique. Radiother
Oncol. 93:593–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Munck Af Rosenschöld P, Engelholm S,
Ohlhues L, Law I, Vogelius I and Engelholm SA: Photon and proton
therapy planning comparison for malignant glioma based on CT,
FDG-PET, DTI-MRI and fiber tracking. Acta Oncol. 50:777–783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guckenberger M, Richter A, Krieger T,
Wilbert J, Baier K and Flentje M: Is a single arc sufficient in
volumetric-modulated arc therapy (VMAT) for complex-shaped target
volumes? Radiother Oncol. 93:259–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Palma DA, Verbakel WF, Otto K and Senan S:
New developments in arc radiation therapy: A review. Cancer Treat
Rev. 36:393–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verbakel WF, Senan S, Cuijpers JP, Slotman
BJ and Lagerwaard FJ: Rapid delivery of stereotactic radiotherapy
for peripheral lung tumors using volumetric intensity-modulated
arcs. Radiother Oncol. 93:122–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vanetti E, Clivio A, Nicolini G, Fogliata
A, Ghosh-Laskar S, Agarwal JP, Upreti RR, Budrukkar A, Murthy V,
Deshpande DD, et al: Volumetric modulated arc radiotherapy for
carcinomas of the oro-pharynx, hypo-pharynx and larynx: A treatment
planning comparison with fixed field IMRT. Radiother Oncol.
92:111–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clivio A, Fogliata A, Franzetti-Pellanda
A, Nicolini G, Vanetti E, Wyttenbach R and Cozzi L:
Volumetric-modulated arc radiotherapy for carcinomas of the anal
canal: A treatment planning comparison with fixed field IMRT.
Radiother Oncol. 92:118–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ron E, Modan B, Boice JD Jr, Alfandary E,
Stovall M, Chetrit A and Katz L: Tumors of the brain and nervous
system after radiotherapy in childhood. N Engl J Med.
319:1033–1039. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simmons NE and Laws ER Jr: Glioma
occurrence after sellar irradiation: Case report and review.
Neurosurgery. 42:172–178. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsang RW, Laperriere NJ, Simpson WJ,
Brierley J, Panzarella T and Smyth HS: Glioma arising after
radiation therapy for pituitary adenoma. A report of four patients
and estimation of risk. Cancer. 72:2227–2233. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaido T, Hoshida T, Uranishi R, Akita N,
Kotani A, Nishi N and Sakaki T: Radiosurgery-induced brain tumor.
Case report. J Neurosurg. 95:710–713. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shamisa A, Bance M, Nag S, Tator C, Wong
S, Norén G and Guha A: Glioblastoma multiforme occurring in a
patient treated with gamma knife surgery. Case report and review of
the literature. J Neurosurg. 94:816–821. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu JS, Yong WH, Wilson D and Black KL:
Glioblastoma induction after radiosurgery for meningioma. Lancet.
356:1576–1577. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Neglia JP, Robison LL, Stovall M, Liu Y,
Packer RJ, Hammond S, Yasui Y, Kasper CE, Mertens AC, Donaldson SS,
et al: New primary neoplasms of the central nervous system in
survivors of childhood cancer: A report from the childhood cancer
survivor study. J Natl Cancer Inst. 98:1528–1537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Relling MV, Rubnitz JE, Rivera GK, Boyett
JM, Hancock ML, Felix CA, Kun LE, Walter AW, Evans WE and Pui CH:
High incidence of secondary brain tumours after radiotherapy and
antimetabolites. Lancet. 354:34–39. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walter AW, Hancock ML, Pui CH, Hudson MM,
Ochs JS, Rivera GK, Pratt CB, Boyett JM and Kun LE: Secondary brain
tumors in children treated for acute lymphoblastic leukemia at St
Jude Children's Research Hospital. J Clin Oncol. 16:3761–3767.
1998.PubMed/NCBI
|
|
32
|
Followill D, Geis P and Boyer A: Estimates
of whole-body dose equivalent produced by beam intensity modulated
conformal therapy. Int J Radiat Oncol Biol Phys. 38:667–672. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kry SF, Salehpour M, Followill DS, Stovall
M, Kuban DA, White RA and Rosen II: The calculated risk of fatal
secondary malignancies from intensity-modulated radiation therapy.
Int J Radiat Oncol Biol Phys. 62:1195–1203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hall EJ and Wuu CS: Radiation-induced
second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol
Biol Phys. 56:83–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gershkevitsh E, Clark CH, Staffurth J,
Dearnaley DP and Trott KR: Dose to bone marrow using IMRT
techniques in prostate cancer patients. Strahlenther Onkol.
181:172–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Halperin EC, Perez CA and Brady LW:
Principles and Practice of Radiation Oncology (5th). 730,
Lippincott Williams & Wilkins. 2008.
|
|
37
|
Corn BW, Yousem DM, Scott CB, Rotman M,
Asbell SO, Nelson DF, Martin L and Curran WJ Jr: White matter
changes are correlated significantly with radiation dose.
Observations from a randomized dose-escalation trial for malignant
glioma (Radiation therapy oncology group 83-02). Cancer.
74:2828–2835. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang JZ, Li XA, D'Souza WD and Stewart RD:
Impact of prolonged fraction delivery times on tumor control: A
note of caution for intensity-modulated radiation therapy (IMRT).
Int J Radiat Oncol Biol Phys. 57:543–552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Teoh M, Clark CH, Wood K, Whitaker S and
Nisbet A: Volumetric modulated arc therapy: A review of current
literature and clinical use in practice. Br J Radiol. 84:967–996.
2011. View Article : Google Scholar : PubMed/NCBI
|