Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic,
progressive fibrotic lung disease of unknown etiology, with a
median survival from diagnosis of 3–5 years. The clinical course of
individual patients with IPF is variable and unpredictable
(1). In particular, although Collard
et al (2) proposed the
definition of IPF-acute exacerbation (AE) in 2007, the etiology of
IPF-AE remains uncertain. However, there are some reports regarding
the development of IPF-AE after surgical lung resection. Reported
histological findings from lung biopsy in IPF-AE described not only
the typical usual interstitial pneumonia (UIP) pattern, but also
diffuse alveolar damage (DAD) (3).
Several therapies for IPF-AE have been introduced thus far, but
survival remains poor.
Neutrophil elastase (NE) is an elastolytic enzyme
that is released from activated neutrophils and is known to involve
lung injury, such as acute respiratory distress syndrome (ARDS)
(4). Glutathione (GSH) is the major
antioxidant involved in cell metabolism and survival (5). It is also known that IPF is
characterized by GSH deficiency in bronchoalveolar lavage fluid
(6). As described by Teramoto et
al (7), the amount of oxidized
GSH (GSSG) in the blood is significantly increased in patients with
IPF. Moreover, Muramatsu et al (8) reported that inhaled N-acetylcysteine
(NAC) monotherapy was associated with improvement of the redox
imbalance in patients with early IPF. Hence, we investigated
whether novel biomarkers, such as serum NE and redox balance
[reduced GSH (rGSH/GSSG)] were more rapid and highly sensitive
indicators in the postoperative IPF-AE setting. These biomarkers
were analyzed immediately prior to surgery, on the day of onset of
IPF-AE, and 3 days after IPF-AE.
The present study was approved by the local Ethics
Committee of the Toho University Omori Medical Center (no. 23–60;
approval date, 30/09/2011).
Case reports
Case 1
A 67-year-old man was referred to our hospital
complaining of a 2-year history of progressive dyspnea on exertion
(DOE). The patient was initially diagnosed with IPF and received
inhaled NAC monotherapy. Two years later, chest high-resolution
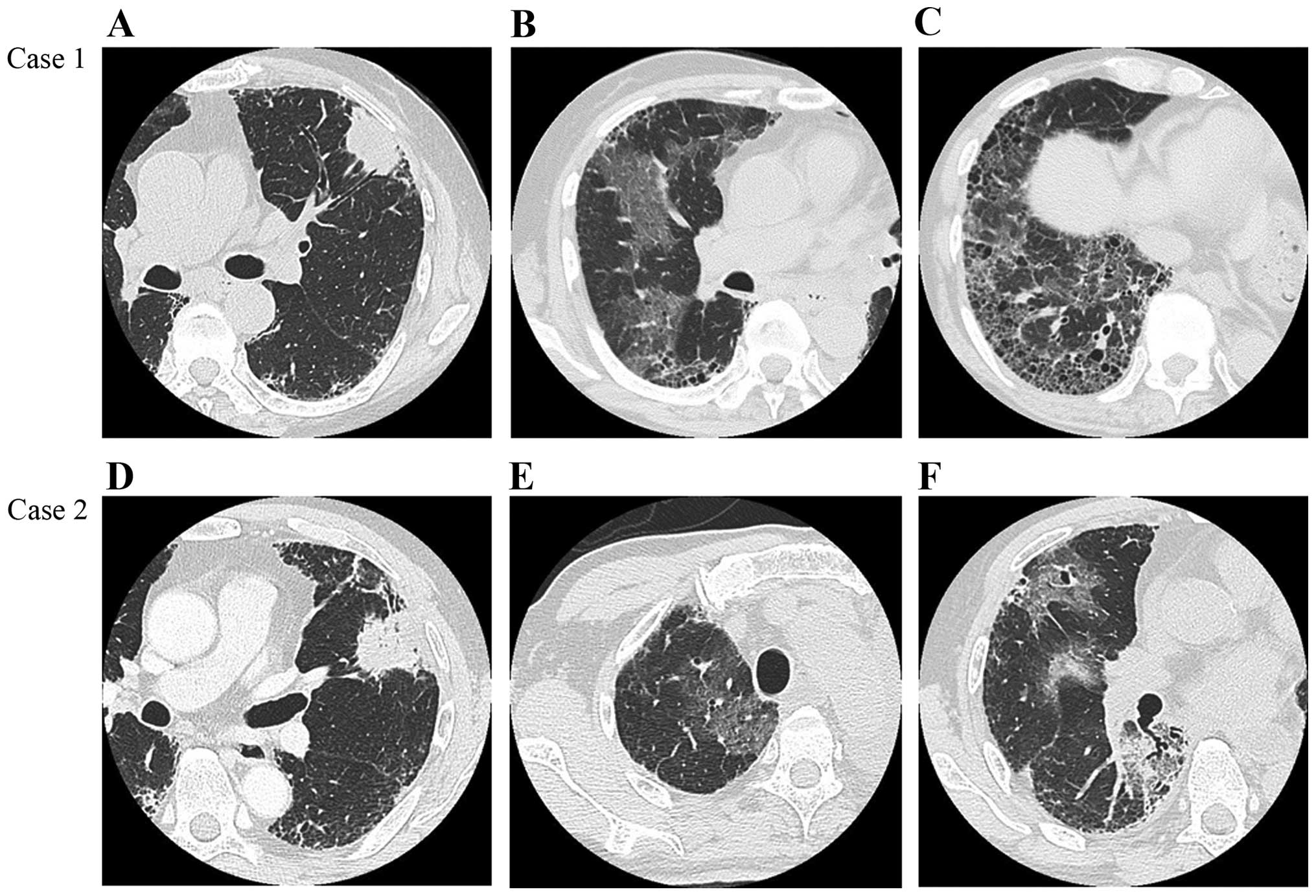
computed tomography (HRCT) scans revealed a 4-cm lobulated
subpleural mass in the left upper lobe on a background of
reticulation, with honeycombing, predominantly in the bilateral
lower lobes (Fig. 1A-C). Pulmonary
function tests (PFT) revealed a vital capacity (VC) of 2.73 l
(79.4% of predicted value), with decreased diffusing capacity of
the lungs for carbon monoxide (DLCO; 53.7% of predicted value). The
severity of IPF was stage I in accordance with the Japanese
Respiratory Society criteria (9).
Left upper lobectomy with lymph node dissection was performed
(operative time, 161 min; mean PaO2 during operation,
89.5 Torr; intraoperative blood loss, 50 ml).
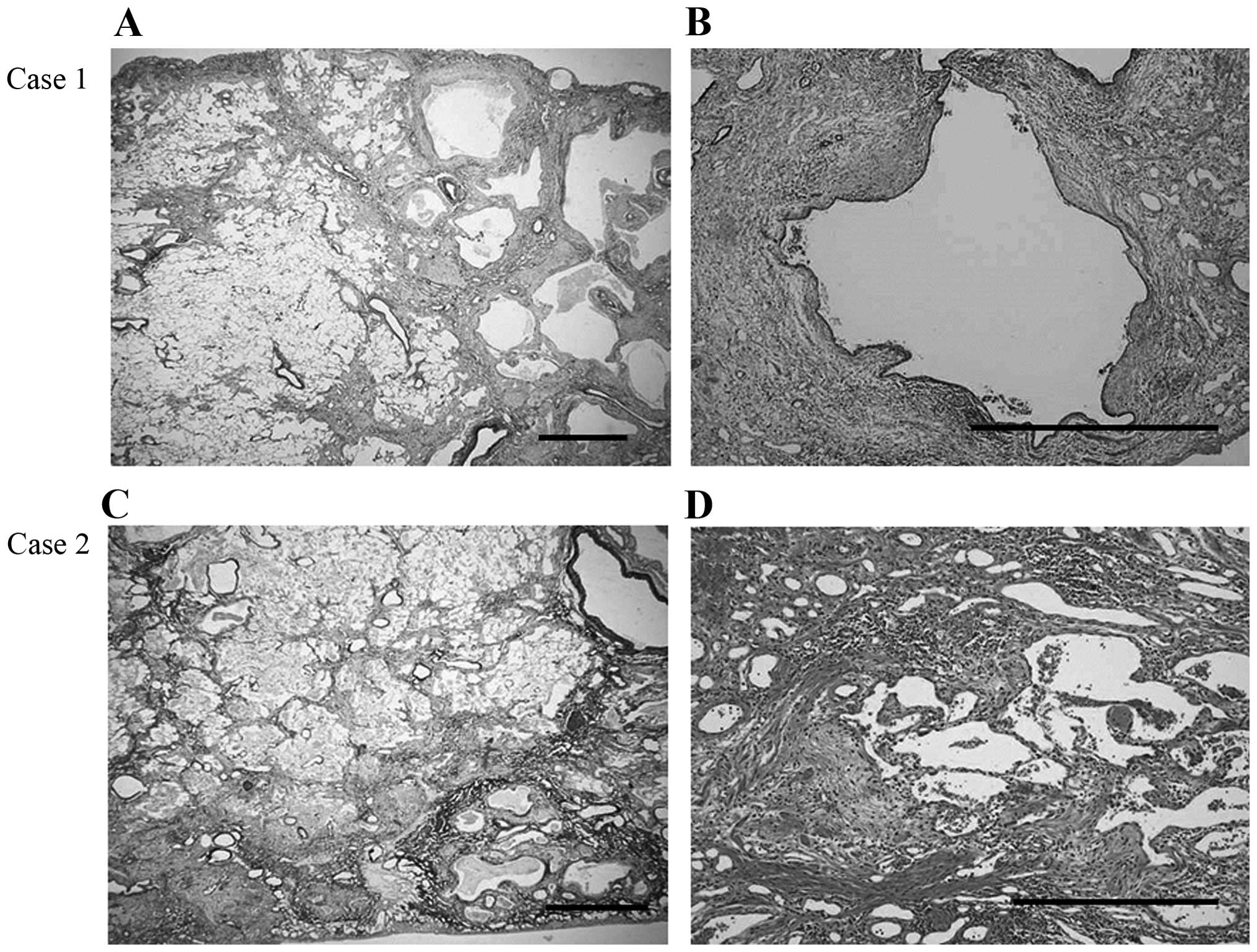
On postoperative histological examination, there was
heterogeneous interstitial fibrosis with honeycombing in a
subpleural and perilobular distribution pattern, alternating with
areas of normal lung tissue. Furthermore, fibroblastic foci were
sporadically present in a dense collagen fibrosis background, with
infiltration by abundant lymphocytes and neutrophils (Fig. 2A and B). Invasive large-cell
neuroendocrine carcinoma adjacent to the honeycomb lesions was
diagnosed (pT2aN2M0, stage IIIA). Five days after lung surgery, the
patient complained of dyspnea with acutely decreased oxygenation
(PaO2/FiO2=215). Chest HRCT scans revealed
multifocal ground-glass opacities (GGO), predominantly in the
non-operated right lung (Fig. 1B and
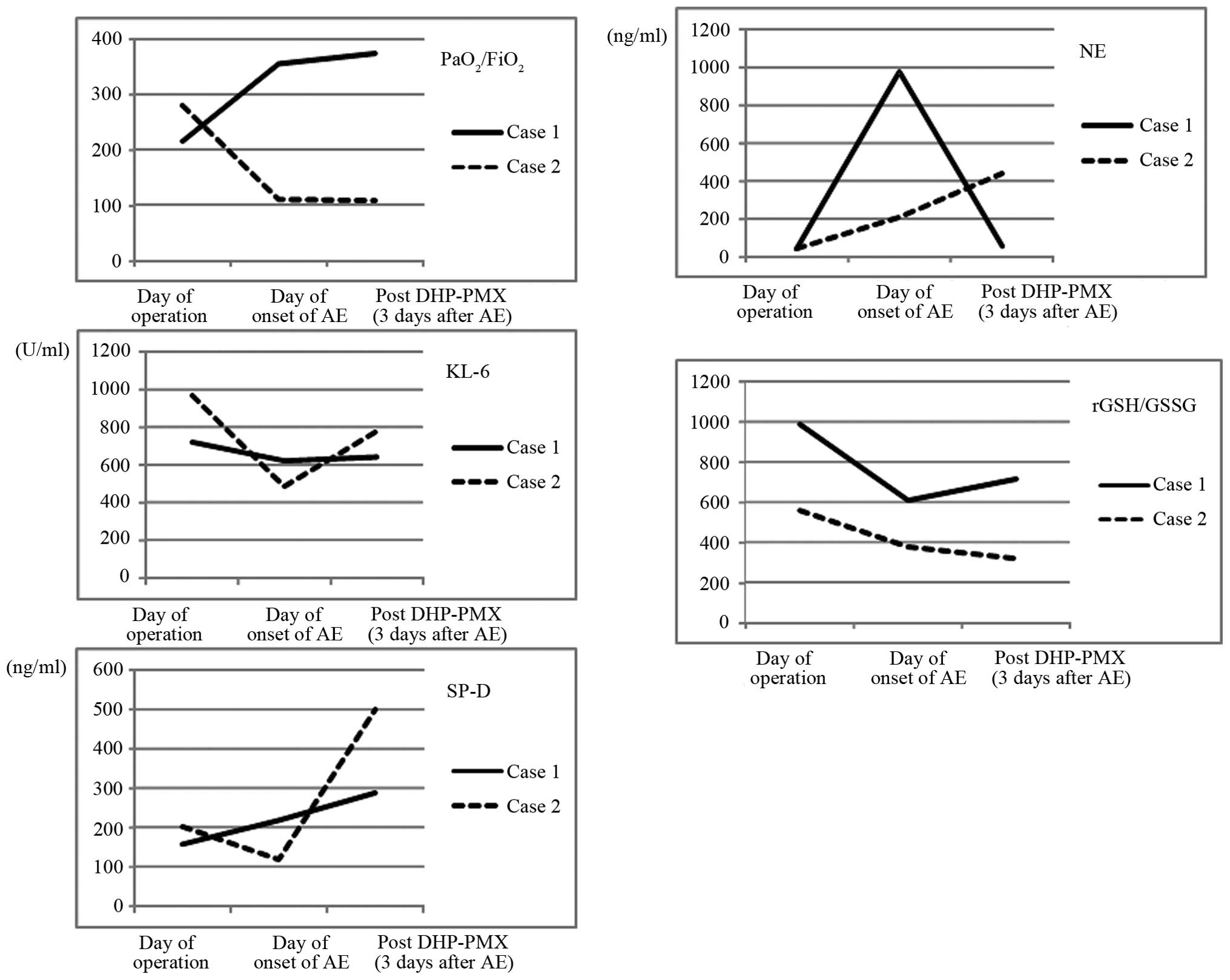
C). In addition, the levels of serum NE and GSSG were
significantly increased, with a decreased rGSH/GSSG ratio. However,
the levels of serum Krebs von den Lungen-6 (KL-6) and surfactant
protein-D (SP-D) remained unchanged (Fig.
3). After the patient was diagnosed with AE, he was treated
with methylprednisolone (1,000 mg/day) intravenously for 3 days,
followed by a tapered dose based on his respiratory condition. At
the same time, the patient received a synthetic NE inhibitor,
recombinant human soluble thrombomodulin, cyclosporine A and
pirfenidone. Furthermore, direct hemoperfusion with an immobilized
polymyxin B column (DHP-PMX) was performed once daily (6 h/day) for
2 days. Three days after the initiation of these combination
therapies, the patient's general condition had significantly
improved, with an increase in the PaO2/FiO2
ratio from 215 to 355. Moreover, the levels of serum NE and the
rGSH/GSSG ratio immediately improved (Fig. 3).
Case 2
A 67-year-old man was referred to our hospital with
suspected lung cancer, complaining of dry cough and DOE. Chest HRCT
scans showed a subpleural mass, sized 7.5 cm, in the left upper
lobe, exhibiting spiculation and air bronchogram, as well as
reticulation and faint honeycombing, predominantly in the bilateral
lower lobes (UIP pattern) (Fig.
1D-F). The PFT indicated restrictive impairment with decreased
diffusion capacity, with a VC of 2.64 l (74.6% of predicted value)
and a DLCO 61.7% of the predicted value. The patient was diagnosed
with stage I IPF and underwent left upper lobectomy with lymph node
dissection (operative time, 239 min; mean intraoperative
PaO2, 387 Torr; intraoperative blood loss, 160 ml).
The postoperative histological examination revealed
a background pattern of UIP with fibrotic changes predominantly
distributed in the subpleural and perilobular areas, and an abrupt
transition between almost-normal alveolar septa and dense fibrosis
with architectural disruption. Additionally, there were prominent
fibroblastic foci and a lymphocyte and neutrophil infiltration of
the interstitium (Fig. 2C and D).
Well-differentiated adenocarcinoma was detected adjacent to the
fibrotic lesions (pT2aN2M0, stage IIIA). Ten days after lung
surgery, the patient complained of dyspnea and the oxygenation
progressively worsened (PaO2/FiO2=281). A
chest HRCT scan revealed multifocal GGO, predominantly in the
non-operated right middle and lower lobes (Fig. 1E and F). Similar to case 1, the levels
of serum NE were increased, with a decreased rGSH/GSSG ratio
(Fig. 3). By contrast, the serum KL-6
and SP-D levels were decreased. The patient was treated with
methylprednisolone (1,000 mg/day) intravenously for 3 days,
followed by a tapered dose of 60 mg/day. At the same time, a
synthetic NE inhibitor was administered and DHP-PMX was performed
once daily (6 h/day) for 2 days. Despite these treatments, the
patient's general condition rapidly deteriorated, with the
PaO2/FiO2 ratio decreasing from 281 to 111.
Additionally, the value of the rGSH/GSSG ratio in the blood
decreased, in addition to elevation of the serum NE, KL-6 and SP-D
levels (Fig. 3). Four days after the
onset of AE, the patient succumbed to acute respiratory failure due
to IPF-AE.
Written informed consent was obtained from the
patients' next-of-kin for the publication of this manuscript and
any accompanying images.
Discussion
The incidence of postoperative IPF-AE has been
reported to range from 0 to 25%, with a mortality rate of 33.3–100%
(10). However, considering the high
risk of IPF-AE following chemotherapy or radiotherapy, surgical
lung resection is the first choice of treatment for IPF patients
with early-stage lung cancer. Sakamoto et al (11) reported that possible inciting factors
in postoperative IPF-AE may include the administration of oxygen
supplementation at a high concentration and/or surgical stress,
including mechanical ventilation-related lung injury. Padley et
al (12) reported that ARDS
following pulmonary resection occurs mainly in the non-operated
lung. In fact, intraoperative high concentrations of inspired
oxygen may have triggered the IPF-AE in case 2. Furthermore, the
present cases exhibited increased GGO on the non-surgical side
receiving single-lung ventilation. These results suggest that
hyperoxia, barotrauma, volutrauma and biotrauma may be involved in
the DAD of IPF-AE.
IPF-AE histologically manifests as acute or
organizing DAD, and less commonly as profuse organizing pneumonia
superimposed on underlying UIP (3).
Tiitto et al (13) reported
that the number of fibroblastic foci (FF) in lung samples prior to
death was associated with poor survival, but not with DAD of
patients with UIP, suggesting that the number of FF cannot predict
an IPF-AE. Although there were significant numbers of FF and
infiltration by lymphocytes and neutrophils in fibrotic lesions in
the cases presented herein, the outcomes after postoperative IPF-AE
differed. These histological findings may indicate that both
patients had highly active IPF.
NE is one of the most destructive enzymes, with the
capability of degrading almost all extracellular matrix, and plays
a crucial role in the pathophysiology of ARDS (4). Furthermore, it has been proven that the
serum NE level, in addition to serum KL-6 and SP-D levels, was
elevated in patients with IPF-AE (4).
We hypothesized that the serial changes of the serum NE level may
be associated with the onset of postoperative IPF-AE and survival.
In addition, a marked deficiency of the major antioxidant GSH has
been previously described in patients with IPF (6). Thus, administration of antioxidants such
as NAC, a precursor of GSH, is a potential treatment option for IPF
patients. Recently, Homma et al (14) reported the clinical efficacy of
inhaled NAC monotherapy in patients with early-stage IPF. More
recently, Muramatsu et al (8)
reported that inhaled NAC monotherapy was associated with improved
redox imbalance in patients with early IPF. NAC administration may
contribute to the restoration of the serum GSH balance and improve
the outcome after developing AE of IPF, as the patient in case 1
who achieved postoperative IPF-AE was treated with inhaled NAC
monotherapy for 2 years. In the 2 cases reported herein, the serial
change in the serum value of the rGSH/GSSG ratio may suggest the
possibility of predicting the onset of postoperative AE and/or
survival, along with serum NE level.
To the best of our knowledge, this is the first
study to evaluate the serial changes of GSH levels in the blood of
patients with postoperative IPF-AE associated with lung cancer.
Further studies are required to validate our methodologies and
results.
Acknowledgements
The authors are grateful to J. Tatebe (Department of
Clinical Laboratory, Toho University Omori Medical Center, Tokyo,
Japan) for the advice and analysis of the patient's GSH and NE
levels. This study was supported by the Practical Research Project
for Rare Intractable Diseases from the Japan Agency for Medical
Research and Development (AMED).
Glossary
Abbreviations
Abbreviations:
|
AE
|
acute exacerbation
|
|
IPF
|
idiopathic pulmonary fibrosis
|
|
NE
|
neutrophil elastase
|
|
GSH
|
glutathione
|
|
rGSH
|
reduced glutathione
|
|
GSSG
|
oxidized glutathione
|
|
DOE
|
dyspnea on exertion
|
|
UIP
|
usual interstitial pneumonia
|
|
DAD
|
diffuse alveolar damage
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
NAC
|
N-acetylcysteine
|
|
HRCT
|
high-resolution computed
tomography
|
|
PFT
|
pulmonary function test
|
|
VC
|
vital capacity
|
|
DLCO
|
diffusing capacity of the lungs for
carbon monoxide
|
|
GGO
|
ground-glass opacities
|
|
KL-6
|
Krebs von den Lungen-6
|
|
SP-D
|
surfactant protein-D
|
|
DHP-PMX
|
direct hemoperfusion with an
immobilized polymyxin B column
|
References
|
1
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J Respir Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Collard HR, Moore BB, Flaherty KR, Brown
KK, Kaner RJ, King TE Jr, Lasky JA, Loyd JE, Noth I, Olman MA, et
al: Acute exacerbations of idiopathic pulmonary fibrosis. Am J
Respir Crit Care Med. 176:636–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parambil JG, Myers JL and Ryu JH:
Histopathologic features and outcome of patients with acute
exacerbation of idiopathic pulmonary fibrosis undergoing surgical
lung biopsy. Chest. 128:3310–3315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawabata K, Hagio T and Matsuoka S: The
role of neutrophil elastase in acute lung injury. Eur J Pharmacol.
451:1–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hunninghake GW: Antioxidant therapy for
idiopathic pulmonary fibrosis. N Engl J Med. 353:2285–2287. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cantin AM, Hubbard RC and Crystal RG:
Glutathione deficiency in the epithelial lining fluid of the lower
respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir
Dis. 139:370–372. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teramoto S, Fukuchi Y, Uejima Y, Shu CY
and Orimo H: Superoxide anion formation and glutathione metabolism
of blood in patients with idiopathic pulmonary fibrosis. Biochem
Mol Med. 55:66–70. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muramatsu Y, Sugino K, Ishida F, Tatebe J,
Morita T and Homma S: Effect of inhaled N-acetylcysteine
monotherapy on lung function and redox balance in idiopathic
pulmonary fibrosis. Respir Investig. 54:170–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clinical diagnostic and treatment guidance
for idiopathic interstitial pneumoniaJapanese Respiratory Society's
Committee Formulating Diagnosis and Treatment Guideline for Diffuse
Lung Diseases. Nankodo; Tokyo: pp. 63–65. 2004, (In Japanese).
|
|
10
|
Watanabe A, Kawaharada N and Higami T:
Postoperative acute exacerbation of IPF after lung resection for
primary lung cancer. Pulm Med. 2011:9603162011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakamoto S, Homma S, Mun M, Fujii T,
Kurosaki A and Yoshimura K: Acute exacerbation of idiopathic
interstitial pneumonia following lung surgery in 3 of 68
consecutive patients: A retrospective study. Intern Med. 50:77–85.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Padley SP, Jordan SJ, Goldstraw P, Wells
AU and Hansell DM: Asymmetric ARDS following pulmonary resection:
CT findings initial observations. Radiology. 223:468–473. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tiitto L, Bloigu R, Heiskanen U, Pääkkö P,
Kinnula VL and Kaarteenaho-Wiik R: Relationship between
histopathological features and the course of idiopathic pulmonary
fibrosis/usual interstitial pneumonia. Thorax. 61:1091–1095. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Homma S, Azuma A, Taniguchi H, Ogura T,
Mochiduki Y, Sugiyama Y, Nakata K, Yoshimura K, Takeuchi M and
Kudoh S: Japan NAC Clinical Study Group: Efficacy of inhaled
N-acetylcysteine monotherapy in patients with early stage
idiopathic pulmonary fibrosis. Respirology. 17:467–477. 2012.
View Article : Google Scholar : PubMed/NCBI
|