Introduction
Solid pseudopapillary tumors (SPTs) of the pancreas
are a rare occurrence, accounting for 1–2% of all solid pancreatic
tumors (1). SPT is more prevalent in
females and is more frequently encountered in adolescence. The
clinical findings of SPT vary considerably (2). The patients are often diagnosed during
work-up for non-specific signs and symptoms, such as
intra-abdominal mass, abdominal pain, and/or discomfort. The
patients rarely present with an acute abdomen resulting from the
rupture of the tumor capsule. In our patient, the tumor had
ruptured as a result of blunt abdominal trauma. The patient
underwent laparotomy with the indication of an acute abdomen,
during which an SPT was identified and completely resected.
Case report
A 9-year-old female patient presented to the
emergency service with severe abdominal pain of sudden onset and
vomiting following blunt abdominal trauma (impact of a swing on her
abdomen). Upon physical examination, the patient was found to have
abdominal distension, tenderness, abdominal guarding and elevated
body temperature (>37.9°C); she was also tachycardic and mildly
hypotensive (blood pressure 80/55 mmHg). The laboratory tests
results revealed anemia (Hb 7.9 mg/dl) and leukocytosis (white
blood cell count 22.800/mm3). An abdominal X-ray
revealed dilated bowel loops and air-fluid levels. On abdominal
ultrasound, a mass measuring 120×90 mm was identified on the
pancreas, together with a generalized intra-abdominal fluid
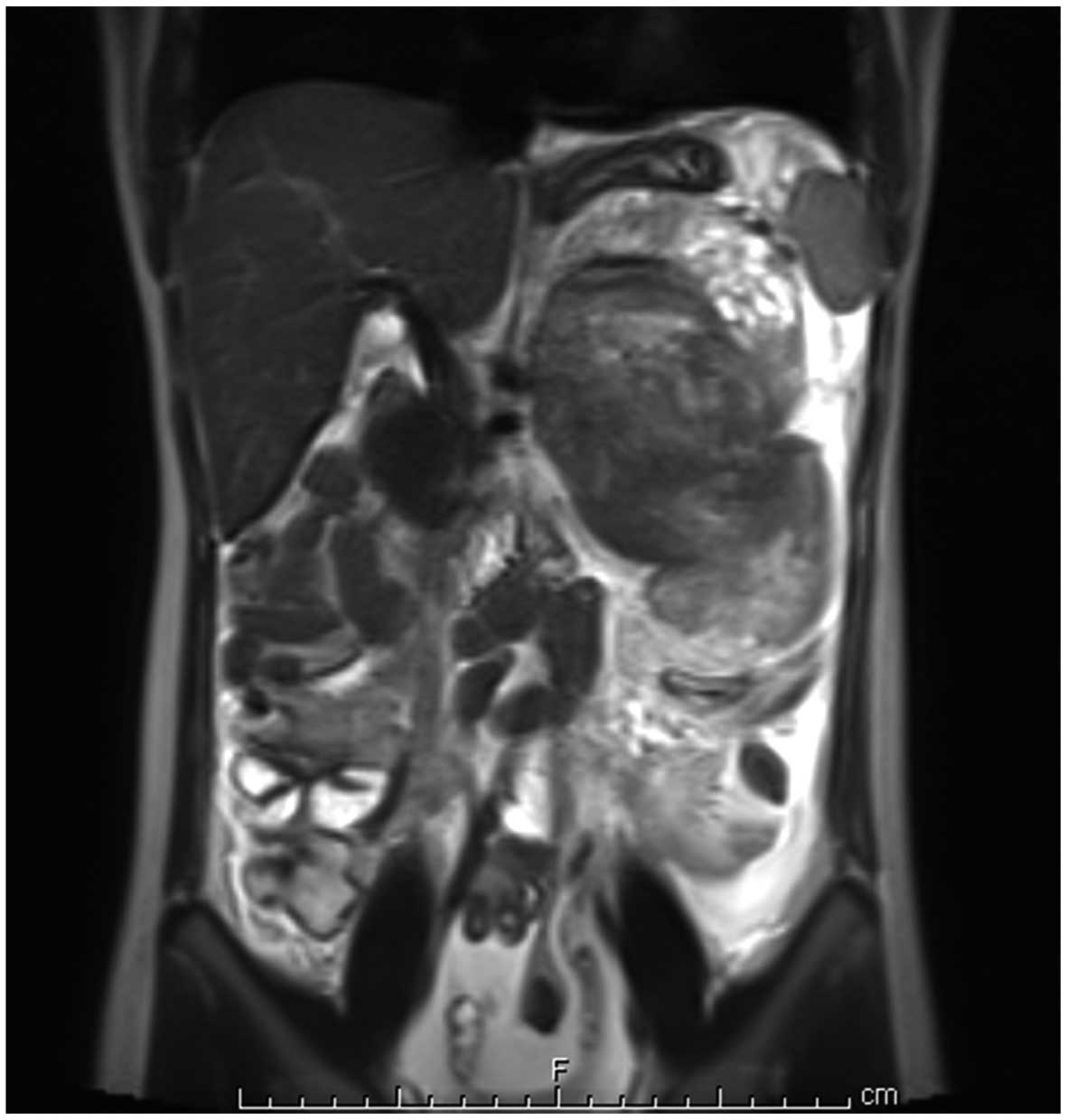
collection. Abdominal magnetic resonance imaging revealed a mass
sized 8×9×12 cm located in the pancreas, with hemorrhagic-necrotic
areas and abundant intra-abdominal fluid. The mass appeared to
originate from the inferolateral aspect of the pancreas (Fig. 1). The patient underwent surgery
following resuscitation. Intra-abdominal exploration revealed an
abundant amount of intraperitoneal hemorrhagic fluid. A mass
originating from the body and tail of the pancreas was identified,
which was adherent to the mesentery of the colon and the hilum of
the spleen, and had ruptured from its inferolateral side. The mass
was dissected from the colonic mesentery but could not be separated
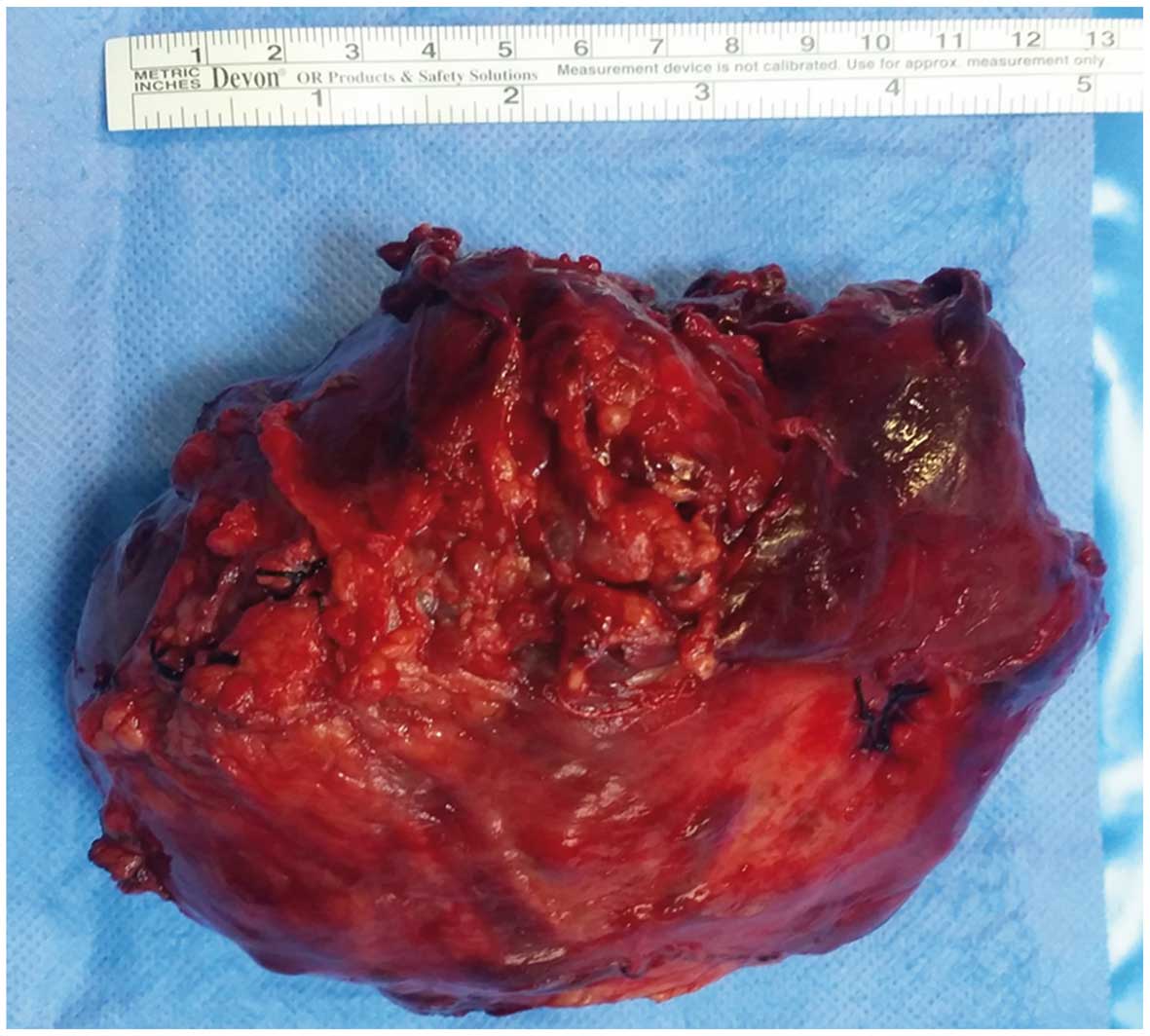
from the splenic vessels. Therefore, the tumor was extirpated
together with the spleen and the involved parts of the pancreas
(Fig. 2). Approximately 40% of
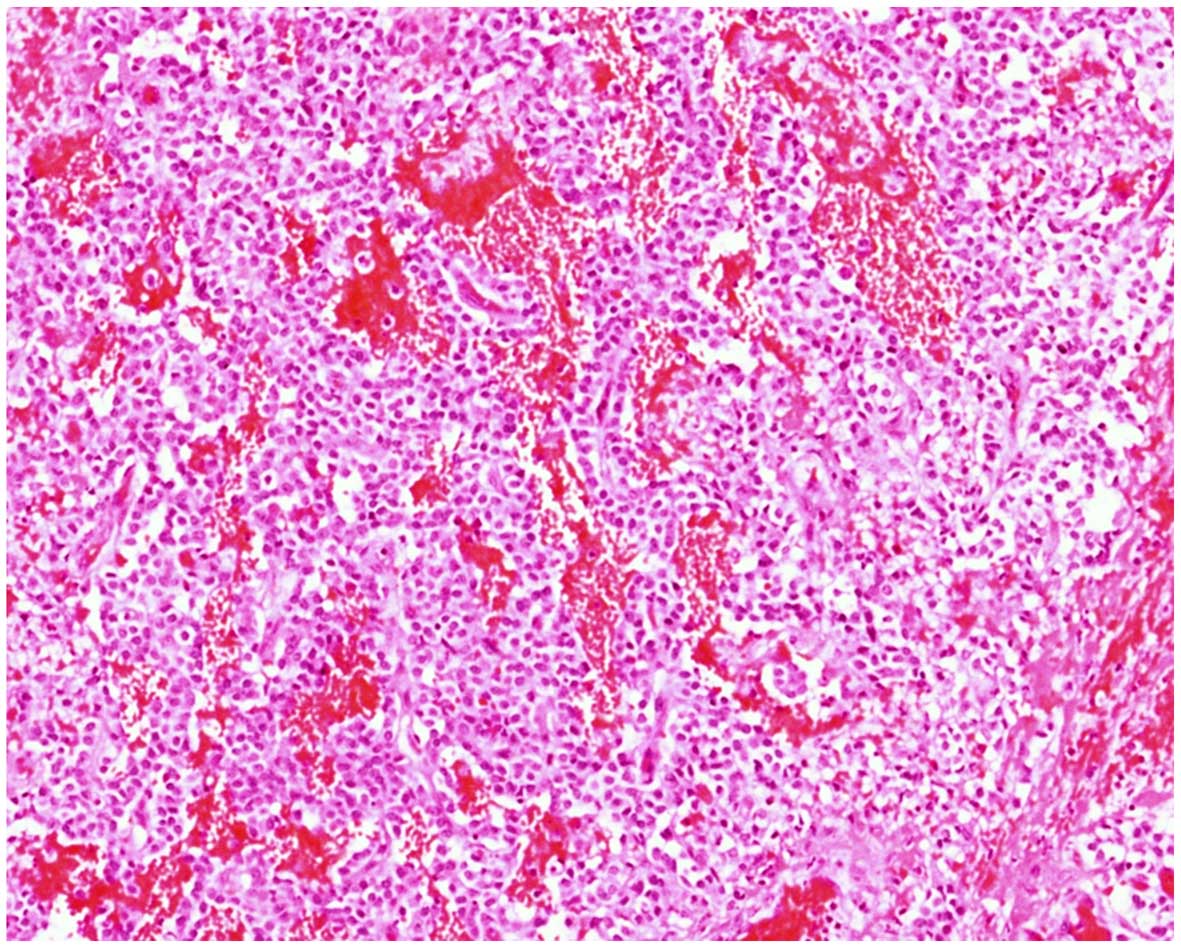
intact, healthy pancreatic tissue remained. Histopathological
examination demonstrated a solid pseudopapillary tumor of
pancreatic origin (Fig. 3). The
patient's postoperative course was clinically and metabolically
uneventful. Written informed consent was obtained from the
patient's parents for the publication of this case report and
related images.
Discussion
Although SPT is usually located in the tail and body
of the pancreas (3–5), the caput pancreas is also a frequent
location (6). Patients with SPT may
present with complaints of abdominal pain and discomfort, and the
diagnosis of SPT may be incidental (3,6). In
addition, a proportion of patients are incidentally diagnosed with
SPT during work-up for hemorrhagic complications and peritonitis
following abdominal trauma (5,7,8). However, spontaneous perforation of the
tumor without a history of trauma has been reported (9). An increased use of radiological
modalities has resulted in a 7-fold increase in the number of
patients diagnosed with SPT since 2000 (3).
According to the World Health Organization
classification, SPT is considered to be a low-grade exocrine
pancreatic malignancy (1,10). Hu et al reported that gender,
preoperative age of the patient and tumor size are not associated
with the malignant potential of SPT (11). However, in a recent study, the authors
stated that a tumor with a diameter of >5 cm as visualized on
computerized tomography (CT) scan may be an indication of
malignancy (10). However, in another
study, the solid component of the tumor was considered to be a
significant predictor of malignant potential (4). Hwang et al emphasized that a SPT
may be classified as benign or malignant based on various criteria,
including the presence/extent of perineural invasion,
angioinvasion, capsular invasion, lymph node involvement, adjacent
organ invasion and distant metastases (4). Rupture and metastasis of the tumor are
the primary causes of recurrence. Vascular involvement has also
been reported in cases with SPT, and the tumor most commonly
metastasizes to the peritoneum, liver and lymph nodes (3,4,10).
The treatment of SPT consists of total surgical
excision of the tumor mass and the adjacent pancreatic tissue.
Enucleation is not recommended, as it carries the risk of
incomplete resection. A statistically significant difference
between laparoscopic and open surgery was not detected with regard
to postoperative complications and prognosis (2,11,12). Local recurrence often occurs when the
tumor is incompletely resected or ruptures (4). In recurrent or metastatic cases,
chemotherapy and/or radiotherapy may be effective, whereas
5-fluorouracil and gemcitabine are used as adjuvant chemotherapy
(3). Since very few cases require
radiotherapy and/or chemotherapy, it is difficult to evaluate the
outcomes of these treatments (5).
Hwang et al reported on their cases of liver transplantation
for patients with multiple liver metastases (4). The average patient follow-up period was
reported to be 36–67 months (3,6,10) whereas in 1 patient the follow-up
period was 7 years (5).
Among pediatric patients, SPT rarely presents with
traumatic rupture. Tajima et al (7) presented the case of a 12-year-old female
patient who presented with an acute abdomen secondary to abdominal
trauma. SPT rupture was detected in the patient, and she underwent
urgent surgery for hemostasis, drainage and biopsy. The biopsy
result was consistent with SPT. Five weeks later, the patient
underwent an exploratory laparotomy, and the mass was found to be
localized to the head of the pancreas; it had not metastasized into
the intra-abdominal cavity, and the surrounding organs were totally
extirpated with pylorus-sparing pancreoduodenectomy. At the 7-year
postoperative follow-up visit, an abdominal CT scan revealed
metastatic nodules; these were subsequently removed with a third
operation and pathologically confirmed to be SPT recurrence.
Park et al (13) reported the case of another pediatric
patient with hemoperitoneum due to SPT rupture. During the first
operation, laparotomy was performed that revealed the presence of
an unresectable mass. Therefore, only hemostatic control and a
biopsy were performed. Subsequently, the patient received 3 cycles
of chemotherapy. Three months later, during a second-look
laparotomy, the patient underwent subtotal pancreatectomy and
splenectomy. During the postoperative follow-up visit, portal vein
thrombosis and liver metastasis were detected, necessitating
additional chemotherapy with radiofrequency ablation. On a
follow-up visit 97 months later, the patient was disease-free,
although she exhibited portal vein obliteration and cavernous
transformation.
Our patient presented with similar manifestations. A
one-stage operation was performed, during which the patient
received subtotal pancreactomy, splenectomy and hemostatic control.
No additional treatment was applied. The 1-year follow-up visit
revealed no medical problems. This patient remains under close
surveillance.
In conclusion, pancreatic SPT is a rare tumor with a
low malignant potential. The optimal treatment for the tumor is
total surgical excision. Patients with metastatic disease or a
ruptured SPT should be closely followed up for tumor recurrence.
Tumor markers have not been found to be helpful for postoperative
monitorıng (14).
References
|
1
|
Bhatnagar R, Olson MT, Fishman EK, Hruban
RH, Lennon AM and Ali SZ: Solid-pseudopapillary neoplasm of the
pancreas: Cytomorphologic findings and literature review. Acta
Cytol. 58:347–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takamatsu S, Nagano H, Ohtsukasa S,
Kawachi Y and Maruyama H: A case of spontaneous ruptured solid
pseudopapillary tumor of pancreas resected by laparoscopic surgery.
Case Rep Med. 2013:9532402013.PubMed/NCBI
|
|
3
|
Law JK, Ahmed A, Singh VK, Akshintala VS,
Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, et al: A
systematic review of solid-pseudopapillary neoplasms: Are these
rare lesions? Pancreas. 43:331–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hwang J, Kim DY, Kim SC, Namgoong JM and
Hong SM: Solid-pseudopapillary neoplasm of the pancreas in
children: Can we predict malignancy? J Pediatr Surg. 49:1730–1733.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang HL, Shih SC, Chang WH, Wang TE, Chen
MJ and Chan YJ: Solid-pseudopapillary tumor of the pancreas:
Clinical experience and literature review. World J Gastroenterol.
11:1403–1409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki S, Hatori T, Furukawa T, Shiratori
K and Yamamoto M: Clinical and pathological features of solid
pseudopapillary neoplasms of the pancreas at a single institution.
Dig Surg. 31:143–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tajima Y, Kohara N, Maeda J, Inoue K,
Kitasato A, Natsuda K, Irie J, Adachi T, Kuroki T, Egochi S and
Kanematsu T: Peritoneal and nodal recurrence 7 years after excision
of a ruptured solid pseudopapillary neoplasm of the pancreas:
Report of a case. Surg Today. 42:776–780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spătaru RI, Enculescu A and Popoiu MC:
Gruber-Frantz tumor: A very rare pathological condition in
children. Rom J Morphol Embryol. 55:1497–1501. 2014.PubMed/NCBI
|
|
9
|
Pattanshetti VM, Vinchurkar K and
Pattanshetti SV: Solid pseudo papillary tumor of pancreas:
Presenting as acute abdomen in a female child. Indian J Med
Paediatr Oncol. 35:184–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim MJ, Choi DW, Choi SH, Heo JS and Sung
JY: Surgical treatment of solid pseudopapillary neoplasms of the
pancreas and risk factors for malignancy. Br J Surg. 101:1266–1271.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu S, Lin X, Song Q and Chen K: Solid
pseudopapillary tumour of the pancreas in children: Clinical and
computed tomography manifestation. Radiol Med. 117:1242–1249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morita K, Urushihara N, Fukumoto K, Miyano
G, Yamoto M, Nouso H, Miyake H and Kaneshiro M: Solid
pseudopapillary tumor of the pancreas in children: Surgical
intervention strategies based on pathological findings. Pediatr
Surg Int. 30:253–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JY, Kim SG and Park J: Solid
pseudopapillary tumor of the pancreas in children: 15-year
experience at a single institution with assays using an
immunohistochemical panel. Ann Surg Treat Res. 86:130–135. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yagcı A, Yakan S, Coskun A, Erkan N,
Yıldırım M, Yalcın E and Postacı H: Diagnosis and treatment of
solid pseudopapillary tumor of the pancreas: Experience of one
single institution from Turkey. World J Surg Oncol. 11:3082013.
View Article : Google Scholar : PubMed/NCBI
|