Introduction
Pancreatic cancer is projected to surpass breast,
prostate, and colorectal cancers to become the second leading cause
of cancer-related death by 2030. It is associated with extremely
poor prognosis (1,2). Patients with advanced pancreatic
carcinoma usually present some extent of accompanying fatigue.
After the introduction of recent stronger regimen of chemotherapy,
patients presented a high incidence of fatigue (3,4). Fatigue
impairs patients' physical and mental energy, directly and
indirectly influencing the sustainability of chemotherapy.
Nab-paclitaxel plus gemcitabine is a standard therapy and a
promising treatment for unresectable pancreatic cancer (URPC)
including metastatic/locally advanced pancreatic cancer (3). In an international multicenter
open-label randomized phase 3 study, Von Hoff et al reported
the considerable frequency of treatment-related adverse events
rated grade 3 or higher of the nab-paclitaxel plus gemcitabine
therapy as neutropenia (38%), leucopenia (31%), fatigue (17%), and
peripheral neuropathy (17%) (3).
Frequency and degree gradually rise, particularly during
chemotherapy, significantly impacting on quality of life of URPC
patients. Quality of life is easily impaired by fatigue and
peripheral neuropathy in patients who receive nab-paclitaxel plus
gemcitabine therapy (5).
Patient-reported outcomes are useful for evaluating adverse effects
that are difficult to assess objectively by physicians. Functional
Assessment of Chronic Illness Therapy-Fatigue (FACIT-F)
questionnaire (6,7) and Patient Neurotoxicity Questionnaire
(PNQ) (8) have been utilized to
assess patient-reported outcomes of cancer treatments such as
chemotherapy or chemoradiotherapy to adjust to sustainable dosage
of agents. However, the feasibility and sensitivity of fatigue
assessment in patients receiving recent stronger chemotherapy
regimen is unknown.
We prospectively investigated the feasibility and
validity of a patient-based scale, FACIT-F Questionnaire, PNQ,
toxicities, and adverse effects evaluated in accordance with Common
Terminology Criteria for Adverse Events (CTCAE) version 4.0
(9) for cumulative fatigue and
peripheral neuropathy in the timing of the introduction of
nab-paclitaxel plus gemcitabine therapy. The data from this study
could be useful for identifying appropriate timing and duration of
medical intervention for supporting therapy in nab-paclitaxel plus
gemcitabine therapy.
Patients and methods
Patients
The present study was approved by the Wakayama
Medical University Hospital Institutional Review Board (approval
no. 1771). This trial is registered on the UMIN Clinical Trials
Registry (UMIN000021758). We excluded patients with severe
comorbidity, such as severe cardiac/renal failure or bowel
obstruction and those unable to intake oral medicine, to facilitate
comparison of data with the next interventional trial
(UMIN000025606) where endpoints are defined identically to this
study. Criteria of eligible patients enrolled in this study were as
follows: Patients with URPC who received nab-paclitaxel plus
gemcitabine therapy as a first line chemotherapy and had an Eastern
Cooperative Oncology Group (ECOG) PS of 0 or 1; ≥20 years old.
Written informed consent to participate in this study was also
required. Additionally, the following criteria had to be satisfied
in laboratory tests within 14 days of registration: WBC count
≥3,500/mm3 and ≤12,000/mm3, neutrophil count
≥1,500/mm3, Hb ≥9.0 g/dl, Plt ≥100,000/mm3,
T.Bil ≤2.0 mg/dl (≤3.0 mg/dl in biliary drainage case), serum Cr
≤1.5 mg/dl, and AST, ALT≤100 IU/l. Patients with URPC during the
period of this study received nab-paclitaxel plus gemcitabine
therapy as a first line chemotherapy in our institute. In this
study, locally advanced pancreatic cancer was defined according to
National Comprehensive Cancer Network (NCCN) version 2.2016
criteria (10).
Endpoints
The primary endpoint of this study was to
investigate the feasibility and validity of fatigue evaluation by
the FACIT-F version 4 questionnaire and additional concerns
(Japanese version) for URPC patients. Secondary endpoints included:
Appetite loss, degree of pain and sensory disorder evaluated by
Numerical Rating Scale (NRS), cumulative sensory/motor
neurotoxicity with Patient Neurotoxicity Questionnaire (PNQ).
Treatment
Enrolled patients were administered a 30-min
intravenous infusion of nab-paclitaxel at a dose of 125
mg/m2, followed by a 30-min intravenous infusion of
gemcitabine at a dose of 1,000 mg/m2, on days 1, 8 and
15 over a four-week period as one cycle of regimen similar to that
previously reported (5). There was
one week of rest between each cycle. The criteria for restart, dose
reduction, and discontinuation of chemotherapy were also as
previously reported (5). Treatment
was repeated until disease progression or toxicity levels became
unacceptable, or when discontinuation was decided by the
investigators or by patient refusal. In the absence of disease
progression, patients continued chemotherapy.
Assessments
Enrolled patients completed FACIT-F (version 4) and
questionnaires about additional concerns, an NRS test about
appetite loss, degree of pain and sensory disorder (cold, burning)
and PNQ before administration of nab-paclitaxel plus gemcitabine.
Questionnaires and tests were completed on registration day and
weekly thereafter during therapy on days 1, 8, 15, 22, 29, 36, 43
and 50 over an eight-week period as the first two cycles of
continuous regimen. FACIT-F was evaluated by degree and each degree
was converted to numerical values as follows: 0: Not at all; 1: A
little bit; 2: Somewhat; 3: Quite a bit; 4: Very much. Total values
were recorded weekly for each questionnaire. Appetite loss, degree
of pain and sensory disorder were evaluated by NRS converting to
0–10, cumulative sensory/motor neurotoxicity with PNQ converting to
0–4.
Toxicities and adverse effects of chemotherapy were
evaluated in accordance with Common Terminology Criteria for
Adverse Events [CTCAE] version 4.0. Complete blood counts and
differential count of leukocytes, blood chemical tests and physical
examinations were carried out at least once per week until the end
of the two cycles and every two weeks thereafter. In cases of grade
4 hematological toxicity, re-examination within four days was
required. We carried out computed tomography when the tumor marker
was extremely elevated. Tumor response was reviewed in accordance
with Response Evaluation Criteria in Solid Tumors (RECIST) version
1.1.
Statistical analysis
We evaluated the total score of questionnaires using
linear-mixed effect modeling with patients as a random effect and
treatment courses and weeks as fixed effects.
Results
Patient characteristics
Between April 2016 and September 2016, 10
consecutive patients with unresectable pancreatic cancer (URPC)
including metastatic (n=4)/locally advanced pancreatic (n=6) cancer
were registered and scheduled for nab-paclitaxel plus gemcitabine
therapy. Baseline compliance with completion of FACIT-F, NRS, and
PNQ revealed no data deficit: 100% were completed in all
questionnaires or tests from all the patients. Table I shows the characteristics of the
analyzed patients. No patients in this study discontinued
chemotherapy.
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
|
Characteristica | Value |
|---|
| Baseline |
|
| Sex
(male/female) | 5/5 |
| Age,
years | 63±10.3 |
| Location
of pancreatic cancer (body-tail/head) | 4/6 |
| Comorbidity, patient
no. |
|
| Diabetes
mellitus | 3 |
|
Hypertension | 1 |
| Biliary
stent or drainage | 6 |
| Performance
status |
|
| 0 | 10 |
| 1 | 0 |
| 2 | 0 |
| 3 | 0 |
| 4 | 0 |
| Frequency of
administration | 5.0±0.9 |
| Skipping of
administration | 1.0±0.9 |
| Dose reduction | 5 |
| Metastatic/locally
advanced | 4/6 |
| UICC stage |
|
| IIA | 1 |
| IIB | 0 |
| III | 5 |
| IV | 4 |
| Discontinuation of
chemotherapy | 0 |
| Response
evaluation |
|
| Partial
response | 1 |
| Stable
disease | 8 |
|
Progressive disease | 1 |
| Decrease rate of CA
19-9 value | 0.50±0.5 |
Endpoints
Table II shows
patient-reported outcomes by means of FACIT-F fatigue evaluation in
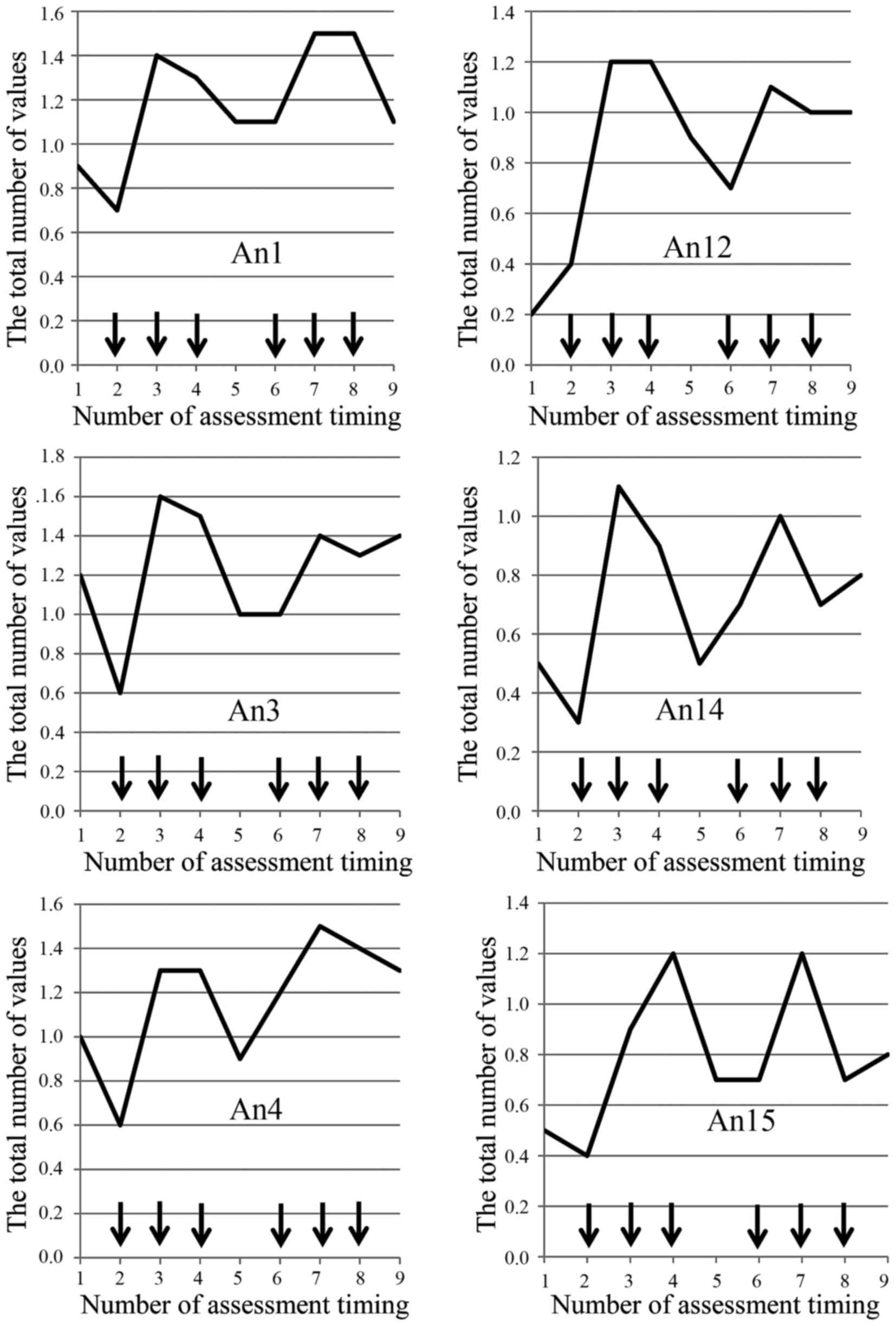
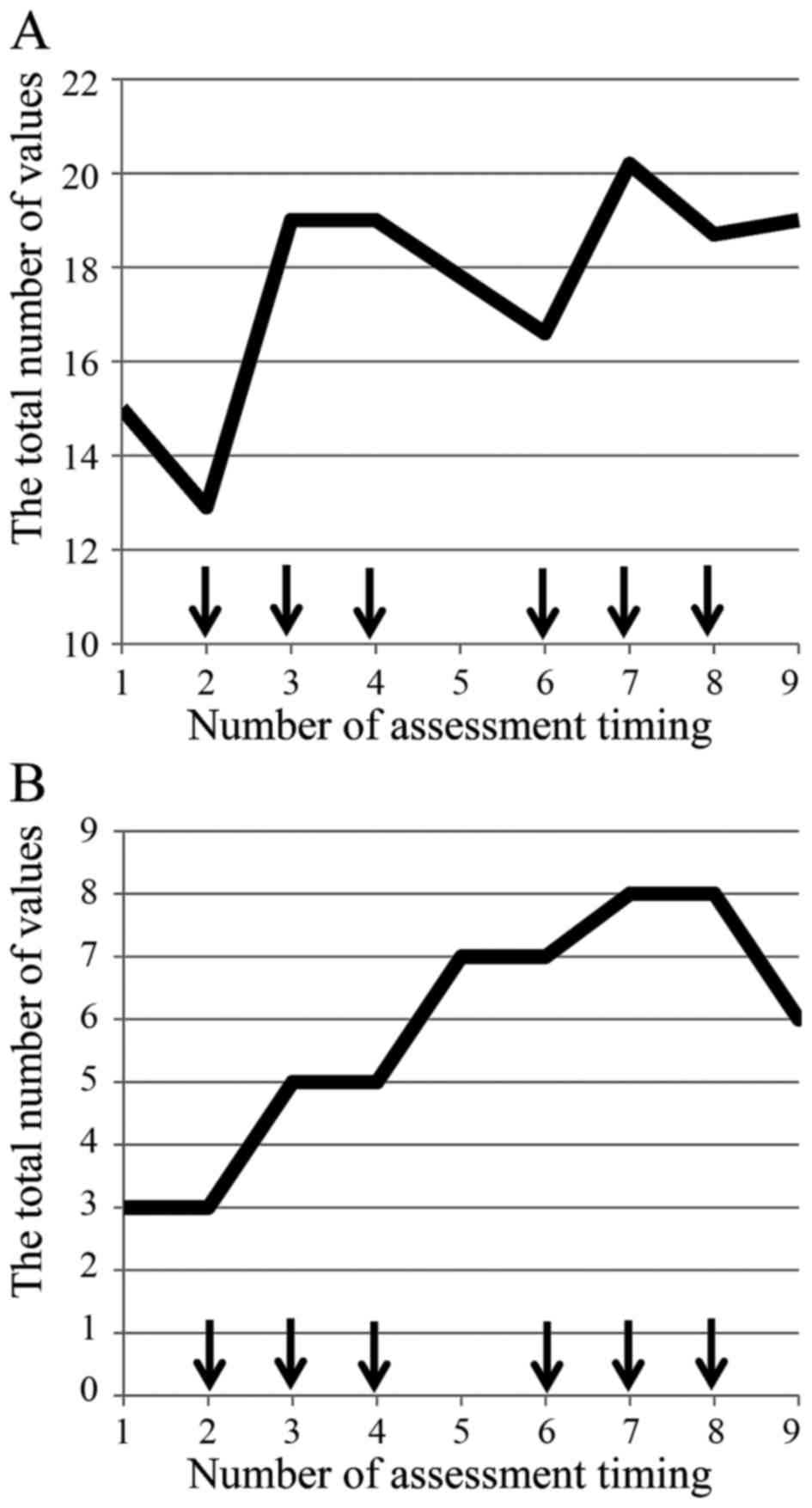
the present study. The total of each mean value of the
questionnaire also showed spike patterns in the time-sequence
diagram (Fig. 1A) and the
fluctuation range based on the maximum and minimum values was 7.3
(12.9–20.2), an increased rate from baseline 35%. The value of the
first day of the second course was elevated compared to that of the
first course.
 | Figure 1.(A) FACIT-F was evaluated by the mean
of degree, and each degree was converted to numerical values as
follows: 0: Not at all; 1: A little bit; 2: Somewhat; 3: Quite a
bit; 4: Very much. Total values were recorded weekly for each
questionnaire. The total value of means in each questionnaire show
a spike pattern in time-sequence diagram. (B) Fatigue evaluated by
CTCAE revealed only an increase in the number of patients who were
evaluated as all grade fatigue without showing any patterns.
Enrolled patients were administered intravenous infusion of
nab-paclitaxel, followed by intravenous infusion of gemcitabine
with two cycles, on days 1, 8 and 15 over a four-week period as one
cycle of regimen (arrow). Vertical lines show the total number of
values assessed by each assessment tool, and horizontal lines
represent the number of assessment timing, i.e., 1–9, on
registration day, and weekly assessment during therapy on days 1,
8, 15, 22, 29, 36, 43 and 50 over an eight-week period as first two
cycles of consecutive regimen. |
 | Table II.FACIT-F (version 4) questionnaire
additional concerns. |
Table II.
FACIT-F (version 4) questionnaire
additional concerns.
| Question | Baseline
(Degree) | Maximum of mean
(Mean) | Increase rate
(%) |
|---|
| HI7 |
|
|
|
| I feel
fatigued | 1.4 (0–2) | 1.9 (1.3–1.9) | 36 |
| HI12 |
|
|
|
| I feel
weak all over | 1.8 (0–4) | 1.9 (1.6–1.9) |
6 |
| An1 |
|
|
|
| I feel
listless (‘washed out’) | 0.9 (0–2) | 1.5 (0.7–1.5) | 67 |
| An2 |
|
|
|
| I feel
tired | 1.6 (1–3) | 1.9 (1.1–1.9) | 19 |
| An3 |
|
|
|
| I have
trouble starting things because I am tired | 1.2 (0–3) | 1.6 (0.6–1.6) | 33 |
| An4 |
|
|
|
| I have
trouble finishing things because I am tired | 1.0 (0–3) | 1.5 (0.6–1.5) | 50 |
| An5 |
|
|
|
| I have
energy | 1.6 (0–2) | 2.3 (1.6–2.3) | 44 |
| An7 |
|
|
|
| I am able
to do my usual activities | 2.3 (0–4) | 2.9 (2.1–2.9) | 26 |
| An8 |
|
|
|
| I need to
sleep during the day | 1.2 (0–2) | 1.8 (1.0–1.8) | 50 |
| An12 |
|
|
|
| I am too
tired to eat | 0.2 (0–1) | 1.2 (0.4–1.2) | 500 |
| An14 |
|
|
|
| I need
help doing my usual activities | 0.5 (0–2) | 1.1 (0.3–1.1) | 120 |
| An15 |
|
|
|
| I am
frustrated by being too tired to do the things I want to do | 0.5 (0–2) | 1.2 (0.4–1.2) | 140 |
| An16 |
|
|
|
| I have to
limit my social activity because I am tired | 1.0 (0–3) | 1.6 (0.8–1.6) | 60 |
Table III shows the
results of the mixed effect model. There were no significant
differences in each of the fixed effects. However, fatigue
evaluated by CTCAE revealed increase only in the number of patients
who were evaluated as all grade fatigue without any patterns
(Fig. 1B). The mean maximum values
of fatigue degree increased from mean baseline values in all
categories of questionnaire (6–500%). In addition, the degree of
fatigue shows a spike pattern over a four-week scheduled period as
one cycle of regimen in time-sequence diagram regarding ten of
thirteen (77%) questionnaires (HI12, An1, An2, An3, An4, An8, An12,
An14, An15, An16) (Fig. 2).
Secondary endpoints did not reveal any specific patterns in
appetite loss, but the degree of pain and sensory disorder
evaluated by NRS revealed a spike pattern in the number of patients
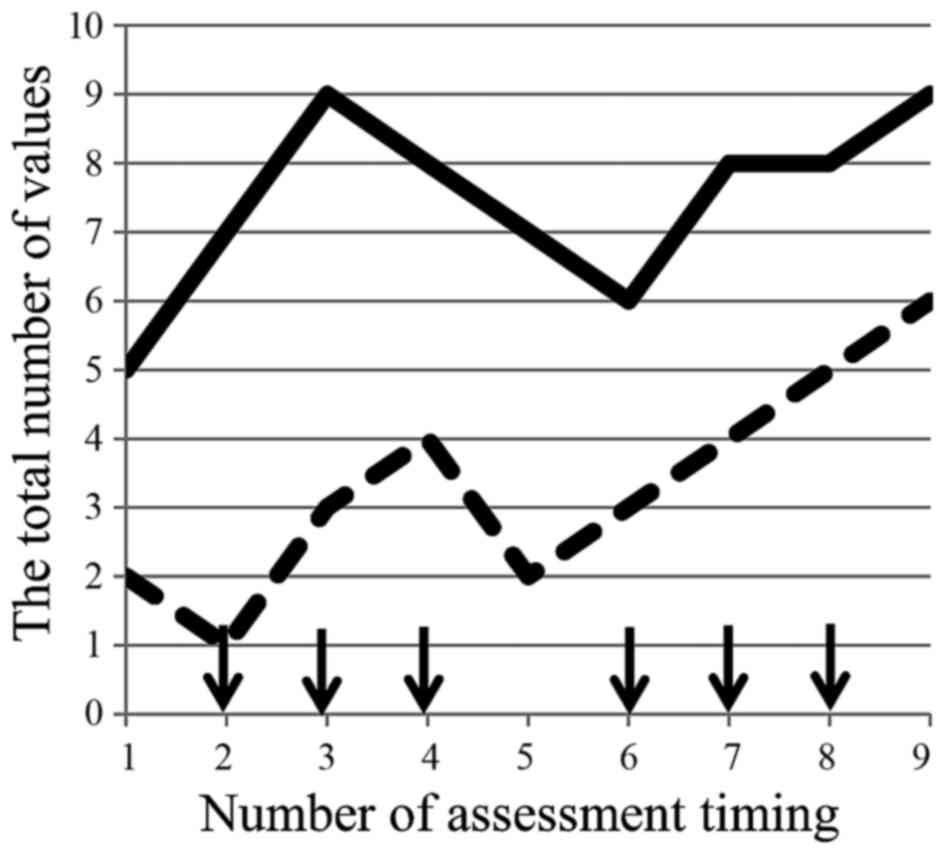
(data not shown). PNQ concerning sensory/motor disorder
demonstrated a spike pattern and increase from the baseline as the
number of administrations (Fig. 3).
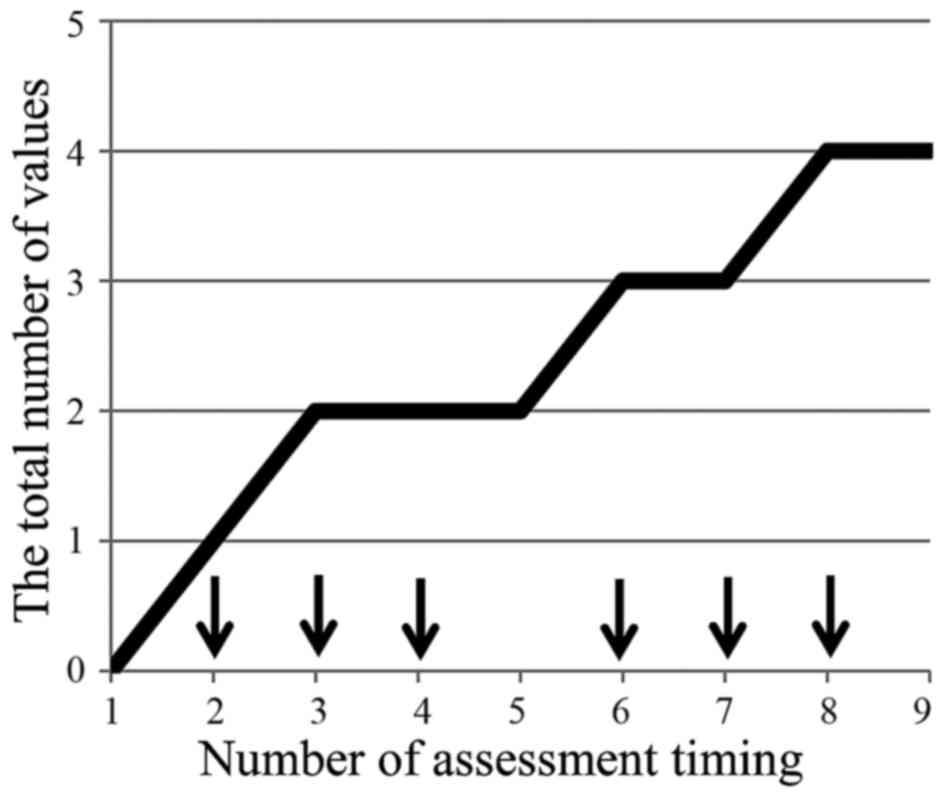
No patients presented burning pain, but the incidence of cold
sensory disorder increased with the number of administrations
(Fig. 4).
 | Table III.Results of mixed effect model. |
Table III.
Results of mixed effect model.
| Fixed effect | P-value | Coefficients |
|---|
| Courses | 0.299 | 1.55 |
| Weeks | 0.137 | 1.04 |
Toxicities and adverse effects
Adverse drug reactions deemed to be potentially
related to the nab-paclitaxel plus gemcitabine therapy are shown in
Table IV. The overall rate of any
grade events (CTCAE ver. 4.0 criteria) during the treatment was
100%. The overall rate of patients who presented grade 3 and 4
events was 80%. The majority of these adverse events represented
leucopenia (80%), appetite loss (80%), hair loss (90%), and fatigue
(90%). There were no incidences of serious adverse events such as
febrile neutropenia, sepsis, grade three or higher interstitial
pneumonia, and no treatment-related deaths in this study (Table IV).
 | Table IV.Toxicity following treatment with
neoadjuvant nab-paclitaxel plus gemcitabine therapy. |
Table IV.
Toxicity following treatment with
neoadjuvant nab-paclitaxel plus gemcitabine therapy.
| Treatment
toxicity | All grade | G3 | G4 |
|---|
| Leucopenia | 8 (80) | 5 (50) | 0 |
| Anemia | 1 (10) | 0 | 0 |
| Thrombocytopenia | 4 (40) | 1 (10) | 0 |
| Neutropenia | 5 (50) | 3 (30) | 1 (10) |
| Liver
dysfunction | 2 (20) | 0 | 0 |
| Appetite loss | 8 (80) | 0 | 0 |
| Nausea | 6 (60) | 0 | 0 |
| Vomit | 1 (10) | 0 | 0 |
| Diarrhea | 0 | 0 | 0 |
| Fatigue | 9 (90) | 2 (20) | 0 |
| Oral
inflammation | 3 (30) | 0 | 0 |
| Hand foot
syndrome | 0 | 0 | 0 |
| Hair loss | 9 (90) | 0 | 0 |
| Febrile
neutropenia | 0 | 0 | 0 |
| Cholangitis | 0 | 0 | 0 |
| Interstitial
pneumonia | 0 | 0 | 0 |
| Peripheral
sensory |
|
|
|
|
Neuropathy | 3 (30) | 0 | 0 |
Discussion
Fatigue is a common lasting symptom in most patients
who receive chemotherapy, its control is a key to the
sustainability of chemotherapy treatment. Although fatigue affects
quality of life in patients with advanced pancreatic cancer,
detailed change of fatigue levels during chemotherapy remains to be
investigated. In the present study, we prospectively demonstrated
the feasibility and validity of the FACIT-F Questionnaire, and its
detailed change in accordance with scheduled cycles of chemotherapy
regimen. Compared with CTCAE assessment of fatigue, FACIT-F
reflects spike patterns in the degree of fatigue in phases with
each cycle of regimen. The presence and its amplitude revealed the
chance of intervention for fatigue. Until now, there were few
effective established preventive measures in the field of
supportive care medicine. Unfortunately, the total score of the
questionnaire revealed no significant increase in using
linear-mixed effect modeling analyses regarding treatment courses
and weeks as fixed effects. This could be due to the short
observation duration of this study. We also observed other adverse
effects such as appetite loss, degree of pain and sensory
disorders, and cumulative sensory/motor neurotoxicity since the
mechanism of fatigue and relationships with other adverse effects
remains unknown.
This study demonstrated fatigue alternated between
stronger and weaker in synch with an on- and-off chemotherapy
schedule, but may be influenced by other adverse effects, such as
the number of administrations. Recently, a website was designed to
provide researchers information about Patient-Reported Outcomes
version of the Common Terminology Criteria for Adverse Events
(PRO-CTCAE™), a patient-reported outcome measurement
system developed by the National Cancer Institute to capture
symptomatic adverse events in patients in cancer clinical trials
(11). Although we mainly intended
to assess quality of life by FACIT-F and by referring to PRO-CTCAE
recommendation, we designed a weekly assessment for the
introductory period of chemotherapy in this study, i.e., weekly
assessment during the first two cycles of consecutive chemotherapy
to compare with CTCAE assessment about fatigue. The FACIT-F
questionnaire is composed of five sections as follows: Well-being,
social/family well-being, emotional well-being, functional
well-being and additional concerns. We limited assessment of the
additional concerns section by considering only appropriate
questions directly reflecting patient's fatigue and the outcomes
originating from fatigue. In addition, we avoided patient burden
from high number of questions to a single assessment in this study
requiring a short time (within 3 min). However, this is also a
major limitation of this study. The results of our study revealed
the feasibility and validity of the FACIT-F questionnaire in
accordance with the CTCAE assessment about fatigue in patients on
cancer clinical trials. In addition to that, FACIT-F assessment
revealed the elevated fatigue status during chemotherapy. Based on
the results of this study, we have just started the next
interventional trial (UMIN000025606) in which patients were
administered Japanese herbal medicines for fatigue, and fatigue
levels were assessed in a similar way. We aim to confirm, not only
the feasibility and validity by means of the FACIT-F questionnaire
in accordance with the CTCAE assessment, but also the differences
between the two assessments about fatigue.
In conclusion, it was feasible to use the FACIT-F
Questionnaire for assessment of patients with URPC who underwent
nab-paclitaxel plus gemcitabine therapy, and to detect detailed
changes in accordance with scheduled cycle of chemotherapy regimen.
The data obtained from this tool are useful for identifying the
timing and duration of medical intervention for supporting therapy
in cancer patients.
Acknowledgements
We would like to thank the Department of Clinical
Study Support Center, Wakayama Medical University, for proofreading
and editing the manuscript.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okada KI, Hirono S, Kawai M, Miyazawa M,
Shimizu A, Kitahata Y, Ueno M, Hayami S and Yamaue H: Phase I study
of nab-paclitaxel plus gemcitabine as neoadjuvant therapy for
borderline resectable pancreatic cancer. Anticancer Res.
37:853–858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salsman JM, Beaumont JL, Wortman K, Yan Y,
Friend J and Cella D: Brief versions of the FACIT-fatigue and FAACT
subscales for patients with non-small cell lung cancer cachexia.
Support Care Cancer. 23:1355–1364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Younossi ZM, Stepanova M, Charlton M,
Curry MP, O'Leary JG, Brown RS and Hunt S: Patient-reported
outcomes with sofosbuvir and velpatasvir with or without ribavirin
for hepatitis C virus-related decompensated cirrhosis: An
exploratory analysis from the randomised, open-label ASTRAL-4 phase
3 trial. Lancet Gastroenterol Hepatol. 1:122–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimozuma K, Ohashi Y, Takeuchi A,
Aranishi T, Morita S, Kuroi K, Ohsumi S, Makino H, Mukai H,
Katsumata N, et al: Feasibility and validity of the Patient
Neurotoxicity Questionnaire during taxane chemotherapy in a phase
III randomized trial in patients with breast cancer: N-SAS BC 02.
Support Care Cancer. 17:1483–1491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
National Cancer Institute, . Common
terminology criteria for adverse events (CTCAE) Version 4.0.
https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdfMarch
3–2017
|
|
10
|
National Comprehensive Cancer Network, .
NCCN practice guidelines for pancreatic cancer, version 2. 2016,
http://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdfOctober
4–2016
|
|
11
|
National Cancer Institute, .
Patient-reported outcomes version of the common terminology
criteria for adverse events (PRO-CTCAE™). https://healthcaredelivery.cancer.gov/pro-ctcae/pro-ctcae_japanese.pdf#search=pro-ctcaeMarch
3–2017
|