Introduction
Conventional magnetic resonance imaging (MRI) has
limited value in the diagnosis of lymph node or distant metastasis
in patients with urothelial cancer. Multiparametric (mp)MRI is
widely used in the imaging of prostate cancer (1). mpMRI is also gaining ground for
detecting and diagnosing breast cancer (2). However, there are scanty reports on the
use of mpMRI in urothelial cancer (UC) (3). In the present study we describe the
visualization of primary tumors and lymph node metastasis in two
cases of UC. It is expected that mpMRI can improve the sensitivity
and specificity of UC imaging. MRI requires no X-ray exposure and
thus may be repeated frequently during follow-up while maintaining
minimal radiation exposure to patient. MRI is becoming increasingly
available, and the cost of mpMRI is only 1.5 times higher than that
of contrast-enhanced computed tomography (CT) in Japan, and thus it
does not result in a markedly higher financial burden for patients
or for the medical insurance system in general.
Case reports
Written informed consent was obtained from all of
the patients and their families. Case 1 was a 78-year-old male who
underwent transurethral resection (TUR) for a bladder tumor in
February 2016 at Yamagata Tokushukai Hospital. Pathological
diagnosis was UC, pT1, low-grade. The surgery was followed by
intravesical instillation of farmorubicin. In June 2016,
intravesical recurrence was suspected based on results of
cystoscopy and urinary cytology (class V). In June 2016, CT
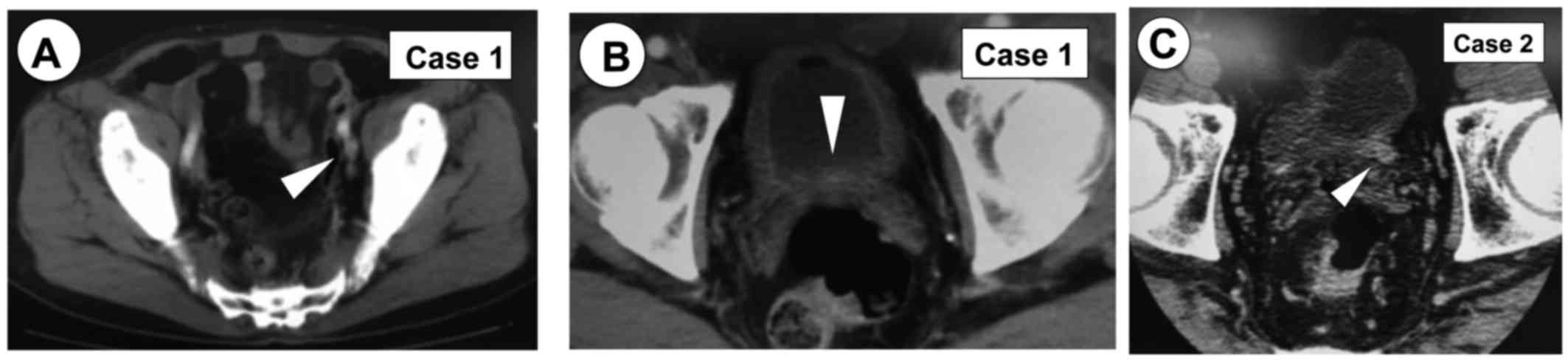
identified an 8 mm left internal iliac lymph node (Fig. 1A). Imaging diagnosis of metastasis
was not performed because of the shape and size of the lymph node.
Dynamic contrast-enhanced MRI (DCE) revealed a highly enhanced
lesion and a diagnosis of lymph node metastasis was made (Fig. 2A-C). The same CT revealed whole
bladder wall thickness (Fig. 1B) but
did not provide enough data to confirm the diagnosis of residual
tumor. On the other hand, mpMRI revealed residual bladder cancer
(Fig. 2D-G).
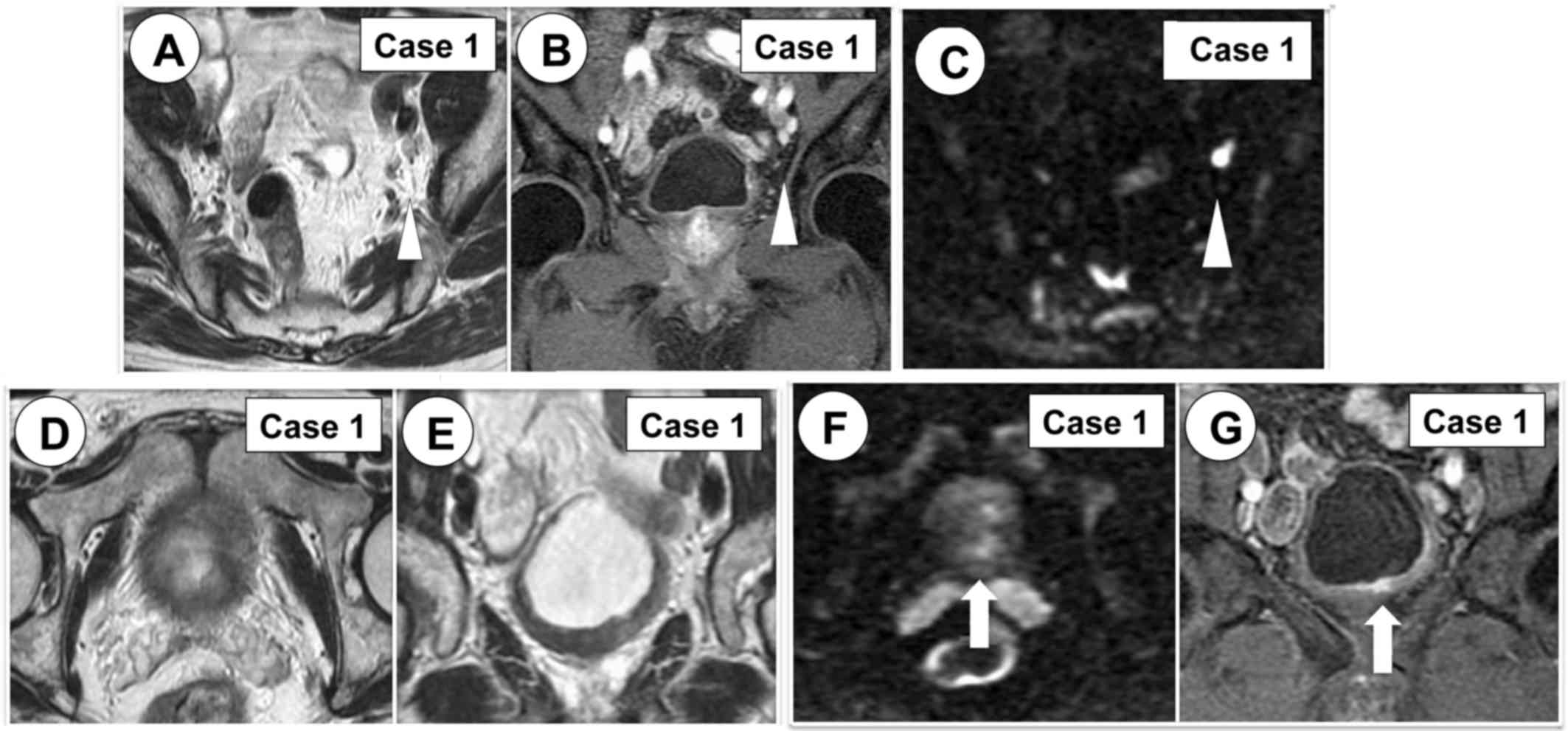 | Figure 2.Lymph node metastasis (white
arrowhead) and residual bladder tumor (white arrow) were diagnosed
by mpMRI. (A) T2W MRI, axial view. (B) DCE MRI, coronal view. (C)
DW MRI, axial view, b-value=1,000 s/mm2. (D) T2W MRI,
axial view. (E) T2W MRI, coronal view. (F) DW MRI, axial view,
b-value=1,000 s/mm2. (G) DCE MRI, coronal view. MRI,
magnetic resonance imaging; mpMRI, multi-parametric magnetic
resonance imaging; T2W, T2-weighted; DCE, dynamic
contrast-enhanced; DW, diffusion-weighted. |
Peritoneum preserving retrograde radical cystectomy
(4) with pelvic lymph node
dissection and bilateral ureterocutaneostomy was performed in June
2016. Pathological diagnosis was UC, high-grade muscle-invasive
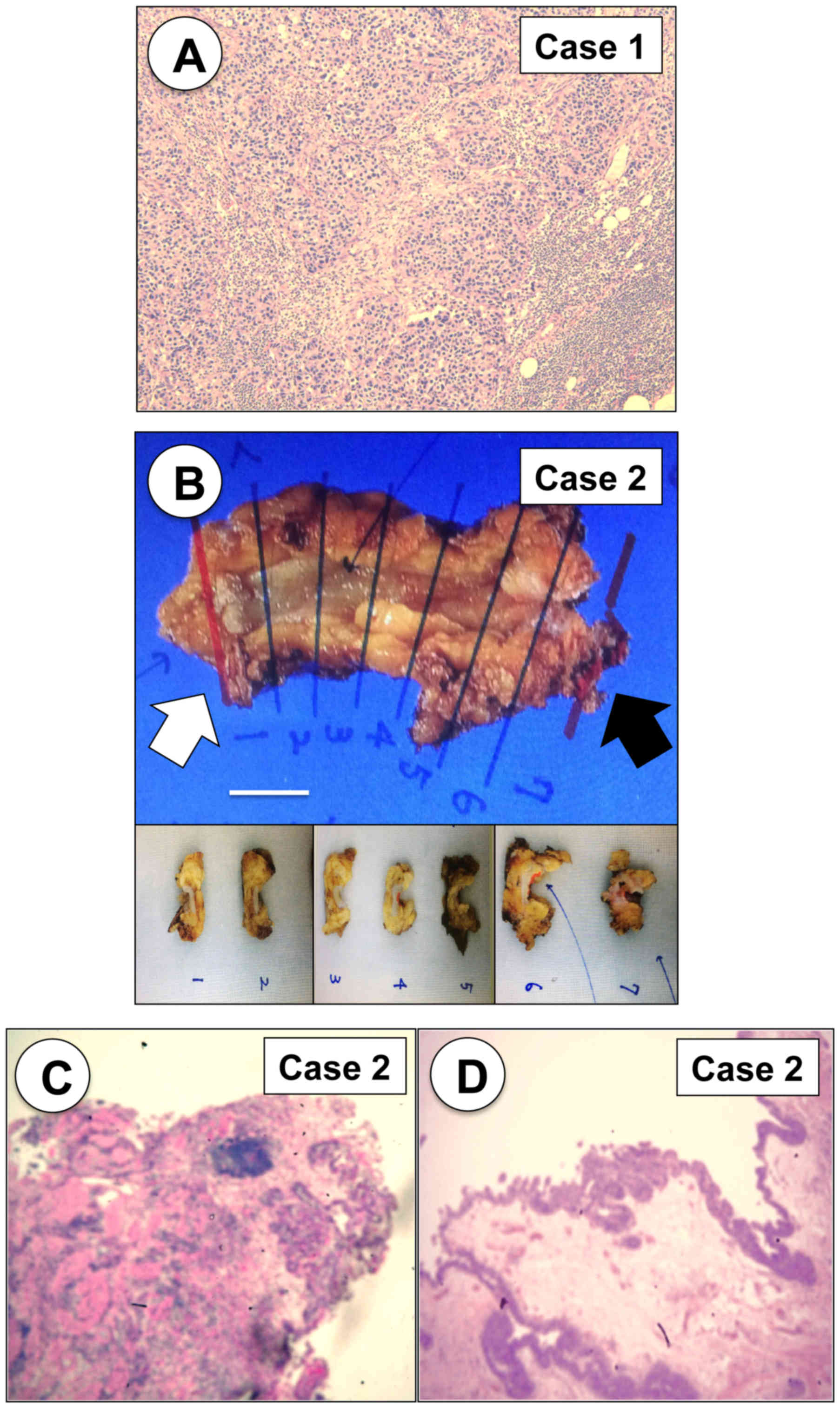
bladder cancer (pT2) and left internal iliac lymph node metastasis
(Fig. 3A). The patient received six
courses of adjuvant chemotherapy with gemcitabine and carboplatin
(5) and was of ‘no evidence of
disease’ 23 months after radical cystectomy.
Case 2 was a 65-year-old male. In August 2016, TURBT
was performed for a bladder tumor located around the left ureteral
orifice at Yamagata Tokushukai Hospital; and the left ureter
orifice was deeply resected. In September 2016, CT revealed lеft
terminal ureter dilatation (Fig. 1C)
and left hydronephrosis. The inflammatory changes of the left
ureter were diagnosed following deep incision of the left ureteral
orifice. In November 2016, ultrasound revealed disappearance of the
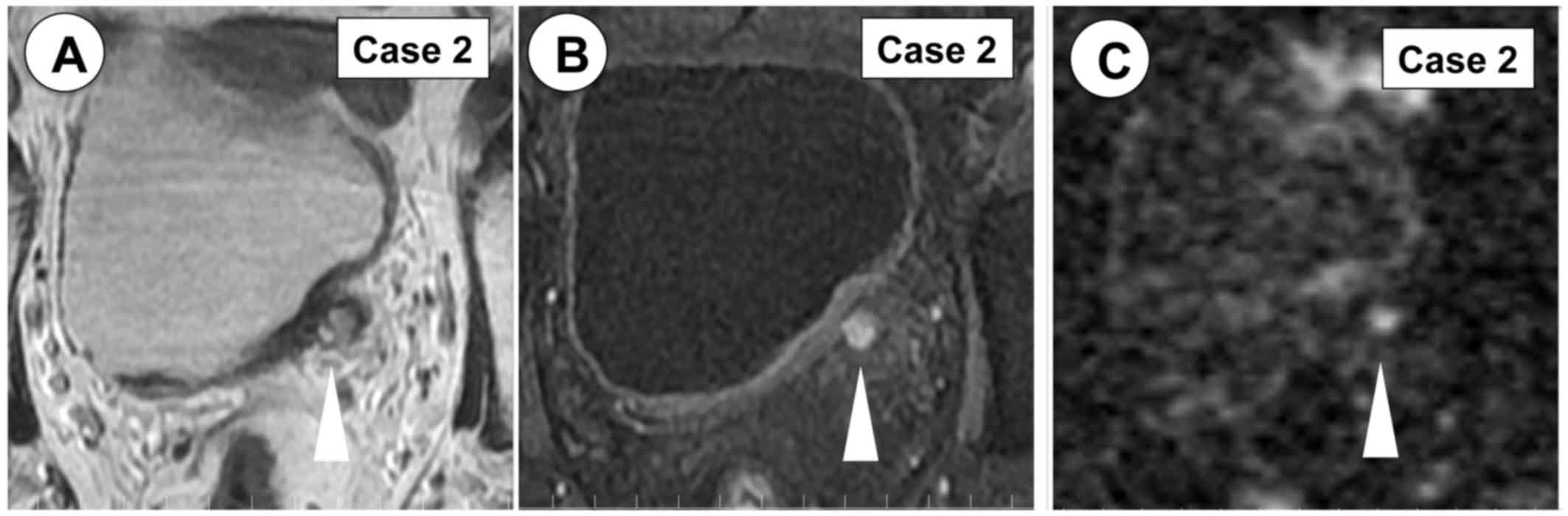
left hydronephrosis. In January 2017, MRI revealed a left lower
ureter tumor (Fig. 4). In February
2017, partial resection of the left terminal ureter was performed
and the pathological diagnosis was UC, high-grade, pT2 (Fig. 3B-D).
Discussion
mpMRI in prostate and breast cancers improves
diagnostic accuracy, eliminates the need for biopsies, and enables
improved assessment, image-guided biopsy targeting and prediction
of response to neoadjuvant therapy (1,2). In
bladder cancer patients, staging and postoperative follow-up
depends on CT scan data. However, the sensitivity and specificity
of CT imaging in detecting lymph node metastasis are relatively
low, and regular CT scans result in an accumulation of radiation.
Lymph node metastasis not resulting in significant lymph node
enlargement typically results in false-negative results while
enlarged non-metastatic lymph nodes lead to false-positive results
in CT scanning. For early diagnosis of pelvic lymph node metastasis
in bladder cancer, our group has previously performed bipedal
lymphography and percutaneous fine needle aspiration biopsy (FNAB)
of pelvic lymph nodes. In a series of 200 bladder cancer patients
at Yamagata Prefectural Central Hospital, a diagnosis of metastasis
to the pelvic lymph nodes was determined by this method in 34
patients (17%). Of these 34 patients, only 12 (35%) were positive
or suspected of having pelvic lymph node metastasis by CT scan. A
total of 16 patients (47%) exhibited positive or highly suspected
positive lymphogram and 18 patients (53%) exhibited normal
lymphogram. A total of 78 cases, including 8 FNAB-positive cases,
were treated by radical cystectomy and regional lymph node
dissection. The sensitivity, specificity, positive predictive value
and negative predictive value of FNAB were 57, 100, 100 and 91%,
respectively (6). With FNAB,
cytopathological diagnosis is possible; however, this technique is
complex, time consuming and exposes patients to ionizing radiation.
On the other hand, bipedal lymphography could not visualize
obturator or internal iliac lymph nodes. mpMRI may improve the
accuracy of tumor detection and staging without the risk of
exposure to ionizing radiation. Additionally, mpMRI is a more
feasible technique than FNAsB for the pelvic lymph node and may be
performed regularly on an out-patient basis.
For high-grade non-muscle invasive bladder cancer
(NMIBC) and muscle invasive bladder cancer (MIBC), MRI may detect
and stage tumors with high sensitivity and specificity (7). mpMRI has demonstrated high diagnostic
accuracy in differentiating NMIBC from MIBC and organ-confined
disease from non-organ confined disease (8); exceeding that of T2 weighted imaging
(T2W) or diffusion weighted imaging (DWI)-MRI used alone (8). Compared with CT, MRI offers improved
soft-tissue resolution, making it easy to distinguish between NMIBC
and MIBC. It has even been proven to be superior to CT in
identifying bladder-wall invasion (7). There are a number of reports on the
usefulness of mpMRI for the detection of tumor recurrence (9) and differentiation of non-muscle
invasive UC from muscle-invasive UC (10,11).
Afifi et al (8) reported on
the usefulness of mpMRI in detecting metastatic lymph nodes. The
largest size of the metastatic lymph nodes detected was 42 mm, and
lymph nodes with low apparent diffusion coefficient values were
considered positive (8). However, in
general, data on lymph node staging from mpMRI remains limited.
Both patients reported presently underwent mpMRI
prior to tumor resection. A total of 4 MRI setsT2W +
perfusion-weighted imaging (PWI), T2W + DWI, T2W + DWI + PWI and
T2W + DWI + PWI + diffusion tensor imaging (DTI) were interpreted
qualitatively. PWI, DWI and DTI were also analyzed quantitatively.
Accuracy was determined using histopathology as the reference
standard. Thus, mpMRI may provide qualitative and quantitative
information on the tumor microenvironment beyond traditional tumor
size measures and/or morphological assessments.
pMRI was useful for the diagnosis of pelvic lymph
node metastasis of bladder cancer and invasive lower ureteral
tumor. mpMRI with DWI and DTI has the potential to become a
reliable staging tool for invasive bladder cancer and lower
ureteral cancer, and to diagnose metastasis of pelvic lymph nodes
when the lymph node is not significantly enlarged.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The raw data used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
SH participated in data acquisition, developed the
concept of the manuscript and was a major contributor in writing
the manuscript. KH analyzed and interpreted MRI images. KN
participated in data acquisition and critically revised the content
of the manuscript. KH was involved in data acquisition and
analysis. VB was involved in drafting the manuscript and revising
it critically for important intellectual content. IS participated
in data acquisition, and also provided administrative support and
supervision. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patients and their families.
Patient consent for publication
Written informed consent was obtained from the
patients and their families.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UC
|
urothelial cancer
|
|
MRI
|
magnetic resonance imaging
|
|
mpMRI
|
multi-parametric magnetic resonance
imaging
|
|
CT
|
computed tomography
|
|
TURBT
|
transurethral resection of bladder
tumor
|
|
FNAB
|
fine needle aspiration biopsy
|
|
NMIBC
|
non-muscle invasive bladder cancer
|
|
MIBC
|
muscle invasive bladder cancer
|
|
T2W
|
T2 weighted imaging
|
|
PWI
|
perfusion-weighted imaging
|
|
DTI
|
diffusion tensor imaging
|
References
|
1
|
Christidis D, McGrath S, Leaney B,
O'Sullivan R and Lawrentschuk N: Interpreting prostate
multiparametric magnetic resonance imaging: Urologists' guide
including prostate imaging reporting and data system. Urology.
111:136–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pinker K, Helbich HT and Morris AE: The
potential of multiparametric MRI of the breast. Br J Radiol.
90:201607152017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bouchelouche K, Turkbey B and Choyke PL:
PET/CT and MRI in bladder cancer. J Cancer Sci Ther. S14:pii: 7692.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoshi S, Yamamuro T, Ogata Y, Numahata K
and Sugano O: 948 Peritoneum preserving retrograde radical
cystectomy for elderly and high risk bladder cancer patients. J
Urol. 183:e3692010. View Article : Google Scholar
|
|
5
|
Hoshi S, Ohyama C, Ono K, Takeda A,
Yamashita S, Yamato T, Itoh A, Satoh M, Saito S, Okada Y, et al:
Gemcitabine plus carboplatin; and gemcitabine, docetaxel, and
carboplatin combined chemotherapy regimens in patients with
metastatic urothelial carcinoma previously treated with a
platinum-based regimen: Preliminary report. Int J Clin Oncol.
9:125–129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoshi S, Orikasa S, Suzuki KI, Takahashi
T, Ohyama C, Sato K and Ono K: Diagnosis and treatment of pelvic
lymph node metastasis in bladder cancer. Int J Urol. 6:400–407.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bagheri MH, Ahlman MA, Lindenberg L,
Turkbey B, Lin J, Civelek Cahid A, Malayeri AA, Agarwal PK, Choyke
PL, Folio LR and Apolo AB: Advances in medical imaging for the
diagnosis and management of common genitourinary cancers. Urol
Oncol. 35:473–491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Afifi AH, Maksoud Abdel TAS, El-noueam KI,
Ataa MA and Abdallah DM: Multiparametric-MRI as a comprehensive
study in evaluation, characterization & local staging of
urinary bladder carcinomas. Egyptian J Radiol Nuclear Med.
48:493–507. 2017. View Article : Google Scholar
|
|
9
|
Wang HJ, Pui MH, Guo Y, Yang D, Pan BT and
Zhou XH: Diffusion-weighted MRI in bladder carcinoma: The
differentiation between tumor recurrence and benign changes after
resection. Abdom Imaging. 39:135–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panebianco V, De Berardinis E, Barchetti
G, Simone G, Leonardo C, Grompone MD, Del Monte M, Carano D,
Gallucci M, Catto J and Catalano C: An evaluation of morphological
and functional multi-parametric MRI sequences in classifying
non-muscle and muscle invasive bladder cancer. Eur Radiol.
27:3759–3766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HJ, Pui MH, Guo Y, Li SR, Guan J,
Zhang XL and Cai HS: Multiparametric 3-T MRI for differentiating
low-versus high-grade and category T1 versus T2 bladder urothelial
carcinoma. AJR Am J Roentgenol. 204:330–334. 2015. View Article : Google Scholar : PubMed/NCBI
|