Introduction
Cholangiocarcinoma (CCA) is a malignant tumor that
originates from the epithelium of the bile ducts. Jaundice and
abdominal pain are the most common presentations. The tumor
commonly metastasizes via lymphatic spread to the regional lymph
nodes, followed by hematogenous metastasis to the liver, lungs and
peritoneum (1). Distant metastasis
is uncommon, although bone, muscle and brain metastasis have also
been reported (2–5). Patients usually develop distant
metastasis in the late stages of the disease.
Appendicular bone metastasis from CCA as a first
presentation is an extremely rare event, considering the very small
number of published case reports. Bone scan is not usually
routinely performed in all patients first diagnosed with CCA
(6), but only in those presenting
with bone pain and pathological fractures.
The aim of the present study was to report a case of
CCA with multiple osseous metastases, with the imaging findings
(severe cortical bone destruction and a large soft tissue mass)
mimicking osteosarcoma.
Case report
A 61-year-old female with a history of
well-controlled hypertension presented on September 2016 to the
Khon Kaen University Hospital (Khon Kaen, Thailand) with a history
of pain in the right scapular area for 5 months. The pain was
described as dull aching, and was more severe at night. Two months
prior to visiting our hospital, the patient noticed a lump in the
right side of her back, gradually increasing in size. She started
to feel numbness in her right arm and forearm, and reported a
history of asthenia for ~1 month. On physical examination, the
patient was not icteric, with impalpable cervical and
supraclavicular lymph nodes. The liver was found to be mildly
enlarged, with no chronic liver stigmata. A 12-cm mass was
identified in the right shoulder area.
A magnetic resonance imaging examination of the
right shoulder revealed aggressive osseous destruction of nearly
the entire right scapula, with an adjacent extraosseous soft tissue
mass encasing the neurovascular bundle (Fig. 1A). Bone scintigraphy also
demonstrated multiple bone metastases to the skull, mid-cervical
vertebrae, T4, L1-2 and L4-5 vertebrae, pelvis, left proximal
femur, right scapula, right humerus, proximal right forearm,
lateral aspect of the right 8th rib and posterior aspect of the
right 9th rib (Fig. 1B). A computed
tomography scan of the whole abdomen revealed an ill-defined
hypodense lesion at hepatic segment 7/6 with subcapsular
retraction, compatible with CCA (Fig.
1C).
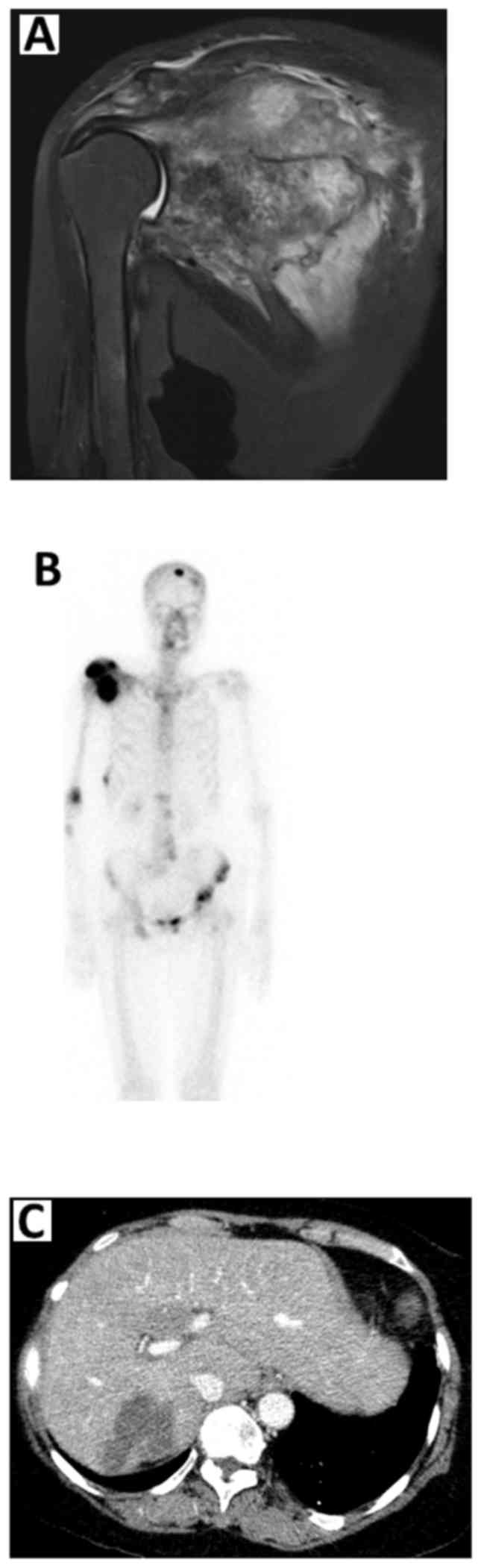 | Figure 1.(A) Magnetic resonance imaging
examination of the right shoulder showing destruction of nearly the
entire right scapula, with an adjacent extraosseous soft tissue
mass encasing the neurovascular bundle. (B) Bone scintigraphy
revealed multiple bone metastases to the skull, mid-cervical
vertebrae, T4, L1-2 and L4-5 vertebrae, pelvis, left proximal
femur, right scapula, right humerus, proximal right forearm,
lateral aspect of the right 8th rib and posterior aspect of the
right 9th rib. (C) A computed tomography scan of the whole abdomen
revealed an ill-defined hypodense lesion at hepatic segment 7/6
with subcapsular retraction, compatible with
cholangiocarcinoma. |
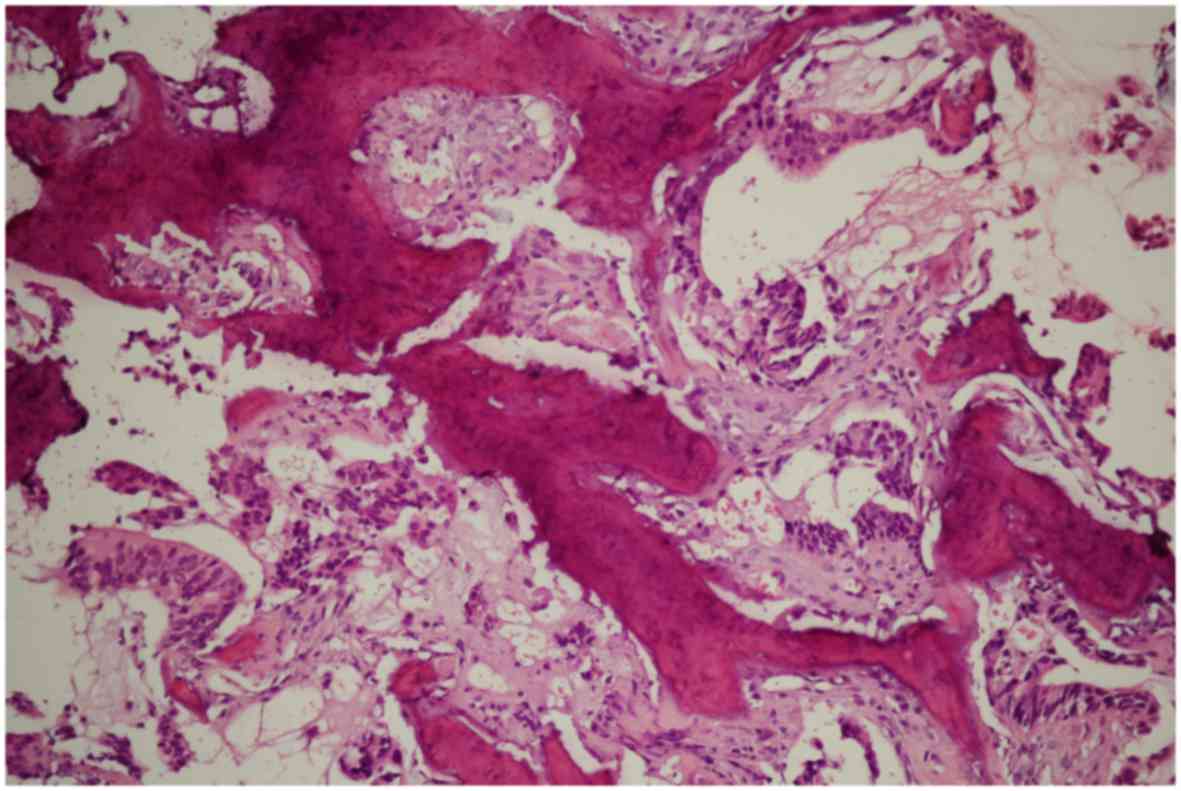
A biopsy of the scapular mass was performed, and the
histological examination revealed bone invasion by neoplastic cells
arranged in cords and glandular formations, with a desmoplastic
stromal reaction, resembling metastatic adenocarcinoma (Fig. 2). Immunohistochemical examination
revealed that the tumor cells were positive for cytokeratin (CK)7,
CK20 and carbohydrate antigen 19-9, and negative for thyroid
transcription factor-1, confirming the diagnosis of CCA.
The patient received external beam radiation (30 Gy
in 10 fractions) of the right scapular metastasis. One month after
radiation treatment, the pain improved. Chemotherapy with
carboplatin (AUC 5) and fluorouracil (1,000 mg/m2) every
4 weeks was also administered, but the tumor did not respond to the
treatment and progressed after 3 cycles. The patient received
palliative end-of-life care with pain control and eventually
succumbed to the disease at home, 10 months after the
diagnosis.
Discussion
The pattern of metastasis in CCA usually starts with
lymphatic spread to the regional lymph nodes, and further spread
via the hematogenous route to other organs, including the liver,
lung, peritoneum and, occasionally, to the bone and brain (2–5). Bone is
a common site of metastasis from several tumors, including
prostate, breast and lung cancer. However, appendicular bone
metastasis from CCA is a rare occurrence. The underlying reason for
this rarity has not yet been elucidated.
Bone scan is not routinely performed in patients
first diagnosed with CCA (6). It is
only recommended for patients with bone symptoms, such as bone pain
and pathological fractures. Since the majority of the patients
exhibit elevated alkaline phosphatase (ALP) levels due to the
primary liver pathology, ALP is not a useful marker for bone
metastasis in this condition.
Spinal metastases are more frequent compared with
appendicular bone metastasis. Dowsiriroj et al reported 55
cases of spinal metastases from CCA, with a median survival of only
4.0 months, despite palliative spinal surgery and radiation
treatment (3).
The characteristics of the scapular mass in our
patient mimicked those observed in osteosarcoma, a malignant
primary bone tumor; both types of tumor are associated with severe
cortical destruction of the bone and a large soft tissue mass.
Furthermore, although osteosarcoma usually occurs around the knee
area, the incidence of trunk and girdle tumors increases with age
(7). However, the majority of adult
osteosarcomas arise as secondary lesions in patients with Paget's
disease of bone (7) which was not
the case in this patient.
Only five cases of metastasis to the appendicular
skeleton from CCA have been reported in the literature to date
(Table I). The most commonly
reported sites of bone metastasis are the humerus, fibula and femur
(8–12). To the best of our knowledge, this is
the first report of a scapular metastasis from CCA to date.
 | Table I.Characteristics of patients with
appendicular skeletal metastasis. |
Table I.
Characteristics of patients with
appendicular skeletal metastasis.
| First author,
year | Age, years | Sex | Country | Ethnicity | Primary | Metastatic site | Type | Treatment | Survival | (Refs.) |
|---|
| Carlisle, 1999 | 60 | F | USA | NA | IHC | Humerus | Osteolytic | Intramedullary
nail | >4 weeks | (8) |
| Lahrach, 2010 | 58 | NA | Morocco | NA | NA | Humerus | Osteolytic | Plate and screw
osteosynthesis and bone cement filling | 3 months | (11) |
| Federico, 2013 | 71 | M | Italy | NA | IHC | Humerus | Osteolytic | Gemcitabine,
zoledronic acid, EBRT | NA | (9) |
| Karanjia, 2013 | 75 | F | USA | NA | IHC | Fibula | Osteolytic | EBRT | >12 months | (10) |
| MacKenzie, 2017 | 61 | F | UK | Caucasian | IHC | Femur | Osteolytic | En bloc excision and
distal femoral replacement | 4 weeks | (12) |
In conclusion, metastatic CCA to the bone is rare;
however, oncologists should consider this as a differential
diagnosis in patients who present with bone pain. Since the
prognosis is extremely poor due to the minimal response to
conventional chemotherapy or radiotherapy, symptom control,
particularly pain, is the mainstay of treatment for improving the
quality of life of the patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PC and NT drafted the manuscript. JP interpreted the
radiological data. PU provided the pathological data. JC managed
the treatment for this patient and supervised the entire work. All
authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Faculty of Medicine of
Khon Kaen University provided ethics approval under the guidelines
of the Helsinki Declaration and Good Clinical Practice
(HE601304).
Patient consent for publication
The legal representative of the patient in this case
report provided informed consent and permission for the publication
of the case details and associated images.
Competing of interests
The authors declare that they have no competing
interests to disclose.
References
|
1
|
Sripa B and Pairojkul C:
Cholangiocarcinoma: Lessons from Thailand. Curr Opin Gastroenterol.
24:349–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chindaprasirt J, Sookprasert A,
Sawanyawisuth K, Limpawattana P and Tiamkao S: Brain metastases
from cholangiocarcinoma: A first case series in Thailand. Asian Pac
J Cancer Prev. 13:1995–1997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dowsiriroj P, Paholpak P, Sirichativapee
W, Wisanuyotin T, Laupattarakasem P, Sukhonthamarn K, Kosuwon W and
Jeeravipoolvarn P: Cholangiocarcinoma with spinal metastasis:
Single center survival analysis. J Clin Neurosci. 38:43–48. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee J, Lee SW, Han SY, Baek YH, Kim SY and
Rhyou HI: Rapidly aggravated skeletal muscle metastases from an
intrahepatic cholangiocarcinoma. World J Gastroenterol.
21:1989–1993. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katayose Y, Nakagawa K, Yamamoto K,
Yoshida H, Hayashi H, Mizuma M, Ohtsuka H, Fukase K, Onogawa T,
Motoi F, et al: Lymph nodes metastasis is a risk factor for bone
metastasis from extrahepatic cholangiocarcinoma.
Hepatogastroenterology. 59:1758–1760. 2012.PubMed/NCBI
|
|
6
|
National Comprehensive Cancer Network, .
Hepatobiliary Cancers (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdfJuly
15–2018.
|
|
7
|
Ek ET, Ojaimi J, Kitagawa Y and Choong PF:
Outcome of patients with osteosarcoma over 40 years of age: Is
angiogenesis a marker of survival? Int Semin Surg Oncol. 3:72006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carlisle RT and Roberts CS: Pathologic
fracture of the humerus due to metastatic cholangiocarcinoma. South
Med J. 92:1216–1219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Federico A, Addeo R, Cerbone D, Iodice P,
Cimmino G and Bucci L: Humerus metastasis from cholangiocarcinoma:
A case report. Gastroenterology Res. 6:39–41. 2013.PubMed/NCBI
|
|
10
|
Karanjia H, Abraham JA, O'Hara B, Shallop
B, Daniel J, Taweel N and Schick FA: Distal fibula metastasis of
cholangiocarcinoma. J Foot Ankle Surg. 52:659–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lahrach K, Chbani B, Amar F, Bennani A,
Marzouki A and Boutayeb F: Humerus pathological fracture revealing
biliary carcinoma. Orthop Traumatol Surg Res. 96:910–912. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
MacKenzie SA, Goffin JS, Rankin C and
Carter T: Rare progression of cholangiocarcinoma: Distal femoral
metastasis. BMJ Case Rep. 2017:pii: bcr20162186162017. View Article : Google Scholar
|