Introduction
HCV infection is one of the principal causes of
chronic liver disease, with ~170 million individuals infected
worldwide (1). The 5-year incidence
of HCC from HCV patients is reported to be 13.4%, with a mortality
rate of 15.3% (2). Therefore,
suppression of HCV is critical, and HCV treatments have been
continually developed and improved.
Previously, IFN was the mainstream treatment for
HCV. The SVR rate for two drugs (e.g. peginterferon and ribavirin)
against HCV genotype 1, which is considered to cause the highest
incidence of HCC (3), is ~50%,
whereas the use of three drugs (e.g. peginterferon, ribavirin and
protease inhibitor) increases the SVR rate to ~70% (1). The SVR from IFN treatment has been
identified to decrease the incidence of HCC (3–5).
Compared with patients without SVR, the incidence of HCC following
SVR from IFN treatment is reportedly decreased by 19.1% (6). In addition, randomized control trials
have revealed that the SVR from IFN treatment in patients following
HCC treatment decreases tumor recurrence (7,8).
Recurrence within 2 years is particularly decreased following HCC
treatment (9), as is the rate of
liver disease-associated mortality (10,11).
However, one study demonstrated that SVR from IFN treatment did not
decrease the incidence of HCC in patients with cirrhosis because of
background fibrosis (10).
Conversely, it is unclear whether non-SVR following
IFN treatment decreases the incidence of HCC. One study
demonstrated that non-SVR following IFN treatment decreases the
incidence of HCC (12), whereas
another indicated no decrease in HCC incidence from non-SVR
(6).
Currently, direct-acting antiviral agents (DAAs) are
used worldwide as an alternative to interferon (IFN) for the
treatment of hepatitis C virus (HCV) infections. DAA treatment has
a higher sustained virological response (SVR) rate and fewer side
effects compared with IFN treatment, so it is acceptable for many
elderly patients with HCV infections (13–16).
However, several studies have indicated that the rate of
hepatocellular carcinoma (HCC) recurrence may be increased
following DAA treatment in patients with a history of HCC treatment
(17–19). Conversely, there have been several
studies indicating that DAAs do not raise the recurrence rate, even
following HCC treatments, and instead have a suppressive effect on
carcinogenesis (20–22). This discrepancy has not yet been
resolved.
Therefore, the aim of the present study was to
retrospectively investigate patients with a history of HCC
treatments to whom DAAs were administered at Shiga University of
Medical Science (Otsu, Japan).
Materials and methods
Patient selection and data
collection
Between January 2015 and April 2017, 184 patients
with HCV were administered DAAs in Shiga University of Medical
Science. Among them, 19 had been treated for HCC prior to
commencing DAA treatment. Clinical data were compared between the 9
patients in whom recurrence of HCC was observed following SVR of
DAA treatment (recurrence group), and the 10 patients for whom no
HCC recurrence was observed following SVR of DAA treatment
(no-recurrence group).
Statistical analysis
χ2 tests were performed for nominal
variables, and the Mann-Whitney U test was performed to compare
continuous variables between the two groups. The Cox proportional
hazards model was used for multivariate analysis. The Kaplan-Meier
method was used to analyze the cumulative incidence rate, followed
by log-rank tests for comparisons between the two groups. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using the R statistical
package (version 3.4.4; The R Project for Statistical Computing,
Vienna, Austria; www.r-project.org).
Results
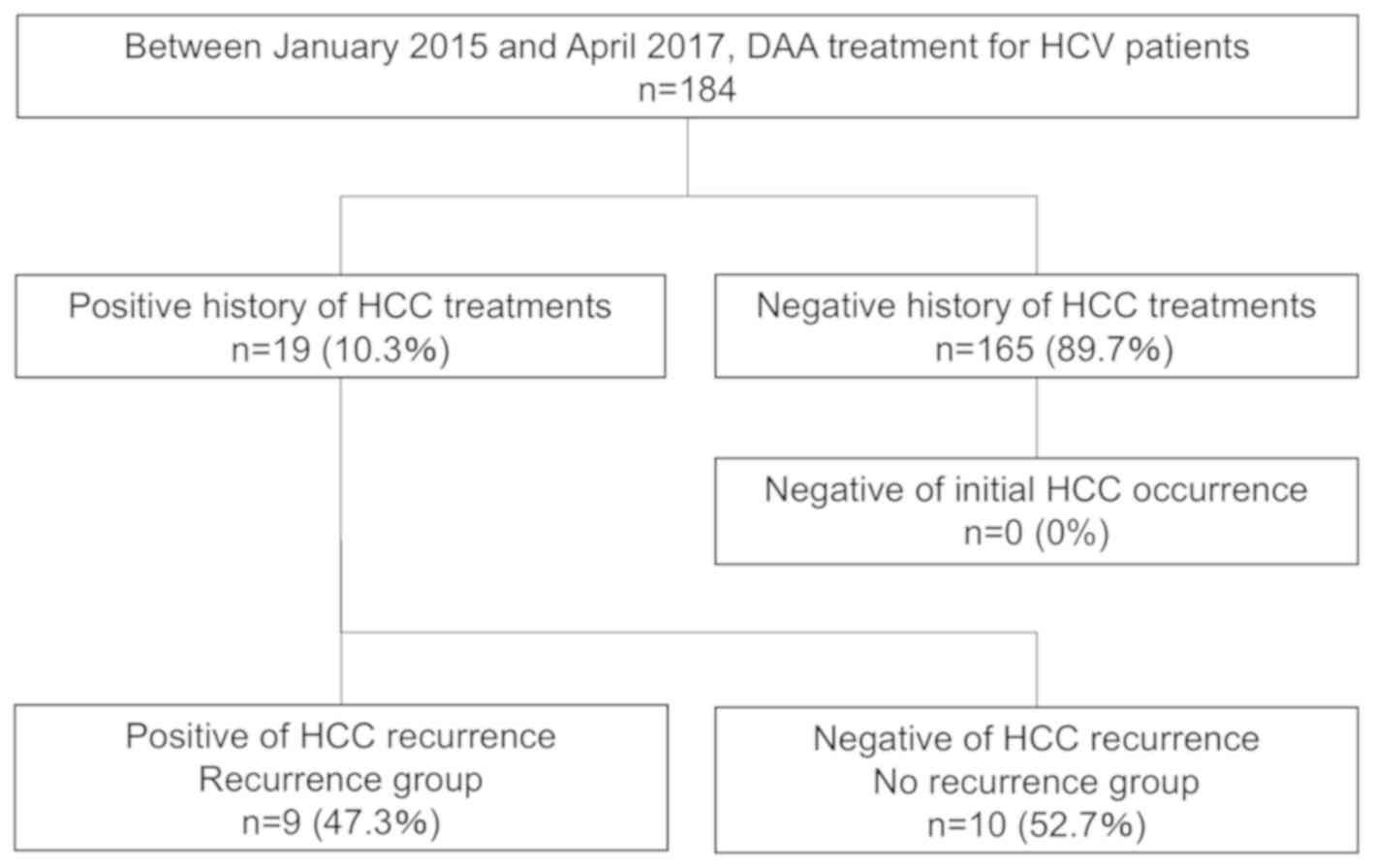
The decision tree for patients in the present study
is presented in Fig. 1. Between
January 2015 and April 2017, 184 patients underwent DAA treatment,
and they all achieved SVR. Among them, 165 patients (89.7%) had no
history of HCC treatment, and no patients experienced initial HCC
occurrence following DAA treatment. In total, 19 patients (10.3%)
had a history of HCC treatment, and 9 of them (47.3%) had HCC
recurrence following DAA treatment.
The results of univariate analysis between the
recurrence and no-recurrence groups are presented in Table I. No significant differences in age,
sex, hemoglobin A1c, serotype of HCV, regimen of DAAs or history of
IFN treatment were identified. In the laboratory data, no
significant differences were observed in α-fetoprotein (AFP),
des-γ-carboxyprothrombin (DCP), albumin, alanine aminotransferase,
aspartate aminotransferase, bilirubin, hemoglobin, platelet count,
or prothrombin activity. Among the tumor-associated factors, there
were no significant differences in the number of tumors, the
maximum tumor diameter, the number of HCC treatments or the final
treatment method for HCC prior to DAA treatment. The only
difference observed was in the median interval between final HCC
treatment and DAA treatment, which was significantly shorter in the
recurrence group compared with in the no-recurrence group (88 and
790 days, respectively; P=0.018).
 | Table I.Univariate analysis between the
no-recurrence and recurrence groups. |
Table I.
Univariate analysis between the
no-recurrence and recurrence groups.
| Factor | No-recurrence group
(n=10) | Recurrence group
(n=9) | P-value |
|---|
| Mean age, years | 74±9.4 | 74±6.5 | 0.961 |
| Sex (male), n
(%) | 6 (60.0) | 4 (44.4) | 0.656 |
| Median AFP, ng/ml
(IQR) | 5.60 (4.15,
10.55) | 10.90 (7.50,
29.50) | 0.111 |
| Median DCP, mAU/ml
(IQR) | 22.50 (19.50,
40.50) | 27.00 (25.00,
31.00) | 0.54 |
| Median albumin,
g/dl (IQR) | 3.55 (3.23,
3.97) | 3.50 (3.10,
3.60) | 0.389 |
| Median AST, IU/l
(IQR) | 44.50 (32.00,
55.25) | 52.00 (40.00,
73.00) | 0.653 |
| Median ALT, IU/l
(IQR) | 31.50 (26.50,
34.50) | 45.00 (27.00,
49.00) | 0.683 |
| Median bilirubin,
mg/dl (IQR) | 0.74 (0.60,
0.93) | 0.69 (0.53,
0.78) | 0.87 |
| Median hemoglobin
A1c, g/dl (IQR) | 12.00 (11.62,
12.38) | 11.60 (11.20,
12.80) | 0.87 |
| Median platelet
count, ×104 µl (IQR) | 12.65 (8.62,
16.52) | 11.40 (10.10,
15.60) | 0.87 |
| Median prothrombin
activity, % (IQR) | 86.00 (81.00,
90.00) | 90.00 (84.00,
95.00) | 0.479 |
| Multiple HCC, n
(%) | 3 (30.0) | 3 (33.3) | >0.999 |
| Maximum tumor size,
mm | 25.7±10.3 | 21.6±17.5 | 0.573 |
| Treatment history
of IFN, % | 3 (30.0) | 3 (33.3) | >0.999 |
| RFA prior to DAA,
% | 3 (30.0) | 4 (44.4) | 0.65 |
| Hepatectomy prior
to DAA, % | 4 (40.0) | 1 (11.1) | 0.303 |
| TACE prior to DAA,
% | 3 (30.0) | 4 (44.4) | 0.65 |
| Median no. of HCC
treatments (IQR) | 1.50 (1.00,
5.50) | 2.00 (1.00,
2.00) | 0.898 |
| HCV serotype, n
(%) |
|
| 0.303 |
| 1 | 6 (60.0) | 8 (88.9) |
|
| 2 | 4 (40.0) | 1 (11.1) |
|
| Regimen of DAA, n
(%) |
|
| 0.371 |
|
DCV+ASV | 2 (20.0) | 4 (44.4) |
|
|
SOF+LDV | 4 (40.0) | 4 (44.4) |
|
|
SOF+RBV | 4 (40.0) | 1 (11.1) |
|
| Median interval
between final HCC treatment and DAA, days (IQR) | 790 (308,
2075) | 88 (20, 120) | 0.018 |
The results of univariate and multivariate analysis
to identify risk factors of tumor recurrence following DAA
treatment are presented in Table
II. Factors of continuous variables were divided into two
groups by their median values. In univariate analysis, a
significant difference was observed in the interval between the
final HCC treatment and DAA treatment (≥120 vs. ≤119 days;
P=0.028). Multivariate analysis was performed using tumor markers
(AFP and DCP), the number of HCCs, and the interval between the
final HCC treatment and the DAA treatment. From this analysis, only
the interval between the final HCC treatment and the DAA treatment
was identified as an independent risk factor of HCC recurrence
following DAA treatment (P=0.045).
 | Table II.Univariate and multivariate analysis
for evaluation of risk factors of HCC recurrence. |
Table II.
Univariate and multivariate analysis
for evaluation of risk factors of HCC recurrence.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factor | n | Median
survival | P-value | Hazard ratio (95%
confidence interval) | P-value |
|---|
| Age, years |
|
| 0.703 |
|
|
|
<75 | 9 | NA (60-NA) |
|
|
|
|
≥75 | 10 | 365 (20-NA) |
|
|
|
| Sex |
|
| 0.783 |
|
|
|
Female | 9 | 365 (150-NA) |
|
|
|
|
Male | 10 | NA (20-NA) |
|
|
|
| AFP, ng/ml |
|
| 0.783 | 0.50
(0.11–2.28) | 0.37 |
|
<9 | 10 | NA (20-NA) |
|
|
|
| ≥9 | 9 | 365 (150-NA) |
|
|
|
| DCP, mAU/ml |
|
| 0.323 | 0.92
(0.18–4.57) | 0.92 |
|
<25 | 9 | NA (60-NA) |
|
|
|
|
≥25 | 10 | 329 (20-NA) |
|
|
|
| Albumin, mg/dl |
|
| 0.783 |
|
|
|
<3.5 | 9 | 365 (150-NA) |
|
|
|
|
≥3.5 | 10 | NA (20-NA) |
|
|
|
| AST, IU/l |
|
| 0.223 |
|
|
|
<50 | 9 | NA (20-NA) |
|
|
|
|
≥50 | 10 | 300 (30-NA) |
|
|
|
| ALT, IU/l |
|
| 0.624 |
|
|
|
<35 | 10 | NA (20-NA) |
|
|
|
|
≥35 | 9 | 329 (30-NA) |
|
|
|
| Bilirubin,
mg/dl |
|
| 0.267 |
|
|
|
<0.7 | 10 | 272.5 (20-NA) |
|
|
|
|
≥0.7 | 9 | NA (150-NA) |
|
|
|
| Hemoglobin,
g/dl |
|
| 0.861 |
|
|
|
<12 | 10 | 365 (150-NA) |
|
|
|
|
≥12 | 9 | NA (20-NA) |
|
|
|
| Platelet count,
×104 µl |
|
| 0.592 |
|
|
|
<11 | 9 | NA (150-NA) |
|
|
|
|
>11 | 10 | 300 (20-NA) |
|
|
|
| Prothrombin
activity, % |
|
| 0.506 |
|
|
|
<90 | 10 | NA (20-NA) |
|
|
|
|
≥90 | 9 | 300 (30-NA) |
|
|
|
| Number of HCC |
|
| 0.907 | 1.92
(0.39–9.40) | 0.42 |
|
Single | 13 | 329 (160-NA) |
|
|
|
|
Multiple | 6 | 365 (20-NA) |
|
|
|
| Maximum size of
HCC, mm |
|
| 0.108 |
|
|
|
<20 | 11 | 329 (60-NA) |
|
|
|
|
>20 | 8 | NA (20-NA) |
|
|
|
| History of IFN |
|
| 0.765 |
|
|
|
Absence | 13 | NA (60-NA) |
|
|
|
|
Presence | 6 | 365 (160-NA) |
|
|
|
| RFA prior to
DAA |
|
| 0.389 |
|
|
|
Absence | 12 | NA (60-NA) |
|
|
|
|
Presence | 7 | 300 (30-NA) |
|
|
|
| Hepatectomy prior
to DAA |
|
| 0.181 |
|
|
|
Absence | 14 | 329 (150-NA) |
|
|
|
|
Presence | 5 | NA (60-NA) |
|
|
|
| TACE prior to
DAA |
|
| 0.642 |
|
|
|
Absence | 12 | NA (60-NA) |
|
|
|
|
Presence | 7 | 365 (20-NA) |
|
|
|
| Number of HCC
treatments |
|
| 0.38 |
|
|
| 1 | 8 | NA (60-NA) |
|
|
|
| ≥2 | 11 | 365 (30-NA) |
|
|
|
| Interval between
final HCC treatment and DAA, days |
|
| 0.0284 | 8.25
(1.05–65.18) | 0.045 |
|
≤119 | 9 | 300 (20-NA) |
|
|
|
|
≥120 | 10 | NA (30-NA) |
|
|
|
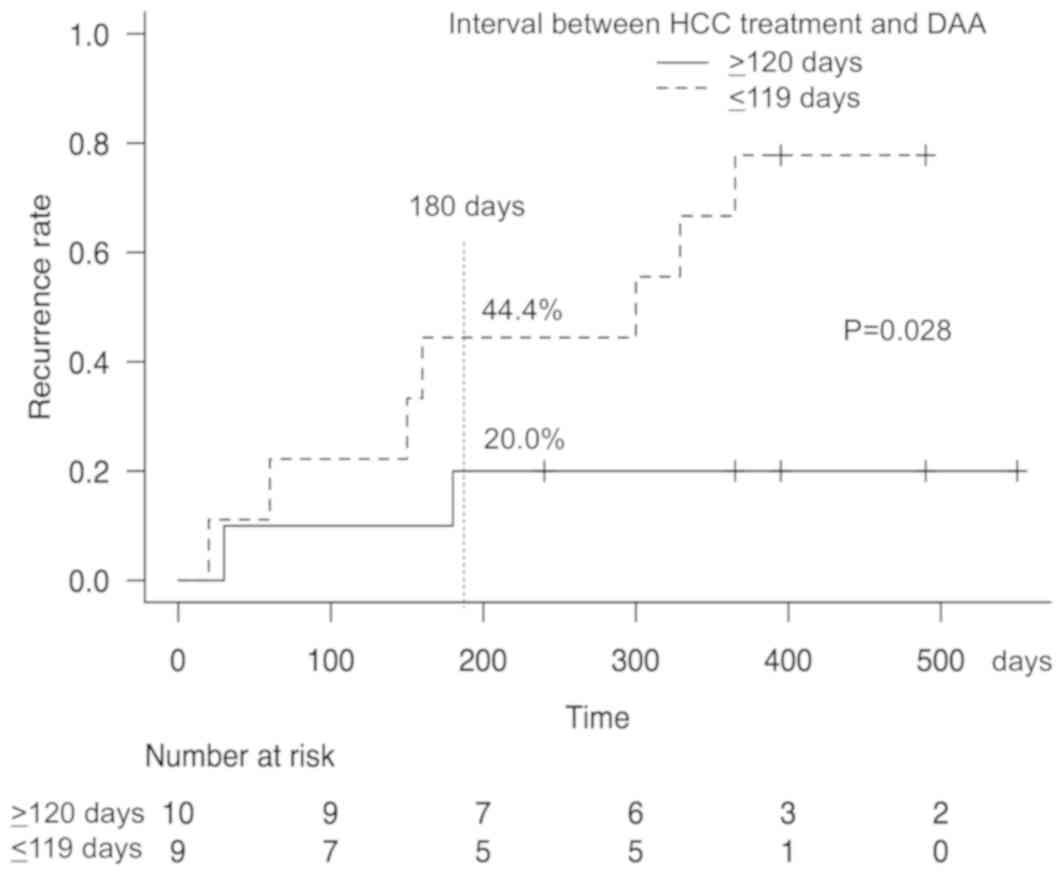
The cumulative recurrence rate of HCC are shown in
Fig. 2. The 180-day cumulative
recurrence rate in patients with a period ≤119 days between final
HCC treatment and DAA treatment was 44.4%, which was significantly
higher compared with the rate for patients with a period of ≥120
days (P=0.028).
Discussion
Recently, IFN-free DAA treatment has been developed,
and consequently the SVR rate has increased further to 90% or more
(13–16). Use of DAAs is currently the
mainstream treatment worldwide (18,23), not
only because of its high rate of SVR, but also because fewer side
effects occur compared with using IFN. Owing to this decrease in
the number of side effects, DAA treatment is also widely used
following hepatectomy for HCC in elderly patients, whose numbers
have been increasing in recent years (24).
However, there have been several reports of DAA
treatment increasing the incidence of HCC. Initially, one study
identified that 16/58 (27.6%) of patients with HCC following
treatment had a median recurrence of 5.7 months (17). Subsequently, several other studies
have identified recurrence rates close to 30% following HCC
treatment, <6 months after DAA treatment (18,19).
These results suggest that DAA treatment may promote carcinogenesis
in patients with HCC with a history of treatment. However, it has
not yet been determined whether DAA treatment promotes
carcinogenesis in patients with no HCC treatment history (1,18,19,25–27).
Several reasons have been proposed for the increase
in HCC recurrence by DAA treatment, as described below. It is
considered that the mechanism of carcinogenesis following DAA
treatment involves changes in IFN gene expression and natural
killer cell function (28). IFN has
anticancer effects and acts in immunoregulation by prolonging all
phases of the cell cycle (28). In
contrast, DAA treatment causes downregulation of IFN genes and
increases cell proliferation without appropriate regulation by
checkpoints. Consistently, it has been reported that serum vascular
endothelial growth factor, angiogenesis and the size of HCC are
increased 4 weeks after the administration of DAAs (29). Although these results have led to
concern that DAAs cause carcinogenesis, this has not yet been
demonstrated, and further research is required. Of relevance, the
results of the present study indicated that patients treated for
HCC had a high recurrence rate. The statistical analyses indicated
that an interval of 4 months after HCC treatment is required to
prevent recurrence. This result is similar to that of a study
published previously (30). It is
not clear why the 4-month interval is required. However, divisions
of 4 months were determined statistically using the median
value.
Currently, IFN has been reported to be
cost-effective because it decreases liver disease-associated
mortality (31), but it is more
expensive compared with DAAs with borderline treatment benefits
(32). If it becomes clear that
there are a number of recurrences of HCC following DAA treatment,
its cost-effectiveness may be very poor. Therefore, further
consideration of the relative value of IFN and DAA treatment is
required. We hypothesize that the residual lesion following HCC
treatment may be associated with recurrence following DAA
treatment.
A decrease in the incidence of HCC following DAA
treatment was identified in a large cohort study of ~17,000
patients with HCV (33). However,
the incidence of HCC by DAA treatment in patients with a history of
HCC was unclear in the conclusion of this paper. It was concluded
that DAAs were administered to patients at high risk of developing
HCC. In addition, several meta-analyses and review articles have
been published. All concluded that DAA treatment decreased the
incidence of initial HCC (34–36).
However, DAA treatment for HCC recurrence has not yet verified. The
findings of these studies highlighted the requirement for
high-quality prospective studies, because the studies included
heterogeneous cohorts, potential misclassification of HCC absence
prior to administration of DAAs, ascertainment bias for recurrence
and short durations of follow-up (34–36).
One meta-analysis focused on the interval from
treatment of HCC to DAA administration. The results indicated that
patients with a 6-month interval between treatment of HCC and DAA
administration decreased the recurrence rate of HCC. Between the
treatment of liver cancer and administration of DAAs, cases with a
period of ~6 months resulted in lowering the recurrence rate of HCC
(37). The results of the present
study indicated that a 4-month interval was required, but it should
be determined whether or not the interval is vital, and, if so, for
how long it is required.
The limitations of the present study are that the
number of patients was small, and it was a single institutional
study. Larger prospective studies with large multicenter cohorts
are required.
In conclusion, the recurrence rate following DAA
treatment may be high in patients with a history of HCC treatment.
To prevent recurrence, an interval of ≥4 months between HCC
treatment and the administration of DAAs is advised. However, the
results are preliminary, and a larger cohort study or much longer
observation period may be required to obtain reliable
conclusions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data used in the present study are available
from the corresponding author on reasonable request.
Authors' contributions
HI designed the research and analyzed the patient
data. HI, RO, TF, HMa, HMo, NK, AA and MT performed the
interventions. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study conformed to the Clinical Research
Guidelines and was approved by the ethical committee of Shiga
University of Medical Science (Otsu, Japan; approval no. 27-233).
Informed consent was obtained from all patients or members of their
families prior to surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
DCP
|
des-γ-carboxyprothrombin
|
|
HCC
|
hepatocellular carcinoma
|
|
IFN
|
interferon
|
|
DAA
|
direct-acting antiviral agent
|
|
HCV
|
hepatitis C virus
|
References
|
1
|
Asselah T and Marcellin P: New
direct-acting antivirals' combination for the treatment of chronic
hepatitis C. Liver Int. 31 (Suppl 1):S68–S77. 2011. View Article : Google Scholar
|
|
2
|
Degos F, Christidis C, Ganne-Carrie N,
Farmachidi JP, Degott C, Guettier C, Trinchet JC, Beaugrand M and
Chevret S: Hepatitis C virus related cirrhosis: Time to occurrence
of hepatocellular carcinoma and death. Gut. 47:131–136. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Imai Y, Kawata S, Tamura S, Yabuuchi I,
Noda S, Inada M, Maeda Y, Shirai Y, Fukuzaki T, Kaji I, et al:
Relation of interferon therapy and hepatocellular carcinoma in
patients with chronic hepatitis C. Osaka hepatocellular carcinoma
prevention study group. Ann Intern Med. 129:94–99. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mazzella G, Accogli E, Sottili S, Festi D,
Orsini M, Salzetta A, Novelli V, Cipolla A, Fabbri C, Pezzoli A and
Roda E: Alpha interferon treatment may prevent hepatocellular
carcinoma in HCV-related liver cirrhosis. J Hepatol. 24:141–147.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hiramatsu N, Oze T and Takehara T:
Suppression of hepatocellular carcinoma development in hepatitis C
patients given interferon-based antiviral therapy. Hepatol Res.
45:152–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cammà C, Giunta M, Andreone P and Craxì A:
Interferon and prevention of hepatocellular carcinoma in viral
cirrhosis: An evidence-based approach. J Hepatol. 34:593–602. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikeda K, Arase Y, Saitoh S, Kobayashi M,
Suzuki Y, Suzuki F, Tsubota A, Chayama K, Murashima N and Kumada H:
Interferon beta prevents recurrence of hepatocellular carcinoma
after complete resection or ablation of the primary tumor-A
prospective randomized study of hepatitis C virus-related liver
cancer. Hepatology. 32:228–232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kubo S, Nishiguchi S, Hirohashi K, Tanaka
H, Shuto T, Yamazaki O, Shiomi S, Tamori A, Oka H, Igawa S, et al:
Effects of long-term postoperative interferon-alpha therapy on
intrahepatic recurrence after resection of hepatitis C
virus-related hepatocellular carcinoma. A randomized, controlled
trial. Ann Intern Med. 134:963–967. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazzaferro V, Romito R, Schiavo M, Mariani
L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli
G, et al: Prevention of hepatocellular carcinoma recurrence with
alpha-interferon after liver resection in HCV cirrhosis.
Hepatology. 44:1543–1554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morgan TR, Ghany MG, Kim HY, Snow KK,
Shiffman ML, De Santo JL, Lee WM, Di Bisceglie AM, Bonkovsky HL,
Dienstag JL, et al: Outcome of sustained virological responders
with histologically advanced chronic hepatitis C. Hepatology.
52:833–844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Backus LI, Boothroyd DB, Phillips BR,
Belperio P, Halloran J and Mole LA: A sustained virologic response
reduces risk of all-cause mortality in patients with hepatitis C.
Clin Gastroenterol Hepatol. 9:509–516.e1. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lok AS, Seeff LB, Morgan TR, di Bisceglie
AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM,
Bonkovsky HL, et al: Incidence of hepatocellular carcinoma and
associated risk factors in hepatitis C-related advanced liver
disease. Gastroenterology. 136:138–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumada H, Suzuki Y, Ikeda K, Toyota J,
Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, et
al: Daclatasvir plus asunaprevir for chronic HCV genotype 1b
infection. Hepatology. 59:2083–2091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Omata M, Nishiguchi S, Ueno Y, Mochizuki
H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T, et al:
Sofosbuvir plus ribavirin in Japanese patients with chronic
genotype 2 HCV infection: An open-label, phase 3 trial. J Viral
Hepat. 21:762–768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizokami M, Yokosuka O, Takehara T,
Sakamoto N, Korenaga M, Mochizuki H, Nakane K, Enomoto H, Ikeda F,
Yanase M, et al: Ledipasvir and sofosbuvir fixed-dose combination
with and without ribavirin for 12 weeks in treatment-naive and
previously treated Japanese patients with genotype 1 hepatitis C:
An open-label, randomised, phase 3 trial. Lancet Infect Dis.
15:645–653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumada H, Chayama K, Rodrigues L Jr,
Suzuki F, Ikeda K, Toyoda H, Sato K, Karino Y, Matsuzaki Y, Kioka
K, et al: Randomized phase 3 trial of
ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype
1b-infected Japanese patients with or without cirrhosis.
Hepatology. 62:1037–1046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reig M, Mariño Z, Perelló C, Iñarrairaegui
M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, et al:
Unexpected high rate of early tumor recurrence in patients with
HCV-related HCC undergoing interferon-free therapy. J Hepatol.
65:719–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Conti F, Buonfiglioli F, Scuteri A, Crespi
C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi
G, et al: Early occurrence and recurrence of hepatocellular
carcinoma in HCV-related cirrhosis treated with direct-acting
antivirals. J Hepatol. 65:727–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calleja JL, Crespo J, Rincón D,
Ruiz-Antorán B, Fernandez I, Perelló C, Gea F, Lens S,
García-Samaniego J, Sacristán B, et al: Effectiveness, safety and
clinical outcomes of direct-acting antiviral therapy in HCV
genotype 1 infection: Results from a Spanish real-world cohort. J
Hepatol. 66:1138–1148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
ANRS collaborative study group on
hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23
CUPILT cohorts). Electronic address: stanislas.pol@aphp.fr: Lack of
evidence of an effect of direct-acting antivirals on the recurrence
of hepatocellular carcinoma: Data from three ANRS cohorts, . J
Hepatol. 65:734–740. 2016.PubMed/NCBI
|
|
21
|
Prenner SB, VanWagner LB, Flamm SL, Salem
R, Lewandowski RJ and Kulik L: Hepatocellular carcinoma decreases
the chance of successful hepatitis C virus therapy with
direct-acting antivirals. J Hepatol. 66:1173–1181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manthravadi S, Paleti S and Pandya P:
Impact of sustained viral response postcurative therapy of
hepatitis C-related hepatocellular carcinoma: A systematic review
and meta-analysis. Int J Cancer. 140:1042–1049. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beste LA, Green PK, Berry K, Kogut MJ,
Allison SK and Ioannou GN: Effectiveness of hepatitis C antiviral
treatment in a USA cohort of veteran patients with hepatocellular
carcinoma. J Hepatol. 67:32–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iida H, Kaibori M, Matsui K, Ishizaki M
and Kon M: Assessing the feasibility of clinicopathological
features of hepatic resection for hepatocellular carcinoma in
patients over 80 years of age. Mol Clin Oncol. 6:29–38. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi M, Suzuki F, Fujiyama S,
Kawamura Y, Sezaki H, Hosaka T, Akuta N, Suzuki Y, Saitoh S, Arase
Y, et al: Sustained virologic response by direct antiviral agents
reduces the incidence of hepatocellular carcinoma in patients with
HCV infection. J Med Virol. 89:476–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alberti A and Piovesan S: Increased
incidence of liver cancer after successful DAA treatment of chronic
hepatitis C: Fact or fiction? Liver Int. 37:802–808. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagaoki Y, Imamura M, Aikata H, Daijo K,
Teraoka Y, Honda F, Nakamura Y, Hatooka M, Morio R, Morio K, et al:
The risks of hepatocellular carcinoma development after HCV
eradication are similar between patients treated with
peg-interferon plus ribavirin and direct-acting antiviral therapy.
PLoS One. 12:e01827102017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nault JC and Colombo M: Hepatocellular
carcinoma and direct acting antiviral treatments: Controversy after
the revolution. J Hepatol. 65:663–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Villani R, Facciorusso A, Bellanti F,
Tamborra R, Piscazzi A, Landriscina M, Vendemiale G and Serviddio
G: DAAs rapidly reduce inflammation but increase serum VEGF level:
A rationale for tumor risk during Anti-HCV treatment. PLoS One.
11:e01679342016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsai PC, Huang CF and Yu ML: Unexpected
early tumor recurrence in patients with hepatitis C virus-related
hepatocellular carcinoma undergoing interferon-free therapy: Issue
of the interval between HCC treatment and antiviral therapy. J
Hepatol. 66:4642017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan K, Lai MN, Groessl EJ, Hanchate AD,
Wong JB, Clark JA, Asch SM, Gifford AL and Ho SB: Cost
effectiveness of direct-acting antiviral therapy for
treatment-naive patients with chronic HCV genotype 1 infection in
the veterans health administration. Clin Gastroenterol Hepatol.
11:1503–1510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cortesi PA, Mantovani LG, Ciaccio A, Rota
M, Mazzarelli C, Cesana G, Strazzabosco M and Belli LS:
Cost-effectiveness of new direct-acting antivirals to prevent
post-liver transplant recurrent hepatitis. Am J Transplant.
15:1817–1826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li DK, Ren Y, Fierer DS, Rutledge S,
Shaikh OS, Lo Re V III, Simon T, Abou-Samra AB, Chung RT and Butt
AA: The short-term incidence of hepatocellular carcinoma is not
increased after hepatitis C treatment with direct-acting
antivirals: An ERCHIVES study. Hepatology. 67:2244–2253. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guarino M, Viganò L, Ponziani FR, Giannini
EG, Lai Q and Morisco F; Special Interest Group on Hepatocellular
carcinoma and new anti-HCV therapies' of the Italian Association
for the Study of the Liver, : Recurrence of hepatocellular
carcinoma after direct acting antiviral treatment for hepatitis C
virus infection: Literature review and risk analysis. Dig Liver
Dis. 50:1105–1114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singh S, Nautiyal A and Loke YK: Oral
direct-Acting antivirals and the incidence or recurrence of
hepatocellular carcinoma: A systematic review and meta-analysis.
Frontline Gastroenterol. 9:262–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tampaki M, Savvanis S and Koskinas J:
Impact of direct-acting antiviral agents on the development of
hepatocellular carcinoma: Evidence and pathophysiological issues.
Ann Gastroenterol. 31:670–679. 2018.PubMed/NCBI
|
|
37
|
Saraiya N, Yopp AC, Rich NE, Odewole M,
Parikh ND and Singal AG: Systematic review with meta-analysis:
Recurrence of hepatocellular carcinoma following direct-acting
antiviral therapy. Aliment Pharmacol Ther. 48:127–137. 2018.
View Article : Google Scholar : PubMed/NCBI
|