Introduction
Prostate cancer (PCa) is one of the most common
cancers in males. Current guidelines (NCCN) consider radiation
therapy (RT) as a therapeutic option in different disease stages
using three-dimensional conformal RT (3D-CRT) and Intensity
Modulated RT (IMRT) as the standard techniques (1).
The incidence of PCa is lower in developing than in
western countries. However, a progressive increase in the incidence
of PCa due to the prolonged life expectancy has been recorded
(2). Furthermore, available RT
technologies in developing countries have several limitations with
several centres using only standard simulators and cobalt machines
as the treatment planning and delivery technologies, respectively
(3–5).
In the past, PCa irradiation was based on 2D
techniques with treatment fields defined with standard simulators
(6,7). In the ′80s, further population-based
indications for 2D-RT arose from the evaluation of prostate size
and anatomical location using computed tomography (CT) scans
(8). However, nowadays more detailed
information and guidelines are available allowing tailored RT even
with 2D technology.
In fact, RT of PCa is based on: i) clear guidelines
on target definition related to risk categories (9); ii) treatment planning systems (TPS) to
enable 3D dose evaluation with possibilities of computing a
customized treatment plan for individual patients by adapting the
beams geometry to different beam energies; iii) the possibility of
defining standard irradiation geometries based on 3D dose
distribution among a patients population.
Optimized 2D-RT based on these new insights could be
helpful for centres without advanced RT technologies (3D-CRT,
IMRT).
Based on this background, the purpose of this study
was to propose practical guidelines for 2D-RT beams definition
adapted to different PCa risk categories and different available
beam energies.
Materials and methods
From our institution, 20 patients with histological
confirmation of PCa, consecutively treated with RT were identified
(median age: 72 years; range: 58–77 years; clinical T stage: cT2b:
3, cT2c: 5, cT3a: 9, cT3b: 3). Patients underwent CT-simulation in
supine position after 3 days of laxatives to avoid rectal
distension. Before CT-simulation commencement, 10 cc of contrast
medium (Gastrografin) were injected into the rectum. Scans were
performed every 5 mm from 3 cm below the ischial tuberosities to 3
cm above the promontory. Patients underwent pelvic MRI scan. MRI
images were fused with CT-simulation images by using the VelocityAI
system (Velocity Medical Solutions, Atlanta, GA) based on the
B-spine algorithm for deformable registrations. In this way,
delineation of the prostate and seminal vesicles was performed on
MRI images. The delineated targets were then transferred to
CT-simulation images for treatment planning.
Clinical Target Volume definition (CTV) was based on
the EORTC guidelines (9).
Irrespective of the individual patient's tumor stage, CTV
delineation was done for four different categories: i) low-risk
PCa: CTV = prostate; ii) intermediate-risk PCa: CTV = prostate + 5
mm radial margin, with inclusion of the caudal (1 cm) portion of
the seminal vesicles; iii) high-risk PCa without involvement of the
seminal vesicles: Prostate + 5 mm radial margin, with the inclusion
of the caudal (2 cm) portion of the seminal vesicles; iv) high-risk
PCa with involvement of seminal vesicles: prostate + 5 mm radial
margin and inclusion of all the seminal vesicles. All contours were
verified by an experienced operator and a senior consultant (GM,
FD, AGM). Organs at Risk (OaRs) contours were defined according to
the QUANTEC indications (10). The
Planning Target Volume (PTV) was defined by adding a margin of 10
mm to the CTV in all directions (11).
For each patient, eight treatment plans were
generated. For each of the four risk categories, two box technique
treatment plans were calculated using a cobalt source or 10 MV
photons. A fixed Source-Axis Distance (SAD) of 100 cm for Linear
Accelerator and 80 cm for the cobalt unit was used. The beams
weights were 20% (anterior-posterior and posterior-anterior beams)
and 30% (lateral beams) to reduce the dose to the rectum, small
bowel, and bladder. Beams were drawn using the standard collimators
(without multileaf collimators). Standard collimators were
initially placed at 5 mm distance with respect to the PTV margins.
Then the minimum dose (defined as D98%) was evaluated. Fields sizes
were gradually increased in steps of 5 mm to achieve the minimum
PTV dose constraint (D98% >95%). This progressive optimization
was carried out with an iterative procedure, with several
evaluations of cumulative dose/volume histograms and beams eye-view
dose paintings. In this way, it was possible to identify the field
sizes to be increased based on observed ‘cold spots’ sites.
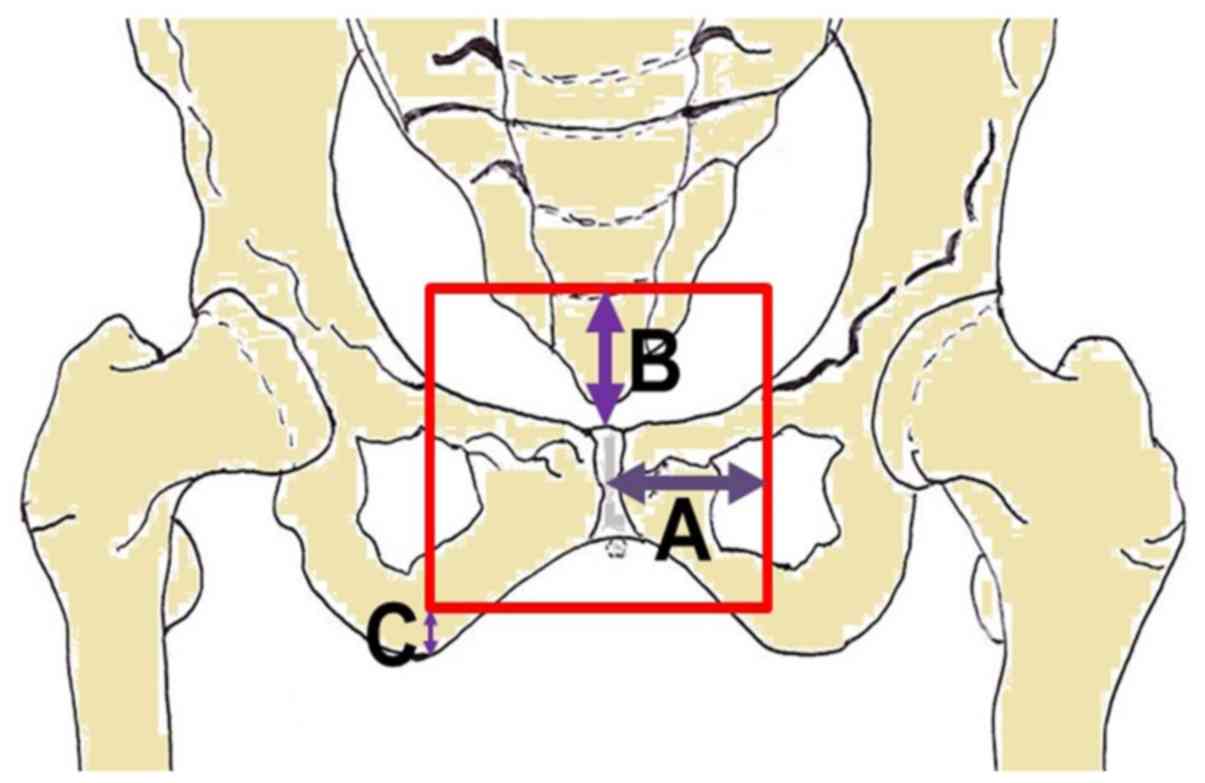
Once the final plan was achieved, distances of the
field edges from a set of reference points (Tables I–IV)
were measured. Both the maximum and the 95th percentile of the
distances were identified. The latter value was taken as the
‘recommended’ value for radiation fields margin.
 | Table I.Field definition: Low risk prostate
cancer. |
Table I.
Field definition: Low risk prostate
cancer.
|
|
|
| Treatment
machine |
|---|
|
|
|
|
|
|---|
| Field | Margin | Description | Cobalt 60 | 10 MV LINAC |
|---|
|
Anterior-posterior | Lateral | From the center of
the symphisis pubis (laterally) [A] | 5.7 (5.7) | 4.5 (5.0) |
|
| Inferior | From the bottom of
ischial tuberosities (above) [B] | 0.9 (0.9) | 1.4 (1.8) |
|
| Superior | From the top of the
symphisis pubis (above) [C] | 5.4 (6.7) | 4.9 (5.7) |
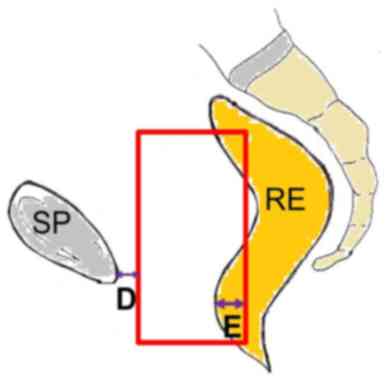
| Lateral | Anterior | From the posterior
margin of the symphisis pubis (posteriorly) [D] | 0.3 (0.4) | 0.4 (0.5) |
|
| Posterior | From the most
anterior point of the rectum (posteriorly) [E] | 4.7 (5.9) | 3.3 (3.7) |
|
| Inferior | From the bottom of
ischial tuberosities (above) | 0.9 (0.9) | 1.4 (1.8) |
|
| Superior | From the top of the
symphisis pubis (above) | 5.4 (6.7) | 4.9 (5.7) |
 | Table IV.Field definition: High-risk prostate
cancer with seminal vesicle involvement. |
Table IV.
Field definition: High-risk prostate
cancer with seminal vesicle involvement.
|
|
|
| Treatment
machine |
|---|
|
|
|
|
|
|---|
| Fields | Margin | Description | Cobalt 60 | 10 MV LINAC |
|---|
|
Anterior-posterior | Lateral | From the center of
the symphisis pubis (laterally) [A] | 7.7 (9.8) | 6.7 (6.9) |
|
| Inferior | From the bottom of
ischial tuberosities (above) [B] | 0.4 (0.6) | 1.0 (1.3) |
|
| Superior | From the top of the
symphisis pubis (above) [C] | 8.7 (9.1) | 6.6 (7.3) |
| Lateral | Anterior | From the posterior
margin of the symphisis pubis (posteriorly) [D] | 0.3 (0.4) | 0.3 (0.4) |
|
| Posterior | From the most
anterior point of the rectum (posteriorly) [E] | 6.7 (8.1) | 5.1 (6.0) |
|
| Inferior | From the bottom of
ischial tuberosities (above) | 0.4 (0.6) | 1.0 (1.3) |
|
| Superior | From the top of the
symphisis pubis (above) | 8.7 (9.1) | 6.6 (7.3) |
The study was approved by the institutional board
High Technology Center for Research and Education-Ethical Committee
(Campobasso, Italy) and it is registered in an international public
registry (ClinicalTrials.gov Identifier:
NCT03339531). Written informed consent was obtained from all of the
enrolled patients for the use of their images in this study prior
to the analysis.
Results
Tables I–IV show the results of the analysis in
terms of fields margins from radiological landmarks margins in the
various patient's categories: Low risk, intermediate risk, high
risk, and high risk with involvement of seminal vesicles. Both the
field margins needed to adequately irradiate all patients of the
analysed sample and distances sufficient to achieve the same result
in 95% of the enrolled patients are reported. The latter dimensions
were defined as the ‘recommended’ margins. Figs. 1 and 2
show the distances to be considered between fields margins and the
radiological landmarks.
Discussion
A planning study on real patients' population was
performed to suggest personalized treatment margins for 2D-RT. A
box technique was used because it is easy to plan with a
conventional simulator and dose conformity produced to the target.
Furthermore, previous analysis showed that this technique produces
planning results comparable to those achieved with more complex
techniques (e.g., 6 beams) (12).
Definition of anatomical structures like seminal vesicles and
prostate apex location was performed with pelvic MRI
co-registration. This integration was used based on the advantages
of MRI in prostatic target definition as previously clearly
demonstrated (13). Particularly, a
study of Villeirs and colleagues showed that the fusion of MRI and
CT in PCa contouring results in a moderate decrease of the CTV but
a relevant decrease of inter-observer variation especially at the
prostate apex (14).
Before CT-simulation, a small amount of contrast
medium was injected into the rectum. This preparation although not
required for CT-simulation was used to attain the same conditions
for conventional simulation. In fact, the purpose of this study was
to provide practical guidelines for this planning method. CTV to
PTV margin of 10 mm was used based on a randomized trial which
demonstrated that this margin produces the same clinical results
compared to larger margins (11).
This result was confirmed by Creak and colleagues who reported no
evidence of a difference in PSA control according to CTV to PTV
margin (1 cm vs. 1.5 cm) (15). It
must be acknowledged that CTV to PTV margin lower than one
centimetre is currently used. However, we considered this margin
appropriate being that our suggestions are mostly addressed to
centres without electronic portal imaging devices or more advanced
image-guided technologies.
In this analysis, irradiation beams of different
energies were simulated including beams produced by a cobalt
machine. We must recognize that the use of cobalt machines is
currently considered obsolete especially for PCa treatment.
However, in many developing countries RT departments it is the only
available treatment device (3,4). The
possibility of effective dose delivery with this type of treatment
unit respecting the current dose/volume constraints remains
uncertain. In particular, it is doubtful the possibility of an
effective delivery to high tumor dose with safe OaRs irradiation
using the box technique despite its practical advantages. It is
generally believed that using only 4 beams, the delivery of >60
Gy doses is impossible without reaching an excessive dosage to
superficial tissues. In fact, PCa treatment with cobalt machines
was often performed with rotational techniques. However, we still
included in this analysis also irradiation with a cobalt machine
due to the following reasons:
i) Doses lower than the ones currently considered
standard (>70–75 Gy) (i) may still be useful in post-operative
treatment; (ii) some randomized studies showed a significant
biochemical and clinical benefit by delivering 60 Gy to the
prostatic bed (16–18); ii) lower standard doses might still
be effective if combined with androgen deprivation therapy (ADT);
several randomized studies demonstrated a significant advantage in
terms of specific or overall survival by combining ADT to RT at
lower doses (65–70 Gy) than those currently considered as standards
(>70–75 Gy) (19–23);
iii) the current recommended ‘standard doses’ were
defined mainly based on biochemical relapse-free survival advantage
and not in terms of overall survival (11,24–28); the
use of high doses in other words was less associated with a
significant improvement of ‘clinical’ outcomes;
iv) in addition, a meta-analysis including 7
randomized clinical trials compared the results achieved with
conventional RT dose and high-dose RT. The latter resulted
significantly associated with improved biochemical control but
there was no difference in terms of mortality rate and specific
prostate cancer mortality rate. Furthermore, a subgroup analysis
showed that a dose of 64 Gy is associated with a 5-year biochemical
relapse-free survival of 72, 61 and 40% in low, intermediate and
high-risk PCa, respectively (29).
Obviously, these results cannot be defined as optimal but may be
acceptable in health systems where other alternative therapies or
RT techniques are not available.
In our analysis we have not dealt with the problem
of OaRs and planning organ at risk volumes. The main reason is that
using a 2D-RT technique it is not possible to calculate the DVHs
and to evaluate the constraints of OaRs including planning organ at
risk volumes. However, the technique proposed by us is intended to
be used with relatively low doses (60–64 Gy). In accordance to the
QUANTEC, the maximum bladder dose should be less than 65 Gy and the
V65 Gy of the rectum must be less than 25%,
it is reasonably likely that these constraints are respected.
Guidelines for PCa 2D-RT were obviously available in the past.
However, these were mainly based on ‘expert's opinion’ or
population-based CT measurements of prostate and seminal vesicles
(6,8). Our study presents obvious differences
in terms of: i) precise MRI-based prostate and seminal vesicles
contouring; ii) use of an additional margin between prostate and
CTV according to risk category; iii) CTV to PTV margin validated by
the results of a clinical trial (11); iv) definition of field margins
adapted to different energy beams.
Probably in clinical practice it is possible to
further optimize our instructions by customizing them to individual
patients even with 2D technology. These optimizations can be
implemented with simple diagnostic integrations feasible with a
standard simulator.
Use of retrograde urethrography and cystography for
example could enable an individualized location of the prostate
apex and base, respectively (30,31). In
addition, it should be noted that the recommended margins in our
study are based on the 95th percentile of the obtained measurement.
This means that they may be considered adequate in 95% of patients.
This choice derives from the need to obtain a compromise between
tumor control probability and the risk of side effects. However, in
the tables also the maximum value of the measured distance, i.e.
the sizes appropriate in 100% of the evaluated sample was
indicated. Therefore, in case of simulation images showing a
reduced OaRs involvement, the planner can use the larger value to
increase the likelihood of complete target ‘coverage’. Although the
limits of PCa irradiation with a cobalt machine have been
previously mentioned, this analysis represents the basis for a
subsequent study that has been planned in our center with the aim
of defining the dose which can be safely administered with this
kind of machine. The study will be conducted based on the current
OaRs dose/volume constraints (10).
Our study was limited to prostate +/- seminal
vesicles irradiation. However, according to current guidelines in
high-risk patients, prophylactic irradiation of the pelvic lymph
nodes is recommended (1). Therefore,
a further study was planned to provide 2D indications for pelvic
fields design based on current guidelines for nodal CTV definition
(32). In conclusion, we aimed at
providing convenient 2D PCa target delineation tools. In the last
years, our team worked on the optimization of 2D-RT in palliative
treatments (33–35). Worth noting is that 2D-RT is still in
use in several centers in the world. Therefore, we think that other
similar studies based on advanced radiological technologies could
be performed to optimize 2D-RT techniques in other tumors for less
equipped departments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
MB, SC, FD, GM, TW, KAFMU, MAS and AGM conceived and
designed the present study. SC, MB, MP, EG, GS, IC, AAW and AGM
planned the treatments, and analyzed and interpreted the data. MB,
FD, IC, AAW, GM, TW, KAFMU, MAS and AGM drafted the article. IC,
AAW, TW, KAFMU, MAS and AGM critically revised the manuscript for
important intellectual content. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
board High Technology Center for Research and Education-Ethical
Committee (Campobasso, Italy), and it is registered in an
international public registry (ClinicalTrials.gov Identifier: NCT03339531). Written
informed consent was obtained from all of the enrolled patients for
the use of their images in this study prior to the analysis.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohler J, Bahnson RR, Boston B, Busby JE,
D'Amico A, Eastham JA, Enke CA, George D, Horwitz EM, Huben RP, et
al: NCCN clinical practice guidelines in oncology: Prostate cancer.
J Natl Compr Canc Netw. 8:162–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adebamowo CA and Akarolo-Anthony S: Cancer
in Africa: Opportunities for collaborative research and training.
Afr J Med Med Sci (38 Suppl 2). S5–S13. 2009.
|
|
3
|
Barton MB, Frommer M and Shafiq J: Role of
radiotherapy in cancer control in low-income and middle-income
countries. Lancet Oncol. 7:584–595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kigula Mugambe JB and Wegoye P: Pattern
and experience with cancers treated with the Chinese GWGP80 cobalt
unit at Mulago Hospital, Kampala. East Afr Med J. 77:523–525.
2000.PubMed/NCBI
|
|
5
|
Page BR, Hudson AD, Brown DW, Shulman AC,
Abdel-Wahab M, Fisher BJ and Patel S: Cobalt, linac, or other: What
is the best solution for radiation therapy in developing countries?
Int J Radiat Oncol Biol Phys. 89:476–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zelefsky MJ, Valicenti RK, Hunt M and
Perez CA: Low-risk prostate cancer. Perez and Brady's Principles
and Practice of Radiation Oncology. Halperin EC, Perez CA, Brady
LW, Wazer DE, Freeman C and Prosnitz LR: 5th. Lippincott Williams
& Wilkins; pp. 1280–1311. 2007
|
|
7
|
Hussey DH: Carcinoma of the prostate.
Textbook of Radiotherapy. Fletcher GH: 3rd. Lea & Febiger;
Philadelphia: pp. 894–914. 1980
|
|
8
|
Pilepich MV, Prasad SC and Perez CA:
Computed tomography in definitive radiotherapy of prostatic
carcinoma, part 2: Definition of target volume. Int J Radiat Oncol
Biol Phys. 8:235–239. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boehmer D, Maingon P, Poortmans P, Baron
MH, Miralbell R, Remouchamps V, Scrase C, Bossi A and Bolla M;
EORTC radiation oncology group, : Guidelines for primary
radiotherapy of patients with prostate cancer. Radiother Oncol.
79:259–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bentzen SM, Constine LS, Deasy JO,
Eisbruch A, Jackson A, Marks LB, Ten Haken RK and Yorke ED:
Quantitative analyses of normal tissue effects in the clinic
(QUANTEC): An introduction to the scientific issues. Int J Radiat
Oncol Biol Phys. 76 (3 Suppl):S3–S9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dearnaley DP, Hall E, Lawrence D, Huddart
RA, Eeles R, Nutting CM, Gadd J, Warrington A, Bidmead M and
Horwich A: Phase III pilot study of dose escalation using conformal
radiotherapy in prostate cancer: PSA control and side effects. Br J
Cancer. 92:488–498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khoo VS, Bedford JL, Webb S and Dearnaley
DP: Class solutions for conformal external beam prostate
radiotherapy. Int J Radiat Oncol Biol Phys. 55:1109–1120. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith WL, Lewis C, Bauman G, Rodrigues G,
D'Souza D, Ash R, Ho D, Venkatesan V, Downey D and Fenster A:
Prostate volume contouring: A 3D analysis of segmentation using
3DTRUS, CT, and MR. Int J Radiat Oncol Biol Phys. 67:1238–1247.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Villeirs GM, Van Vaerenbergh K, Vakaet L,
Bral S, Claus F, De Neve WJ, Verstraete KL and De Meerleer GO:
Interobserver delineation variation using CT versus combined CT +
MRI in intensity-modulated radiotherapy for prostate cancer.
Strahlenther Onkol. 181:424–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Creak A, Hall E, Horwich A, Eeles R, Khoo
V, Huddart R, Parker C, Griffin C, Bidmead M, Warrington J and
Dearnaley D: Randomised pilot study of dose escalation using
conformal radiotherapy in prostate cancer: Long-term follow-up. Br
J Cancer. 109:651–657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bolla M, van Poppel H, Collette L, van
Cangh P, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset
JF, van Velthoven R, et al: Postoperative radiotherapy after
radical prostatectomy: A randomised controlled trial (EORTC trial
22911). Lancet. 366:572–578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Swanson GP, Hussey MA, Tangen CM, Chin J,
Messing E, Canby-Hagino E, Forman JD, Thompson IM and Crawford ED;
SWOG 8794, : Predominant treatment failure in postprostatectomy
patients is local: Analysis of patterns of treatment failure in
SWOG 8794. J Clin Oncol. 25:2225–2229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wiegel T, Bottke D, Steiner U, Siegmann A,
Golz R, Störkel S, Willich N, Semjonow A, Souchon R, Stöckle M, et
al: Phase III postoperative adjuvant radiotherapy after radical
prostatectomy compared with radical prostatectomy alone in pT3
prostate cancer with postoperative undetectable prostate-specific
antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 27:2924–2930. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pilepich MV, Winter K, John MJ, Mesic JB,
Sause W, Rubin P, Lawton C, Machtay M and Grignon D: Phase III
radiation therapy oncology group (RTOG) trial 86-10 of androgen
deprivation adjuvant to definitive radiotherapy in locally advanced
carcinoma of the prostate. Int J Radiat Oncol Biol Phys.
50:1243–1252. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pilepich MV, Winter K, Lawton CA, Krisch
RE, Wolkov HB, Movsas B, Hug EB, Asbell SO and Grignon D: Androgen
suppression adjuvant to definitive radiotherapy in prostate
carcinoma-long-term results of phase III RTOG 85-31. Int J Radiat
Oncol Biol Phys. 61:1285–1290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bolla M, Van Tienhoven G, Warde P, Dubois
JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C,
Billiet I, et al: External irradiation with or without long-term
androgen suppression for prostate cancer with high metastatic risk:
10-year results of an EORTC randomised study. Lancet Oncol.
11:1066–1073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Denham JW, Steigler A, Lamb DS, Joseph D,
Turner S, Matthews J, Atkinson C, North J, Christie D, Spry NA, et
al: Short-term neoadjuvant androgen deprivation and radiotherapy
for locally advanced prostate cancer: 10-year data from the TROG
96.01 randomised trial. Lancet Oncol. 12:451–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nguyen PL, Chen MH, Beard CJ, Suh WW,
Renshaw AA, Loffredo M, McMahon E, Kantoff PW and D'Amico AV:
Radiation with or without 6 months of androgen suppression therapy
in intermediate- and high-risk clinically localized prostate
cancer: A postrandomization analysis by risk group. Int J Radiat
Oncol Biol Phys. 77:1046–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shipley WU, Verhey LJ, Munzenrider JE,
Suit HD, Urie MM, McManus PL, Young RH, Shipley JW, Zietman AL,
Biggs PJ, et al: Advanced prostate cancer: The results of a
randomized comparative trial of high dose irradiation boosting with
conformal protons compared with conventional dose irradiation using
photons alone. Int J Radiat Oncol Biol Phys. 32:3–12. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pollack A, Zagars GK, Starkschall G,
Antolak JA, Lee JJ, Huang E, von Eschenbach AC, Kuban DA and Rosen
I: Prostate cancer radiation dose response: Results of the M. D.
Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys.
53:1097–1105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zietman AL, DeSilvio ML, Slater JD, Rossi
CJ Jr, Miller DW, Adams JA and Shipley WU: Comparison of
conventional-dose vs. high-dose conformal radiation therapy in
clinically localized adenocarcinoma of the prostate: A randomized
controlled trial. JAMA. 294:1233–1239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sathya JR, Davis IR, Julian JA, Guo Q,
Daya D, Dayes IS, Lukka HR and Levine M: Randomized trial comparing
iridium implant plus external-beam radiation therapy with
external-beam radiation therapy alone in node-negative locally
advanced cancer of the prostate. J Clin Oncol. 23:1192–1199. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peeters ST, Heemsbergen WD, Koper PC, van
Putten WL, Slot A, Dielwart MF, Bonfrer JM, Incrocci L and Lebesque
JV: Dose-response in radiotherapy for localized prostate cancer:
Results of the Dutch multicenter randomized phase III trial
comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol.
24:1990–1996. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Viani GA, Stefano EJ and Afonso SL:
Higher-than-conventional radiation doses in localized prostate
cancer treatment: A meta-analysis of randomized, controlled trials.
Int J Radiat Oncol Biol Phys. 74:1405–1418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roach M III, Pickett B, Holland J,
Zapotowski KA, Marsh DL and Tatera BS: The role of the urethrogram
during simulation for localized prostate cancer. Int J Radiat Oncol
Biol Phys. 25:299–307. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu YM, Ling S, Langen KM, Shinohara K,
Weinberg V, Pouliot J and Roach M III: Prostate movement during
simulation resulting from retrograde urethrogram compared with
‘natural’ prostate movement. Int J Radiat Oncol Biol Phys.
60:470–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lawton CA, Michalski J, El-Naqa I,
Buyyounouski MK, Lee WR, Menard C, O'Meara E, Rosenthal SA, Ritter
M and Seider M: RTOG GU Radiation oncology specialists reach
consensus on pelvic lymph node volumes for high-risk prostate
cancer. Int J Radiat Oncol Biol Phys. 74:383–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buwenge M, Marinelli A, Deodato F, Macchia
G, Wondemagegnhu T, Salah T, Cammelli S, Uddin AFMK, Sumon MA,
Donati CM, et al: Definition of fields margins for palliative
radiotherapy of pancreatic carcinoma. Mol Clin Oncol. 8:715–718.
2018.PubMed/NCBI
|
|
34
|
Morganti AG, Marinelli A, Buwenge M,
Macchia G, Deodato F, Massaccesi M, Kigula-Mugambe J, Wondemagegnhu
T, Dawotola D, Caravatta L, et al: Palliative two-dimensional
radiotherapy of pancreatic carcinoma: A feasibility study. Tumori.
99:488–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Buwenge M, Cilla S, Cammelli S, Macchia G,
Arcelli A, Farina E, Frakulli R, Panni V, Wondemagegnhu T, Uddin
AFMK, et al: Feasibility of 2D-conformal radiotherapy for
pancreatic carcinoma. Oncol Lett. 16:5939–5945. 2018.PubMed/NCBI
|