Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a fatal
disease with an overall survival (OS) rate of <6% (1,2).
Although the advent of nab-paclitaxel (nab-PTX) plus
gemcitabine (GEM) therapy (GnP therapy) and 5-FU, leucovorin,
irinotecan and oxaliplatin (FOLFIRINOX) therapy improved treatment
outcome of unresectable PDAC (3,4), surgery
is the only method to achieve long-term survival. Curative (R0)
resection comprising wide lymph node dissection and complete
removal of the extrapancreatic nerve plexus of the superior
mesenteric artery (SMA) or celiac axis (5–10) has
been shown to be one of the key factors influencing survival of
patients with PDAC. However, even in patients who undergo
resection, 5-year survival is poor at <30% and the prognosis of
PDAC has not improved. Nimura et al (11), reported that extended lymphadenectomy
does not improve prognosis in pancreatic head cancer. These
disappointing results indicate that surgery alone is inadequate and
the poor survival is likely attributable to early hematogenous
spread, because in most patients' metastases are present at the
time of surgery (12). Investigation
of postoperative adjuvant chemotherapy is based on this hypothesis.
Oettle et al (13), reported
that adjuvant chemotherapy with GEM produced a statistically
significant improvement in OS. Recently, the JASPAC-01 study in
Japan showed that S-1, an oral fluoropyrimidine analogue, confers
significantly improved OS and recurrence-free survival after
pancreatic cancer resection compared with GEM (14).
A major drawback of adjuvant therapy for PDAC is
that 20–30% of patients are ineligible to receive the designated
therapy because of postoperative complications, such as delayed
surgical recovery, patient refusal, comorbidity, or early disease
recurrence (15–17). This could be overcome by the
preoperative (neoadjuvant) chemotherapy (NAC) or chemoradiotherapy
so that more patients can receive potentially beneficial treatment.
Other theoretical advantages of this approach include the
following: Early treatment of micrometastases; sparing those who
already have occult metastases the morbidity and mortality
associated with major surgery if disseminated disease becomes
apparent at the time of reassessment; reduced risk of tumor seeding
at the time of surgery; and improved tolerance compared with
postoperative therapys.
Potential disadvantages of neoadjuvant therapy
include the following: A requirement for biliary decompression
before chemotherapy and the potential for complications associated
with biliary stents; delayed surgery, allowing progression to an
unresectable stage in patients whose disease does not respond to
therapy; and the potential for an increase in postoperative
complications. Recently, results of randomized clinical trials and
data analyses of preoperative therapy for borderline resectable and
locally advanced PDAC have been reported (18–22).
However, there have been few reports with high evidence levels on
preoperative therapy for resectable PDAC.
We have used neoadjuvant chemotherapy (NAC) for
resectable PDAC since December 2006, and previously conducted some
clinical studies of NAC with a GEM plus S-1 (GS) regimen for
resectable PDAC as a pilot study and phase I trial (23,24).
From August 2013, NAC with a GnP protocol has been used for
resectable PDAC in a pilot clinical trial. GEM monotherapy was
performed at the transition of two regimens. We report our local
experience and long-term outcomes with NAC with GEM-based regimens
for resectable PDAC, compared with those treated with upfront
surgery retrospectively. In addition, we evaluate risk factors for
recurrence after surgery for potentially resectable PDAC cases in
the same period.
Materials and methods
Patients and NAC regimens
From January 2006 to December 2015, 91 patients with
radiologically-proven PDAC considered ‘resectable’ according to the
National Comprehensive Cancer Network (NCCN) guidelines and 86 (50
males and 36 female) patients were operated on at the Department of
Gastroenterological Surgery, Kanazawa University Hospital. Five
patients did not undergo surgery due to rapid tumor local
progression in two cases, distant metastasis detected after
preoperative chemotherapy in two cases and a case of portal vein
thrombosis caused by biliary drainage during preoperative
chemotherapy. In this period, NAC with GEM-based regimens was
performed in 52 cases (NAC group) of the 86 resectable PDAC cases,
and in the remaining 34 cases, surgery was performed without
preoperative chemotherapy (Control group) at the discretion of the
attending physician. In 52 cases of NAC group, there were 31
pancreatic head cancer and 21 body and tail cancer. Control group
obtained 20 pancreatic head cancer and 14 body and tail cancer.
Three types of GEM based regimens, GEM alone, GS, and GnP therapies
were adopted in the NAC group. The case numbers of each treatment
was 10 cases of GEM alone, 33 cases of GS and 9 cases of GnP. In
GEM monotherapy, 1,000 mg/m2 of GEM was administered as
a 30 min intravenous infusion on days 8, 15 and 22 of each cycle.
The cycle was performed twice every 28 days (Fig. 1A). In GS therapy, S-1 was
administered orally postprandially for 14 consecutive days at the
dose of 20 mg/m2/day (from the evening of day 1 to the
morning of day 15), and 1,000 mg/m2 of GEM was
administered as a 30 min intravenous infusion on days 8 and 15 of
each cycle. The cycle was performed twice every 21 days (Fig. 1B). In GnP therapy, 25 or 50
mg/m2 of nab-PTX and 1,000 mg/m2 of
GEM were administered as 60 and 30 min intravenous infusions,
respectively, on days 8, 15 and 22 of each cycle. The cycle was
performed twice every 28 days (Fig.
1C). In all the NAC regimens, surgery was performed >14 days
after the two cycles of chemotherapy ended.
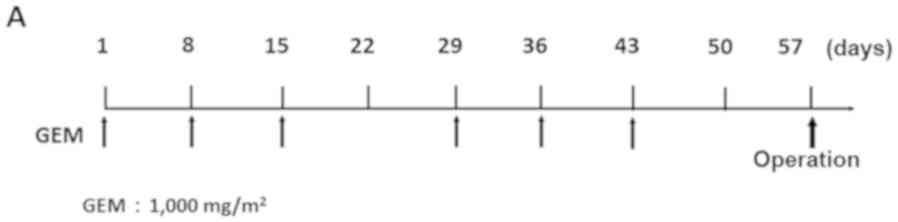 | Figure 1.Treatment protocol for NAC with
GEM-based regimens. (A) GEM monotherapy. GEM (1,000 mg/m2) was
administered on days 1, 8 and 15 of the first cycle, and on days
29, 36 and 43 in the second cycle. (B) GEM plus S-1 therapy: S-1
(20–40 mg/m2/day) was given orally for 14 consecutive days, and GEM
(1,000 mg/m2) was administered intravenously on days 8 and 15 in
the first cycle, and on days 29 and 36 in the second cycle. (C)
nab-PTX plus GEM therapy: 25–50 mg/m2 of nab-PTX and 1,000 mg/m2 of
GEM were administered intravenously on days 1, 8 and 15 in the
first cycle, and on days 29, 36 and 43 in the second cycle. In all
NAC regimens, surgery was performed >2 weeks after the 2
treatment cycles. NAC, neoadjuvant chemotherapy; GEM, gemcitabine;
nab-PTX, nab-paclitaxel. |
Written informed consent was obtained from each
patient prior to treatment, and the present study was approved by
the Ethics Committee of Kanazawa University Hospital (review
number: 2799-1). The authors declare that they have no competing
interests about this study.
Assessments of efficacy
All the patients with resectable PDAC were diagnosed
by multidetector computed tomography (MDCT), magnetic resonance
imaging enhanced with gadolinium ethoxybenzyl diethylenetriamine
pentaacetic acid (Gd-EOB-DTPA) (EOB-MRI), and
18-fluorodeoxyglucose-positron emission tomography/computed
tomography (18FDG-PET/CT) imaging. In the NAC group
tumor response was by comparing pretreatment and posttreatment
images, and was graded according to the Response Evaluation
Criteria in Solid Tumors (RECIST) version 1.0 (25). Complete response (CR) was defined as
the disappearance of all clinical evidence of the measurable tumor.
Partial response (PR) was defined as a 30% or greater reduction in
the sum of the products of 2 perpendicular diameters of all
measurable lesions compared with the baseline values, with no
evidence of new lesions. Stable disease (SD) was defined as <30%
reduction or <20% increase in the sum of the products of 2
perpendicular diameters of all measurable lesions compared with the
baseline values, with no evidence of new lesions. Progressive
disease (PD) was defined as an increase of 20% or more in the sum
of the products of 2 perpendicular diameters of all measurable
lesions compared with the baseline values, the appearance of any
new lesion, or deterioration in clinical status consistent with
disease progression. To assess objective treatment responses,
patients were reevaluated with MDCT, EOB-MRI and
18FDG-PET/CT after two cycles of preoperative
chemotherapy. Carcinoembryonic antigen (CEA), carbohydrate antigen
(CA) 19-9, and sialyl-lcat-N-tetraose (DUPAN-2) were measured
before and after chemotherapy. In the control group, the same
markers were measured before operation.
Pathological diagnosis
All surgically resected specimens were immediately
fixed in 10% neutral-buffered formaldehyde solution. After the
specimens had been cut horizontally into 5-mm tissue blocks
(26), they were dehydrated and
embedded in paraffin. Finally, 5-µm sections were cut and stained
with hematoxylin and eosin. Each section was carefully examined
using light microscopy. The tumors were evaluated according to the
NCCN guidelines of Pancreatic Adenocarcinoma version 3.2017. The
grading system of Evans et al (27), was used to assess the pathological
effects of preoperative chemotherapy. The degrees of cytological
changes and tumor destruction were graded on a scale of I–IV, as
follows: Grade I, presence of characteristics cytological changes
of malignancy, but little (<10%) or no evident tumor cells
destruction; grade IIa, destruction of 10–50% of tumor cells; grade
IIb, destruction of 51–90% of tumor cells; grade III, presence of
few (<10%) viable tumor cells; grade IIIM, presence of sizeable
pools of mucin; grade IV, presence of no viable tumor cells; and
grade IVM, presence of acellular pools of mucin.
Patient follow up
After operation, patients were examined for
recurrence with enhanced MDCT every 3–4 months and blood tests
including tumor marker analysis every month. When recurrence was
suspected, 18FDG-PET/CT was performed. Two radiologists
reviewed the scans. The tumor relapse day was the date when CT
confirmed the recurrence.
Risk factors for early recurrence
after surgery
There were 35 recurrences within 1 year after
surgery (E group) out of 86 resected PDAC cases from 2006 to 2015.
We compared the remaining 51 patients with no relapse or recurrence
after >1 year (L group), looking for differences in risk
factors.
Statistical analyses
Categorical variables were compared using the
chi-squared test, Student's t-test, and the paired t-test. The OS
and disease free survival (DFS) rates were calculated from the
start of the study treatment until death or the final date of
follow up and determined by the Kaplan-Meier method, and the
log-rank test was applied for comparison of survival rates between
groups. A CA19-9 cutoff value as a risk factor for early recurrence
was calculated with receiver operating characteristics (ROC)
analysis. All the analyses were performed using commercial software
(SPSS® v.23, SPSS Inc., Chicago IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The characteristics of the NAC group and Control
(surgery-only) groups are listed Table
I. There were not significant differences in terms of gender,
age, tumor location, tumor size on CT, 18FDG maximum
standardized uptake value (SUVmax), or in tumor marker (CA19-9)
values. Comparisons of resected histopathological findings are
shown in Table II. There were no
significant differences between the NAC group and the Control group
in any histopathological findings (tumor size; serosal,
retroperitoneal, neural and plexus invasion rate; lymph node
metastasis rate; and R0 rate).
 | Table I.Patient characteristics of the NAC
and Control groups. |
Table I.
Patient characteristics of the NAC
and Control groups.
|
Characteristics | NAC group
(n=52) | Control group
(n=34) | P-value |
|---|
| Gender,
Male:Female | 29:23 | 21:13 | 0.582 |
| Median age, years
(range) | 65.4 (48–82) | 68.0 (52–84) | 0.636 |
| Tumor location,
Head:Body and tail | 31:21 | 20:14 | 0.942 |
| Tumor size via CT,
mm |
|
| 0.180 |
| Before
NAC | 23.3±6.5 | 26.6±9.8 |
|
| After
NAC | 22.0±6.2 |
|
|
| SUVmax |
|
| 0.624 |
| Before
NAC | 4.7±4.2 | 5.6±3.7 |
|
| After
NAC | 3.0±3.2a |
|
|
| CA19-9, U/ml |
|
| 0.699 |
| Before
NAC | 183.5±329.0 | 155.9±223.9 |
|
| After
NAC | 93.2±198.1b |
|
|
 | Table II.Histopathological characteristics of
the NAC and Control groups. |
Table II.
Histopathological characteristics of
the NAC and Control groups.
|
Characteristics | NAC group
(n=52) | Control group
(n=34) | P-value |
|---|
| Tumor size, mm | 29.8±15.6 | 30.4±10.1 | 0.862 |
| Serosal invasion,
% | 67.3 | 52.9 | 0.180 |
| Retroperitoneal
invasion, % | 76.9 | 58.8 | 0.074 |
| Lymph node
metastasis, % | 67.3 | 61.8 | 0.598 |
| Neural invasion,
% | 84.6 | 88.2 | 0.636 |
| Plexus invasion,
% | 42.3 | 32.4 | 0.353 |
| R0 rate, % | 80.8 | 79.4 | 0.901 |
Intraoperative bleeding was 471.9±344.2 ml in the
NAC group and 502.1±374.6 ml in the Control group. There were no
significant differences in the amount of bleeding between the two
group (P=0.702), and the overall postoperative mortality rate was
0% in both groups. No differences in rates of postoperative
complications were found between the two groups. Postoperative
adjuvant chemotherapy was performed in 43 of 52 cases (82.7%) in
the NAC group, and in 13 of 34 cases (38.2%) in the Control group
(P<0.0001). There was no difference about side effects among
three chemotherapeutic regimens except for epilation of GnP
therapy.
Efficacy of NAC
Five of 52 NAC group patients (9.6%) showed a PR, 45
(86.5%) showed SD and 2 (3.9%) showed PD. In comparison of the
clinical objective treatment effects before and after NAC listed in
Table I, no significant tumor
shrinkage on CT scan was observed after NAC. Of the tumor markers,
only the CA 19-9 mean value significantly decreased from
183.5±329.0 to 93.2±198.1 IU/ml (P<0.001).
18FDG-PET/CT was performed in 34 of 52 patients, before
and after NAC. Significant decreases in the SUVmax value from
4.7±4.2 to 3.0±3.2 were documented after preoperative chemotherapy
(P=0.003). All the tumor specimens showed histopathological
evidence of tumor cell injury, although none of the patients
exhibited a pathological CR. The NAC treatment effect, as judged by
the Evans grading system, was grade I in 41 patients (78.8%), grade
IIa in 8 (15.4%), and grade IIb in 3 (5.8%). There was no
statistically significant difference in the 5-year OS rates of
pancreatic head cancer patients in the NAC group compared with the
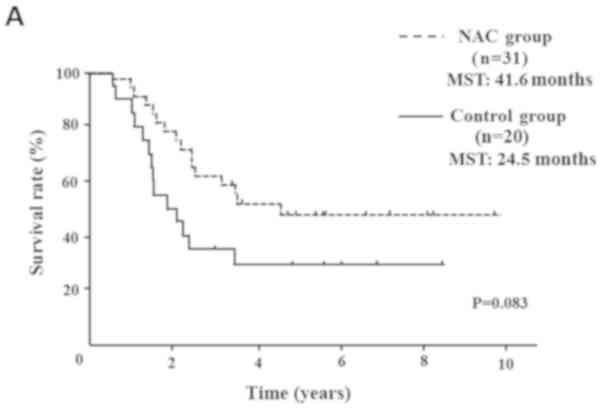
Control group (median survival time (MST): 41.6 months vs. 24.5
months) (P=0.083; Fig. 2A). OS rate
was significantly improved only in node-positive (UICC stage IIB)
pancreatic head cancer patients (MST: 29.8 months vs. 19.1 months)
(P=0.016; Fig. 2B). In pancreatic
body and tail cancer patients, OS rates of the NAC group were not
significantly improved compared with the Control group (MST: 37.2
months vs. 45.2 months) (P=0.772; Fig.
2C). Subgroup analysis stratified according to postoperative
adjuvant chemotherapy demonstrated significant difference only in
the 5-year OS rates of pancreatic head cancer patients. In
pancreatic head cancer patients who received postoperative adjuvant
chemotherapy, OS rate of the NAC group was significantly improved
compared with the Control group (P=0.012; Fig. 2D).
There were no significant differences in DFS rates
of pancreatic head cancer and body and tail cancer patients between
the two groups.
Risk factors for early recurrence
after surgery
There were 35 (40.7%) early recurrent PDAC cases
within a year after surgery (E group). The remaining 51 cases (L
group) consisted of 34 patients without recurrence and 17 patients
with recurrence >1 year after surgery. In the E and L groups,
there were no significant differences in gender, age, tumor
location, or pre- and post-operative adjuvant chemotherapy rates.
In comparison of tumor size on CT, 18FDG PET SUVmax and
tumor marker values as listed in Table
III, significantly higher CA19-9 values were observed in the E
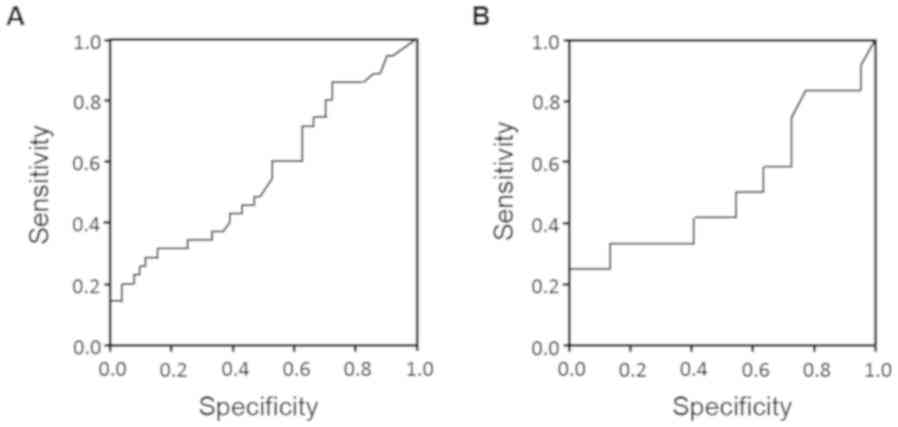
group than the L group (P=0.021). The cut-off value of CA19-9
values of 30 U/ml in the NAC group and 88 U/ml in the Control group
were arrived at with ROC analysis. According to the results of the
ROC curve analysis, the optimal cut-offs for risk of early
recurrence were as follows in the NAC group and the Control group
(Fig. 3). When CA19-9 in the NAC
group was 30.0 U/ml, it had a sensitivity of 47.8%, specificity of
44.8%, positive predictive value (PPV) of 45.8%, and negative
predictive value (NPV) of 57.1%, and the area under the ROC curve
(AUC) was 0.63 [95% confidence interval (CI): 0.48–0.79]. When
CA19-9 in the Control group was 88 0U/ml, it had a sensitivity of
41.7%, specificity of 54.5%, PPV of 29.4%, and NPV of 58.8%, and
the AUC was 0.51 [95% CI: 0.29–0.74]. In pathological comparison,
significantly larger tumor size, higher rates of lymph node
metastasis, nerve, and plexus invasion rates were observed in the E
group (Table IV). Differences in
tumor size were also significant by multivariate analysis. In
comparing recurrence sites (Table
V), the frequency of liver metastasis and peritoneal
dissemination was significantly higher in the E group than in the L
group. Of course OS rate of the E group was significantly poor
compared with the L group (MST: 55.6 months vs. 20.9 months).
However surprisingly, the prognosis of the E group was similar to
NAC dropped out patients (MST: 20.0 months).
 | Table III.Clinical factors of the E and L
groups. |
Table III.
Clinical factors of the E and L
groups.
| Clinical
factors | E group (n=35) | L group (n=51) | P-value |
|---|
| Tumor size on CT,
mm | 23.1±6.3 | 22.7±8.1 | 0.814 |
| 18FDG-PET
SUVmax | 4.8±3.2 | 4.0±3.5 | 0.403 |
| CEA, ng/ml | 4.0±5.0 | 3.4±3.1 | 0.468 |
| CA19-9, U/ml | 180.5±302.2 | 75.1±90.0 | 0.021 |
| DUPAN-2, U/ml | 456.5±1,833.8 | 841.1±4,343.6 | 0.623 |
 | Table IV.Histopathological features of the E
and L groups. |
Table IV.
Histopathological features of the E
and L groups.
| Feature | E group (n=35) | L group (n=51) | P-value
(univariate) | P-value
(multivariate) |
|---|
| Tumor size, mm | 35.3±15.7 | 26.5±10.8 | 0.003 | 0.037 |
| Serosal invasion,
% | 65.7 | 58.8 | 0.519 | 0.490 |
| Retroperitoneal
invasion, % | 74.3 | 66.7 | 0.450 | 0.443 |
| Lymph node
metastasis, % | 82.9 | 53.0 | 0.004 | 0.132 |
| Node positive
number | 4.2 | 1.7 | 0.005 | 0.980 |
| Neural invasion,
% | 97.1 | 78.4 | 0.014 | 0.159 |
| Plexus invasion,
% | 51.4 | 29.4 | 0.039 | 0.626 |
| R0 rate, % | 71.4 | 86.3 | 0.089 | 0.714 |
 | Table V.Recurrent sites of E and L
groups. |
Table V.
Recurrent sites of E and L
groups.
| Site | E group (n=35), n
(%) | L group (n=51), n
(%) | P-value |
|---|
| Liver | 19 (54.3) | 5 (9.8) | <0.0001 |
| Peritoneum | 11 (31.4) | 4 (7.8) | 0.005 |
| Local | 7 (22.9) | 5 (9.8) | 0.097 |
| Lung | 3 (11.4) | 4 (7.8) | 0.574 |
| Lymph node | 2 (5.7) | 4 (7.8) | 0.703 |
Discussion
Surgical resection is the only way to cure PDAC.
However, the majority of pancreatic cancer resections are reported
to be R1 (28), and even after
undergoing curative resection, patients with pancreatic cancer face
a 50–80% local recurrence rate and a 25–50% chance of developing
distant metastases (27). We have
developed a surgical procedure for PDAC with emphasis on anatomy
and embryology (9,10,26,29).
Whereby the long-term prognosis of PDAC was improved to some extent
(9,10), it is not yet satisfactory even for
resectable cases, because of distant metastasis. For this reason,
postoperative adjuvant chemotherapy with S-1 (14) was introduced and hepatic arterial
infusion chemotherapy with GEM for early postoperative liver
metastasis was developed (30–32).
Although, the adaptation of NAC to resectable PDAC is still
controversial, we made a policy to introduce preoperative
chemotherapy even for resectable PDAC to improve treatment outcomes
(23,24).
This study retrospectively analyzed patients who
underwent resection for resectable PDAC at a single center. Between
the NAC group and the Control group, there were no significant
differences in patients' clinicopathological characteristics
including perioperative factors. We have confirmed all cases are
adenocarcinomas, but many cases had plural pathological subtype.
Therefore, we did not consider about pathological subtypes of the
patients. In the NAC group, significant reduction of CA19-9 value
and FDG-PET SUVmax were observed after preoperative chemotherapy.
The effect of NAC according to RECIST guidelines was SD in 86.5%
cases and the pathological effect judged with Evans grade was I in
78.8% cases. In the survival analysis of this study, only the
patients with node-positive pancreatic head cancer receiving NAC
had significantly longer survival time than those in the Control
group. Subgroup analysis in postoperative adjuvant chemotherapy
group showed that pancreatic head cancer of the NAC group had
significantly longer survival time than the Control group. In the
analysis of early recurrent cases, there was no correlation with
pre- or postoperative chemotherapy; however, a significantly higher
CA19-9 value was observed in the E group compared with the L group
(P=0.021). Moreover, cut-off values of CA19-9 were calculated to be
30 U/ml in the NAC group and 88 U/ml in the Control group,
respectively, with ROC analysis. In pathological comparison, a
significantly higher rate of lymph node metastasis, nerve, and
plexus invasion rates were observed in the E group. However, it is
difficult to accurately grasp lymph node metastasis and plexus
infiltration from preoperative image findings. Even in resectable
PDAC cases, if elevated CA19-9 value is not normalized after
preoperative chemotherapy, extension of the preoperative
chemotherapy period should be considered as for borderline
resectable or unresectable PDAC cases (33,34).
The most important purpose of NAC is prevention of
metastasis form the primary site and treatment of occult
metastasis. However, it has been recently described that cancer
stem cells (CSCs) and cancer associated fibroblasts (CAFs) play an
important role in tumor invasion, metastasis, and
chemoradioresistance in pancreatic cancer (35). These resistant cells often change the
expression of several proteins while acquiring resistance to the
therapies. One of the common changes is epithelial mesenchymal
transition (EMT). EMT is a biological process that allows
epithelial cells to undergo multiple changes, enabling them to
assume a mesenchymal cell phenotype, and is positively associated
with the malignancy of cancer cells, and their invasiveness,
motility, and resistance to apoptosis (36). It has recently been reported that
anti-cancer treatments can also induce EMT in cancer cells
(37–39). We reported that residual pancreatic
cancer tissues resected after preoperative chemotherapy are rich in
chemoresistant cancer stem-like cells (40). If this theory is correct, the
effectiveness of preoperative therapy for occult metastatic lesions
is moot. It will be necessary to select agents with EMT inhibitory
effect for the primary lesion during preoperative treatment. It is
well known that low-dose paclitaxel (41–44),
metformin (45–47), angiotensin receptor blocker (48,49),
statins (50,51), and histone deacetylase inhibitors
(52,53) have all been indicated as agents that
can inhibit the EMT of tumor cells or activation of stromal cells.
Many papers have focused on the inhibition of tumor EMT and CAFs
activation with paclitaxel (44,54,55).
These data corroborate that tumor shrinkage and a decrease in
stroma was observed in tumors treated with GnP therapy (56–58).
Hence, GnP therapy comprising paclitaxel seems to be most suitable
for preoperative chemotherapy theoretically.
Several authors reported that predictors of poor
prognosis after surgery for PDAC include early recurrence, elevated
serum CA19-9, lymph node metastasis, positive surgical margin
(59,60). Serum risk factors for early
recurrence have been reported to be elevated Span-1 and CA19-9
(61,62). Kurahara et al (63) reported that serum CA19-9 >85 U/ml
was independent risk factor for recurrence within 6 months after
upfront surgery. This result is almost the same CA19-9 value (85
U/ml) of the Control group as this study. This study demonstrated
that high serum CA19-9, larger tumor size, lymph node metastasis
and plexus invasion would be the risk factors for early recurrence
after surgery. However, CA19-9 recognition is affected by the
patient's Lewis phenotype (64), and
preoperative diagnosis of the extent of tumor spread, lymph node
metastasis and plexus invasion with CT image is not accurate
enough. Even in this study, lymph node metastasis was found to be
more than 60%, nerve infiltration was found to be more than 80%,
and tumor spread was also larger than preoperative diagnosis in
many cases. Therefore, preoperative chemotherapy is considered
necessary even for resectable cases, and PDAC with the
preoperatively diagnosable risk factors for early recurrence
requires preoperative chemotherapy as does borderline resectable
PDAC.
In this study period, five patients could not
undergo surgery because of tumor progression during preoperative
chemotherapy or complication of biliary drainage. In four tumor
progression cases, chemotherapy could be continued by changing to
another treatment regimen. If upfront surgery was performed in
these cases, there is a high possibility that the prognosis was
still poor. Preoperative chemotherapy for PDAC may be valuable in
the selection of patients without chemoresistant metastases or
aggressive local progression. Since this study is a retrospective
cohort and included three regimens, prospective randomize study
with a fixed regimen is necessary in the future.
In conclusion, NAC with GEM prolonged the survival
period of node-positive pancreatic head cancer patients, and SUV
max and serum CA19-9 values are useful for judgment of treatment
effect. However, high serum CA19-9 value, larger tumor size, lymph
node metastasis, and plexus invasion are risk factors for early
tumor recurrence after surgery. Especially, in the NAC group
normalization of CA19-9 after preoperative chemotherapy will be
required. Therefore, preoperative therapy same for borderline
resectable cases should be considered even for resectable PDAC
cases with early recurrence risk factors. Moreover, postoperative
adjuvant chemotherapy is important especially for pancreatic head
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT and TO designed the present study. HN and SF
performed the statistical and pharmacokinetic analyses. MO, TY, SN,
JK, IM, YO, KO and KN conducted chemotherapy on patients. TM, HTak
and IN contributed to the clinical trial operation. All the authors
approved the final version of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient prior to treatment, and the present study was approved by
the Ethics Committee of Kanazawa University Hospital (review no.
2799-1).
Patient consent for publication
Written informed consent was obtained from each
patient for the publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gillen S, Schuster T, Meyer Zum
Büschenfelde C, Friess H and Kleeff J: Preoperative/neoadjuvant
therapy in pancreatic cancer: A systematic review and meta-analysis
of response and resection percentages. PLoS Med. 7:e10002672010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishii H, Furuse J, Boku N, Okusaka T,
Ikeda M, Ohkawa S, Fukutomi A, Hamamoto Y, Nakamura K, Fukuda H, et
al: Phase II study of gemcitabine chemotherapy alone for locally
advanced pancreatic carcinoma: JCOG0506. Jpn J Clin Oncol.
40:573–579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Von Hoff DD, Ramanathan RK, Borad MJ,
Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias
JL, et al: Gemcitabine plus nab-paclitaxel is an active regimen in
patients with advanced pancreatic cancer: A phase I/II trial. J
Clin Oncol. 29:4548–5454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagner M, Redaelli C, Lietz M, Seiler CA,
Friess H and Bűcher MW: Curative resection is the single most
important factor determining outcome in patients with pancreatic
adenocarcinoma. Br J sSurg. 91:586–594. 2004. View Article : Google Scholar
|
|
6
|
Verbeke CS and Menon KV: Redefining
resection margin status in pancreatic cancer. HPB (Oxford).
11:282–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagakawa T, Kurachi M, Konishi K and
Miyazaki I: Translateral retroperitoneal approach in radical
surgery for pancreatic carcinoma. Jpn J Surg. 12:229–33. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagakawa T, Nagamori M, Futakami F,
Tsukioka Y, Kayahara M, Ohta T, Ueno K and Miyazaki I: Result of
extensive surgery for pancreatic carcinoma. Cancer. 77:640–645.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kitagawa H, Tajima H, Nakagawara H, Makino
I, Miyashita T, Shoji M, Nakanuma S, Hayashi N, Takamura H, Ohta T
and Ohtake H: En bloc vascular resection for the treatment of
borderline resectable pancreatic head carcinoma. Mol Clin Oncol.
2:369–374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitagawa H, Tajima H, Nakagawara H, Makino
I, Miyashita T, Terakawa H, Nakanuma S, Hayashi H, Takamura H and
Ohta T: A modification of radical antegrade modular
pancreato-splenectomy for adenocarcinoma of the left pancreas:
Significance of en bloc resection including the anterior renal
fascia. World J Surg. 38:2448–2454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nimura Y, Nagino M, Takao S, Takada T,
Miyazaki K, Kawarada Y, Miyagawa S, Yamaguchi A, Ishiyama S, Takeda
Y, et al: Standard versus extended lymphadenectomy in radical
pancreato-duodenectomy for ductal adenocarcinoma of the head of the
pancreas: Long-term results of a Japanese multicenter randomized
controlled trial. J Hepatobiliary Pancreat Sci. 19:230–2341. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Evans DB, Abbruzzese JL and Willett CG:
Cancer of the pancreas. In: DeVita VT, Hellman S and Rosenberg SA
(eds.): Cancer: Priciples and Practice of Oncology. 6th edition.
Philadelphia, Lippincott, Williams and Wilkins. 1:pp1126–1161.
1997.
|
|
13
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative-intent resection of pancreatic cancer:
A randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: Adjuvant chemotherapy of S-1 versus gemcitabine for
resected pancreatic cancer: A phase 3, open-label, randomised,
non-inferiority trial (JASPAC 01). Lancet. 388:248–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klinlenbijl JH, Jeekel J, Sahmoud T, van
Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT,
Hennipman A, et al: Adjuvant radiotherapy and 5-fluorouracil after
curative resection of cancer of the pancreas and periampullary
region: Phase III trial of the EORTC gastrointestinal tract cancer
cooperative group. Ann Surg. 230:776–782. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spitz FR, Abbruzzese JL, Lee JE, Pisters
PW, Lowy AM, Fenoglio CJ, Cleary KR, Janjan NA, Goswitz MS, Rich
TA, et al: Preoperative and postoperative chemoradiation strategies
in patients treated with pancreaticoduodenectomy for adenocarcinoma
of the pancreas. J Clin Oncol. 15:928–937. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeo CJ, Abrams RA, Grochow LB, Sohn TA,
Ord SE, Hruban RH, Zahurak ML, Dooley WC, Coleman J, Sauter PK, et
al: Pancreaticoduodenectomy for pancreatic adenocarcinoma:
Postoperative adjuvant chemoradiation improves survival. A
prospective, single-institution experience. Ann Surg. 225:621–633.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JL, Kim SC, Kim JH, Lee SS, Kim TW,
Park DH, Seo DW, Lee SK, Kim MH, Kim JH, et al: Prospective
efficacy and safety study of neoadjuvant gemcitabine with
capecitabine combination chemotherapy for borderline-resectable or
unresectable locally advanced pancreatic adenocarcinoma. Surgery.
152:851–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Itchins M, Arena J, Nahm CB, Rabindran J,
Kim S, Gibbs E, Bergamin S, Chua TC, Gill AJ, Maher R, et al:
Retrospective cohort analysis of neoadjuvant treatment and survival
in resectable and borderline resectable pancreatic ductal
adenocarcinoma in a high volume referral center. Eur J Surg Oncol.
43:1711–1717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh
Rde W, Collisson E, Schwartz L, Frankel W, Martin R, Conway W, et
al: Preoperative modified FOLFIRINOX treatment followed by
capecitabine-based chemoradiation for borderline resectable
pancreatic cancer: Alliance for clinical trials in oncology Trial
A021101. JAMA Surg. 151:e1611372016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shubert CR, Bergquist JR, Groeschl RT,
Habermann EB, Wilson PM, Truty MJ, Smoot RL, Kendrick ML, Nagorney
DM and Farnell MB: Overall survival is increased among stage III
pancreatic adenocarcinoma patients receiving neoadjuvant
chemotherapy compared to surgery first and adjuvant chemotherapy:
An intention to treat analysis of the National Cancer Database.
Surgery. 160:1080–1096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagakawa Y, Hosokawa Y, Nakayama H, Sahara
Y, Takishita C, Nakajima T, Hijikata Y, Kasuya K, Katsumata K,
Tokuuye K and Tsuchida A: A phase II trial of neoadjuvant
chemoradiotherapy with intensity-modulated radiotherapy combined
with gemcitabine and S-1 for borderline-resectable pancreatic
cancer with arterial involvement. Cancer Chemother Pharmacol.
79:951–957. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tajima H, Ohta T, Kitagawa H, Okamoto K,
Sakai S, Makino I, Kinoshita J, Furukawa H, Nakamura K, Hayashi H,
et al: Pilot study of neoadjuvant chemotherapy with gemcitabine and
oral S-1 for resectable pancreatic cancer. Exp Ther Med. 3:787–792.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tajima H, Kitagawa H, Tsukada T, Nakanuma
S, Okamoto K, Sakai S, Makino I, Furukawa H, Nakamura K, Hayashi H,
et al: A phase I study of neoadjuvant chemotherapy with gemcitabine
plus oral S-1 for resectable pancreatic cancer. Mol Clin Oncol.
1:768–772. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Makino I, Kitagawa H, Ohta T, Nakagawara
H, Tajima H, Ohnishi I, Takamura H, Tani T and Kayahara M: Nerve
plexus invasion in pancreatic cancer. Spread patterns on
histopathologic and embryological analysis. Pancreas. 37:358–365.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Evans DB, Rich TA, Byrd DR, Cleary KR,
Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ and Ames FC:
Preoperative chemoradiation and pancreaticoduodenectomy for
adenocarcinoma of the pancreas. Arch Surg. 127:1335–1339. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Esposito I, Kleeff J, Bergmann F, Reiser
C, Herpel E, Friess H, Schirmacher P and Büchler MW: Most
pancreatic cancer resections are R1 resections. Ann Surg Oncol.
15:1651–1660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Terakawa H, Kitagawa H, Makino I, Hayashi
H, Oyama K, Nakagawara H, Miyashita T, Tajima H, Takamura H,
Fushida S, et al: Location of the meso-pancreatoduodenum as a
regional lymphatic basin for pancreatic head carcinoma. Oncol Lett.
14:397–403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tajima H, Ohta T, Kitagawa H, Sakai S,
Makino I, Hayashi H, Nakagawara H, Onishi I, Takamura H, Ninomiya
I, et al: Hepatic arterial infusion chemotherapy for post-operative
liver metastases from pancreatic cancer in a patient with
leukocytopenia: A case report. Exp Ther Med. 1:987–990. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tajima H, Ohta T, Kitagawa H, Sakai S,
Makino I, Hayashi H, Oyama K, Nakagawara H, Fujita H, Onishi I, et
al: Pilot study of hepatic arterial infusion chemotherapy with
gemcitabine and 5-fluorouracil for patients with postoperative
liver metastases from pancreatic cancer. Exp Ther Med. 2:265–269.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tajima H, Kitagawa H, Tsukada T, Okamoto
K, Nakanuma SI, Sakai S, Makino I, Furukawa H, Hayashi H, Oyama Ku,
et al: Hepatic arterial infusion chemotherapy with gemcitabine and
5-fluorouracil or oral S-1 improves the prognosis of patients with
postoperative liver metastases from pancreatic cancer. Mol Clinic
Oncol. 1:869–874. 2013. View Article : Google Scholar
|
|
33
|
Satoi S, Yamaue H, Kato K, Takahashi S,
Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H, et al: Role
of adjuvant surgery for patients with initially unresectable
pancreatic cancer with a long-term favorable response to
non-surgical anti-cancer treatments: Results of a project study for
pancreatic surgery by the Japanese society of
hepato-biliary-pancreatic surgery. J Hepatobiliary Pancreat Sci.
20:590–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eguchi H, Yamada D, Iwagami Y, Gotoh K,
Kawamoto K, Wada H, Asaoka T, Noda T, Takeda Y, Tanemura M, et al:
Prolonged neoadjuvant therapy for locally advanced pancreatic
cancer. Dig Surg. 35:70–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Valle S, Martin-Hijano L, Alcalá S,
Alonso-Nocelo M and Sainz B Jr: The ever-evolving concept of the
cancer stem cell in pancreatic cancer. Cancers (Basel). 10(pii):
E332018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsubouchi K, Minami K, Hayashi N, Yokoyama
Y, Mori S, Yamamoto H and Koizumi M: The CD44 standard isoform
contributes to radioresistance of pancreatic cancer cells. J Radiat
Res. 58:816–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxaliplatin resistance induces epithelial-mesenchymal transition in
colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsukamoto H, Shibata K, Kajiyama H,
Terauchi M, Nawa A and Kikkawa F: Irradiation-induced
epithelial-mesencymal transition (EMT) related to invasive
potential in endometrial carcinoma cells. Gynecol Oncol.
107:500–504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tajima H, Ohta T, Makino I, Hayashi H,
Nakagawara H, Onishi I, Takamura H, Ninomiya I, Kitagawa H, Fushida
S, et al: Expression of epithelial-mesenchymal transition markers
in locally recurrent hepatocellular carcinoma after radiofrequency
ablation. Exp Therap Med. 1:347–350. 2010. View Article : Google Scholar
|
|
40
|
Tajima H, Ohta T, Kitagawa H, Okamoto K,
Sakai S, Kinoshita J, Makino I, Furukawa H, Hayashi H, Nakamura K,
et al: Neoadjuvant chemotherapy with gemcitabine for pancreatic
cancer increases in situ expression of the apoptosis marker M30 and
stem cell marker CD44. Oncol Lett. 3:1186–1190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang D, Sun L, Xian W, Liu F, Ling G,
Xiao L, Liu Y, Peng Y, Haruna Y and Kanwar YS: Low-dose paclitaxel
ameliorates renal fibrosis in rat UUO model by inhibition of
TGF-beta/Smad activity. Lab Invest. 90:436–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou J, Zhong DW, Wang QW, Miao XY and Xu
XD: Paclitaxel ameliorates fibrosis in hepatic stellate cells via
inhibition of TGF-beta/Smad activity. World J Gastroenterol.
16:3330–3334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Choi HS, Savard CE, Choi JW, Kuver R and
Lee SP: Paclitaxel interrupts TGF-beta1 signaling between
gallbladder epithelial cells and myofibroblasts. J Surg Res.
141:183–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hirose A, Tajima H, Ohta T, Tsukada T,
Okamoto K, Nakanuma S, Sakai S, Kinoshita J, Makino I, Furukawa H,
et al: Low-dose paclitaxel inhibits the induction of
epidermal-mesenchymal transition in the human cholangiocarcinoma
CCKS-1 cell line. Oncol Lett. 6:915–920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cufí S, Vazquez-Martin A,
Oliveras-Ferraros C, Martin-Castillo B, Joven J and Menendez JA:
Metformin against TGFβ-induced epithelial-to- mesenchymal
transition (EMT): From cancer stem cells to aging-associated
fibrosis. Cell Cycle. 9:4461–4468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vazquez-Martin A, Oliveras-Ferraros C,
Cufí S, Del Barco S, Martin-Castillo B and Menendez JA: Metformin
regulates breast cancer stem cell ontogeny by transcriptional
regulation of the epithelial-mesenchymal transition (EMT) status.
Cell Cycle. 9:3807–3814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Del Barco S, Vazquez-Martin A, Cufí S,
Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B
and Menendez JA: Metformin: Multi-faceted protection against
cancer. Oncotarget. 2:896–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Okamoto K, Tajima H, Ohta T, Nakanuma S,
Hayashi H, Nakagawara H, Onishi I, Takamura H, Ninomiya I, Kitagawa
H, et al: Angiotensin II induces tumor progression and fibrosis in
intrahepatic cholangiocarcinoma through an interaction with hepatic
stellate cells. Int J Oncol. 37:1251–1259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Okazaki M, Fushida S, Harada S, Tsukada T,
Kinoshita J, Oyama K, Tajima H, Ninomiya I, Fujimura T and Ohta T:
The angiotensin II type 1 receptor blocker candesartan suppresses
proliferation and fibrosis in gastric cancer. Cancer Lett.
355:46–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chang TI, Kang HY, Kim KS, Lee SH, Nam BY,
Paeng J, Kim S, Park JT, Yoo TH, Kang SW and Han SH: The effect of
statin on epithelial-mesenchymal transition in peritoneal
mesothelial cells. PLoS One. 9:e1096282014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang T, Chen M and Sun T: Simvastatin
attenuates TGF-β1-induced epithelial-mesenchymal transition in
human alveolar epithelial cells. Cell Physiol Biochem. 31:863–874.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shoji M, Ninomiya I, Makino I, Kinoshita
J, Nakamura K, Oyama K, Nakagawara H, Fujita H, Tajima H, Takamura
H, et al: Valproic acid, a histone deacetylase inhibitor, enhances
radiosensitivity in esophageal squamous cell carcinoma. Int J
Oncol. 40:2140–2146. 2012.PubMed/NCBI
|
|
53
|
Watanabe T, Tajima H, Hironori H,
Nakagawara H, Ohnishi I, Takamura H, Ninomiya I, Kitagawa H,
Fushida S, Tani T, et al: Sodium valproate blocks the transforming
growth factor (TGF)-β1 autocrine loop and attenuates the
TGF-β1-induced collagen synthesis in a human hepatic stellate cell
line. Int J Mol Med. 28:919–925. 2011.PubMed/NCBI
|
|
54
|
Sun JD, Liu Q, Ahluwalia D, Li W, Meng F,
Wang Y, Bhupathi D, Ruprell AS and Hart CP: Efficacy and safety of
the hypoxia-activated prodrug TH-302 in combination with
gemcitabine and nab-paclitaxel in human tumor xenograft models of
pancreatic cancer. Cancer Biol Ther. 16:438–449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang D, Yang R, Wang S and Dong Z:
Paclitaxel: New uses for an old drug. Drug Des Devel Ther.
8:279–284. 2014.PubMed/NCBI
|
|
56
|
Meng H, Wang M, Liu H, Liu X, Situ A, Wu
B, Ji Z, Chang CH and Nel AE: Use of a lipid-coated mesoporous
silica nanoparticle platform for synergistic gemcitabine and
paclitaxel delivery to human pancreatic cancer in mice. ACS Nano.
9:3540–3557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Oettle H: Progress in the knowledge and
treatment of advanced pancreatic cancer: From benchside to bedside.
Cancer Treat Rev. 40:1039–1047. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Miyashita T, Tajima H, Makino I, Okazaki
M, Yamaguchi T, Ohbatake Y, Nakanuma S, Hayashi H, Takamura H,
Ninomiya I, et al: Neoadjuvant chemotherapy with gemcitabine plus
nab-paclitaxel reduces the number of cancer-associated fibroblasts
through depletion of pancreatic stroma. Anticancer Res. 38:337–343.
2018.PubMed/NCBI
|
|
59
|
Waraya M, Yamashita K, Katagiri H, Ishii
K, Takahashi Y, Furuta K and Watanabe M: Preoperative serum CA19-9
and dissected peripancreatic tissue margin as determiners of
long-term survival in pancreatic cancer. Ann Surg Oncol.
16:1231–1240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ueda M, Endo I, Nakashima M, Minami Y,
Takeda K, Matsuo K, Nagano Y, Tanaka K, Ichikawa Y, Togo S, et al:
Prognostic factors after resection of pancreatic cancer. World J
Surg. 33:104–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hosokawa Y, Nagakawa Y, Sahara Y,
Takishita C, Katsumata K and Tsuchida A: Serum SPan-1 is a
significant risk factor for early recurrence of pancreatic cancer
after curative resection. Dig Surg. 34:125–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shimizu T, Asakuma M, Tomioka A, Inoue Y,
Hirokawa F, Hayashi M and Uchiyama K: Span-1 and CA19-9 as
predictors of early recurrence and lymph node metastasis for
patients with invasive pancreatic cancer after pancreatectomy. Am
Surg. 84:109–113. 2018.PubMed/NCBI
|
|
63
|
Kurahara H, Maemura K, Mataki Y, Sakoda M,
Iino S, Kawasaki Y, Arigami T, Mori S, Kijima Y, Ueno S, et al: A
Therapeutic strategy for resectable pancreatic cancer based on risk
factors of early recurrence. Pancreas. 47:753–758. 2018.PubMed/NCBI
|
|
64
|
Berge AC, Meszoely IM, Ross EA, Watson JC
and Hoffman JP: Undetectable preoperative levels of serum CA 19-9
correlate with improved survival for patients with resectable
pancreatic adenocarcinoma. Ann Surg Oncol. 11:644–649. 2004.
View Article : Google Scholar : PubMed/NCBI
|