Introduction
An estimated 20–30% of all patients with renal cell
carcinoma (RCC) have metastatic disease at the time of diagnosis.
Although multiple guidelines, including those of The National
Comprehensive Cancer Network and the European Association of
Urology, recommend first-line vascular endothelial growth factor
(VEGF)-targeted therapy for patients with clear cell metastatic RCC
(mRCC) (1,2), the response to first-line VEGF-targeted
therapy is generally not durable, and most patients require
additional therapy (3). The role of
immune checkpoint inhibitors is increasingly of interest in cancer
immunotherapeutics, and nivolumab, a novel anti-PD1 antibody, has
been increasingly used in patients with several types of cancer.
Although nivolumab has been most frequently used for melanoma, it
is currently under evaluation for a number of other cancers. In
terms of overall survival (OS), nivolumab has been shown to be
superior to everolimus in a phase III randomized trial, and thus
offers promising potential for use in patients with mRCC after
treatment failure. However, unfamiliar toxicities have also been
recognized with nivolumab use, such as immune-related adverse
events (irAEs) (4). There has been
no initial report about mRCC treated by nivolumab in the Japanese
population yet.
The present study aimed to analyze the clinical
outcomes and toxicities related to nivolumab in Japanese patients
with mRCC in a real-world setting.
Patients and methods
Eligibility criteria
The present study included patients who were
diagnosed with advanced RCC and treated with nivolumab from two
institutes between March 2013 and January 2018. All patients with
histologically-proven mRCC, regardless of Eastern Cooperative
Oncology Group (ECOG) performance status (PS), were eligible for
inclusion. All patients received at least two cycles of nivolumab
and were assessed for treatment efficacy and toxicity. This study
was approved by both institutional review boards, The Research
Ethics board of Akita University Hospital (approval no. 1993) and
Japanese Red Cross Akita Hospital, and all procedures were
performed in accordance with the 1964 Declaration of Helsinki and
its later amendments. Written informed consent was obtained from
each patient.
Treatment and follow-up
examinations
Complete medical history, physical examination, ECOG
PS, CBC with differential and platelet count, biochemical profile
(including electrolytes, renal and hepatic function, coagulation,
pancreatic amylase, and lipase), urinalyses, and chest radiography
were recorded for all patients before treatment was started; these
tests were repeated during therapy according to the attending
physician's decision. Toxicity was graded using the National Cancer
Institute Common Toxicity Criteria version 2.0. Tumors were
measured by computed tomography scans within 4 weeks prior to
starting nivolumab. After starting the drug, the assessment
interval was scheduled for individual patients by attending
physicians. We defined that patients experienced tumor enlargement
or appearance of a new lesion after initiating nivolumab and
intolerable adverse event (AE)as ‘nivolumab failure’. Tumor
response was evaluated using the Response Evaluation Criteria in
Solid Tumors (RECIST) guidelines v.1.1.
Results
Patient characteristics
A total 33 patients who were diagnosed with advanced
RCC and eligible for the present study were enrolled. Patients'
baseline characteristics are summarized in Table I. All patients were Japanese, and the
cohort included 27 (81.8%) men and six (18.2%) women. The median
patient age was 68 (range: 37–79) years. Thirty-two (97.0%)
patients underwent radical nephrectomy before starting systemic
therapies. Twenty-nine (87.9%) patients had clear cell histology,
two (6.1%) patients had Xp11.2 translocation, and one patient each
(3.0%) had papillary, chromophobe, and sarcomatoid histology.
Twelve (36.4%) patients were administered nivolumab as second-line
systemic therapy, nine (27.3%) as third-line therapy, and 12
(36.4%) as fourth-line or later therapy. Fourteen (42.4%) patients
had an ECOG PS of 0, 17 (51.5%) patients had an ECOG PS of 1, and
two (6.1%) patients had an ECOG PS of 2 or higher. Using the
Memorial Sloan-Kettering Cancer Center (MSKCC) risk classification
system, one (3.0%) patient was classified as being at favorable
risk, 24 (72.7%) patients were classified as being at intermediate
risk, and eight (24.2%) patients were classified as being at poor
risk. Twelve (36.4%) patients had one metastatic site, 11 (33%)
patients had two, and 10 (30%) patients had three or more.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Result |
|---|
| Total patient
number | 33 |
| Age, median year
(range) | 68 (37–79) |
| Sex, male:Female | 27:6 |
| Nephrectomy,
yes:No | 32:1 |
| Histology |
|
| Clear
cell | 29 |
| Xp11.2
translocation | 2 |
|
Papillary | 1 |
|
Chromophobe | 1 |
|
Sarcomatoid | 1 |
| MSKCC risk
classification, Favorable:Intermediate:Poor |
|
| At the
beginning of systemic therapy | 4:22:7 |
| At the
starting of nivolumab | 1:24:8 |
| Metastatic site,
1:2:≥3 | 12:11:10 |
| ECOG PS, 0:1:≥2 |
|
| At the
beginning of systemic therapy | 24:8:1 |
| At the
starting of nivolumab | 14:17:2 |
| Number of prior
therapy, 1:2:≥3 | 12:9:12 |
Antitumor effect
An objective response (OR) was found in eight
(24.2%) patients (Table II). The
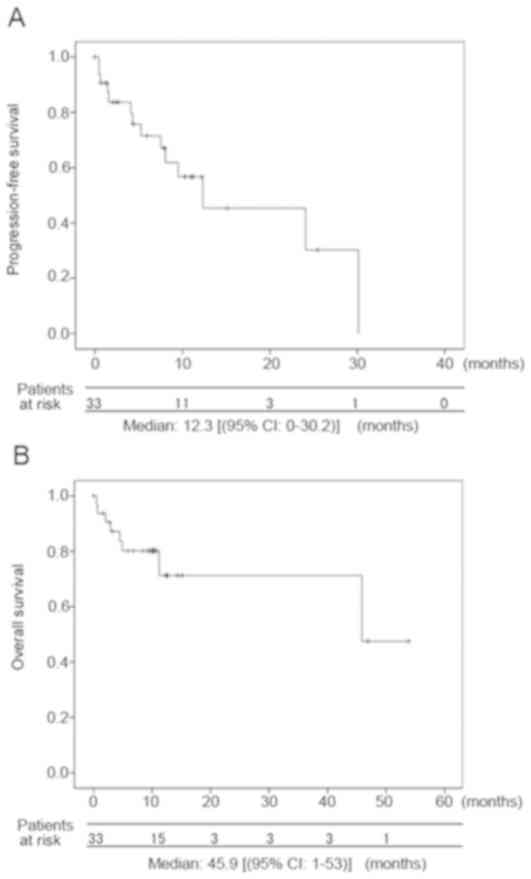
median progression-free survival was 12.3 months (range: 0–30.2),
and the OS was 45.9 months (range: 0–53.9) (Fig. 1). These survival rates might be
equivalent to the median PFS 4.6 months and OS 25.0 months in
CheckMate-025 trial (4). The median
time to best response after starting nivolumab was 3.2 months
(range: 0.5–5.7 months). The median duration after achieving best
response was 2.3 months (range: 2.0–26.6 months) (Table II).
 | Table II.Results of nivolumab management. |
Table II.
Results of nivolumab management.
| Factor | Result |
|---|
| Dose, mg/kg | 3 |
| Median (range) | 11 (3–65) |
| Duration, median
month (range) | 23 (1–131) |
| Current status |
|
|
Continue | 9 |
| Stop | 19 |
|
Pending | 4 |
|
Unknown | 1 |
| Best response |
|
| CR | 1 |
| PR | 7 |
| SD | 11 |
| PD | 10 |
| Not
assessed | 4 |
| Objective response
rate, % | 24.2 |
| Time to best
response, median month (range) | 3.2 (0.5–5.7) |
| Duration of best
response, median month (range) | 2.3 (2.0–26.6) |
Adverse events
At the data cutoff, 15 patients (45.5%) had
experienced an AE (Table III). Six
patients (18.2%) discontinued nivolumab treatment because of AEs.
The most common AE was renal dysfunction (n=3). Grade 3 or higher
AEs presented in six (18.2%) patients. IrAEs (any grade) included
two cases each of interstitial pneumonia, myasthenia gravis, liver
dysfunction, and hypothyroidism, and one case each of
hypopituitarism and organizing pneumonia. Corticosteroid was
administered as primary treatment for all irAEs, except for one
patient with myasthenia gravis. Only one irAE, a case of
hypopituitarism, was not resolved, and the patient has since
required continuous steroid and thyroid hormone supplemental
therapy. There was no relationship between irAE development and
clinical response. Five patients died within 6 months after
initiating nivolumab treatment. Of these five, three patients were
already administered multiple therapies (at least four) and had
advanced RCC before starting nivolumab treatment, and two patients
had an ECOG PS of 2 or worse at the time of starting nivolumab.
 | Table III.Treatment-related adverse events. |
Table III.
Treatment-related adverse events.
| Factor | Result |
|---|
| Patient number | 33 |
| Number of patients
with AEs | 15 (45.5%) |
| Cause of
discontinuation |
|
| PD | 13 |
| AE | 6 |
| Detail of
discontinuation |
|
| G3 or
more | 6 |
|
Myasthenia gravis | 2 |
| Liver
dysfunction | 1 |
| General
edema | 1 |
|
Hypopituitalism | 1 |
| Heart
failure | 1 |
| General AE, any
grade |
|
| Renal
dysfunction | 3 |
| Pleural
effusion | 2 |
| Heart
failure | 2 |
| General
edema | 1 |
| General
fatigue | 1 |
| Hyper
Kalemia | 1 |
| Rash | 1 |
| irAE, any grade |
|
|
Myasthenia gravis | 2 |
|
Interstitial pneumonia | 2 |
| Liver
dysfunction | 2 |
|
Hypothyroidism | 2 |
|
Hypopituitarism | 1 |
|
Organizing pneumonia | 1 |
Treatments after nivolumab
failure
Thirteen patients were administered further
treatment after nivolumab failure during the period of this
investigation. Of these, all patients received VEGF receptor
inhibitor, with five receiving axitinib, four receiving sunitinib,
three receiving pazopanib, and one receiving sorafenib (Table IV). Only three patients (OR rate:
20.8%) showed partial response (PR), but six patients achieved
stable disease (SD) even after nivolumab failure, indicating some
clinical benefit.
 | Table IV.Treatment after nivolumab
failure. |
Table IV.
Treatment after nivolumab
failure.
| Status | Patient number | Best response |
|---|
| Dead | 3 |
|
| Axitinib | 5 | PR 2, SD 2, PD
1 |
| Sunitinib | 4 | PR 1, SD 1, PD
2 |
| Pazopanib | 3 | SD 2, PD1 |
| Sorafenib | 1 | SD 1 |
| Treatment
pending | 4 | SD 2, PD 1, unknown
1 |
| Move to other
hospital | 1 | Unknown 1 |
Discussion
This study found that nivolumab was relatively
effective and showed acceptable results in Japanese patients with
mRCC. The OR rate and survival rate in our cohort were comparable
to those of previous clinical trials (4), despite the fact that our cohort
included patients with worse PS, MSKCC-determined poor risk, and
non-clear cell histology. Nine patients had SD at the time of this
study, and four patients showed durable responses for over 12
months; these findings indicate the potential utility of immune
checkpoint blockade for the treatment of patients with mRCC
(5). This relatively better outcome
than we expected before nivolumab launched despite unfavorable
patient clinical backgrounds could support the assertion that
immune checkpoint inhibitors are a revolutionary therapy (6).
Research efforts to determine a predictive marker,
such as PD-L1 positivity (4),
mutation burden (7), and profile of
immune-related genes (8), are
currently ongoing. Most previous studies have focused on
identifying exceptional responders to immune checkpoint inhibition;
however, even patients with lower expression of PD-L1 showed an OR
rate of over 20% in the CheckMate-025 trial (4). Therefore, immunohistological evaluation
of PD-L1 expression, would not change our clinical decision,
because a substantial number of patients without PD-L1 expression
can still benefit from nivolumab therapy.
The timing of nivolumab administration may be
another important consideration for appropriate clinical decisions.
Although our sample size was small, and we could not assess
statistical significance, we saw a trend between worse survival and
both poor ECOG PS and multiple prior therapies in our cohort. This
suggests that the maximum benefit from nivolumab is likely to occur
early in the sequential course of multiple treatments and prior to
cancer advancement or decline in a patient's general status. Early
exposure to immune checkpoint inhibitors can be reinforced by the
possibility of late immune response with anti-tumor effect
(9). Although in this study there
were no patients who met the criteria for ‘pseudo-progression’
(10) with subsequent response, one
patient with SD did eventually develop PR. Determining the
appropriate time to examine the ‘delayed response’ (11) of an immune-related anti-tumor effect
can be difficult, which emphasizes the importance of earlier
introduction and good PS in nivolumab treatment (10).
Although immune checkpoint-blocking antibodies are
effective in many types of cancers, irAEs may be unavoidable
(12). In addition to relatively
common AEs, such as interstitial pneumonia, colitis, hepatitis,
kidney insufficiency, and hypo-endocrinology, uncommon AEs have
also been reported, such as cardiotoxicity (13), type 1 diabetes (14), and myasthenia gravis (15). In this study, several irAEs were
observed in response to nivolumab despite the small size of the
patient cohort. In the important phase III trial of nivolumab
(4), grade 3–4 toxicities were
reported at a rate of 21%. The rate of grade 3–4 toxicities
observed in this study (18.2%) was similar, despite the short
observation period. Importantly, all grade 3–4 irAEs that occurred
in patients in this study, with the exception of hypopituitarism,
resolved with prompt initiation of corticosteroid administration
followed by a slow steroid taper. This is similar to what has been
described in the management of irAEs in other cancers, and
underscores the necessity for vigilance by the attending physician
concerning these potential events (16). Our results indicate that nivolumab
might be a relatively safe drug for the treatment of mRCC in real
clinical practice.
The proper endpoint for nivolumab treatment has been
controversial (17,18). In several patients in our study, no
tumor progression was observed after cessation of nivolumab
treatment. Based on these cases, we suggest that nivolumab
treatment not be stopped prematurely, even after an initial
therapeutic effect is achieved. However, it is not clear from our
study whether prolongation of treatment would further improve
clinical outcome. It has been reported that long-term application
of anti-PD1 antibody can also cause drug resistance (19). Hence, the duration of nivolumab
application for mRCC treatment needs to be further studied.
Several limitations of this study should be noted,
including the retrospective nature and limited number of patients.
However, our results show promising relevance for nivolumab in
clinical practice and warrant further research of immune checkpoint
inhibitor therapy in advanced RCC.
The present study demonstrated the safety and
acceptable outcomes of anti-PD1 therapy with nivolumab in patients
with advanced RCC. Although nivolumab precipitated unique AEs,
these were treated promptly and all but one were reversible. Our
findings suggest that nivolumab is a reasonable therapeutic option
for patients with mRCC after multiple lines of treatment. Future
prospective studies with larger sample sizes are required to better
understand the appropriate indications of nivolumab for patients
with RCC.
Acknowledgements
The authors would like thank Ms Yoko Mitobe (Akita
University Graduate School of Medicine) for her assistance with the
data collection.
Funding
The present study was supported by Grants-in-Aid for
Scientific Research, Japan (grant no. 17K11121).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KN was involved in the project development, data
collection and management, data analysis, and manuscript writing.
YH collected the data and edited the manuscript. SK was involved in
data collection. AK, TN, SK, MS, SN, TI, TO and SC collected the
data. NS collected the data and edited the manuscript. TH was
involved in the project development, data collection, and
manuscript editing.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and approved by the Research Ethics
board of Akita University Hospital (approval no. 1993) and Japanese
Red Cross Akita Hospital. Written informed consent was obtained
from each patient.
Patient consent for publication
Written informed consent was obtained from each
patient for the publication of their data.
Competing interests
Dr Habuchi received honoraria from Novartis
International AG, Pfizer, Inc., GlaxoSmithKline and Ono
Pharmaceutical Co., Ltd. All other authors declare that they have
no competing interests.
References
|
1
|
Powles T, Staehler M, Ljungberg B,
Bensalah K, Canfield SE, Dabestani S, Giles R, Hofmann F, Hora M,
Kuczyk MA, et al: Updated EAU guidelines for clear cell renal
cancer patients who fail VEGF targeted therapy. Eur Urol. 69:4–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Motzer RJ, Jonasch E, Agarwal N, Bhayani
S, Bro WP, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Fishman
M, et al: Kidney cancer, version 2.2017, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 15:804–834. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vachhani P and George S: VEGF inhibitors
in renal cell carcinoma. Clin Adv Hematol Oncol. 14:1016–1028.
2016.PubMed/NCBI
|
|
4
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomita Y, Fukasawa S, Shinohara N,
Kitamura H, Oya M, Eto M, Tanabe K, Kimura G, Yonese J, Yao M, et
al: Nivolumab versus everolimus in advanced renal cell carcinoma:
Japanese subgroup analysis from the CheckMate 025 study. Jpn J Clin
Oncol. 47:639–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Riaz N, Havel JJ, Makarov V, Desrichard A,
Urba WJ, Sims JS, Hodi FS, Martín-Algarra S, Mandal R, Sharfman WH,
et al: Tumor and microenvironment evolution during immunotherapy
with nivolumab. Cell. 171:934–949, e916. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boutsikou E, Domvri K, Hardavella G,
Tsiouda D, Zarogoulidis K and Kontakiotis T: Tumour necrosis
factor, interferon-gamma and interleukins as predictive markers of
antiprogrammed cell-death protein-1 treatment in advanced non-small
cell lung cancer: A pragmatic approach in clinical practice. Ther
Adv Med Oncol. 10:17588359187682382018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Remon J and Besse B: Immune checkpoint
inhibitors in first-line therapy of advanced non-small cell lung
cancer. Curr Opin Oncol. 29:97–104. 2017.PubMed/NCBI
|
|
10
|
Escudier B, Motzer RJ, Sharma P, Wagstaff
J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski
PG, et al: Treatment beyond progression in patients with advanced
renal cell carcinoma treated with nivolumab in checkmate 025. Eur
Urol. 72:368–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
George S, Motzer RJ, Hammers HJ, Redman
BG, Kuzel TM, Tykodi SS, Plimack ER, Jiang J, Waxman IM and Rini
BI: Safety and efficacy of nivolumab in patients with metastatic
renal cell carcinoma treated beyond progression: A subgroup
analysis of a randomized clinical trial. JAMA Oncol. 2:1179–1186.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Friedman CF, Proverbs-Singh TA and Postow
MA: Treatment of the immune-related adverse effects of immune
checkpoint inhibitors: A review. JAMA Oncol. 2:1346–1353. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarocchi M, Grossi F, Arboscello E,
Bellodi A, Genova C, Dal Bello MG, Rijavec E, Barletta G, Rossi G,
Biello F, et al: Serial troponin for early detection of nivolumab
cardiotoxicity in advanced non-small cell lung cancer patients.
Oncologist. 23:936–942. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gauci ML, Laly P, Vidal-Trecan T,
Baroudjian B, Gottlieb J, Madjlessi-Ezra N, Da Meda L,
Madelaine-Chambrin I, Bagot M, Basset-Seguin N, et al: Autoimmune
diabetes induced by PD-1 inhibitor-retrospective analysis and
pathogenesis: A case report and literature review. Cancer Immunol
Immunother. 66:1399–1410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Makarious D, Horwood K and Coward JIG:
Myasthenia gravis: An emerging toxicity of immune checkpoint
inhibitors. Eur J Cancer. 82:128–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spain L, Diem S and Larkin J: Management
of toxicities of immune checkpoint inhibitors. Cancer Treat Rev.
44:51–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lipson EJ, Sharfman WH, Drake CG, Wollner
I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, et al: Durable
cancer regression off-treatment and effective reinduction therapy
with an anti-PD-1 antibody. Clin Cancer Res. 19:462–468. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martini DJ, Hamieh L, McKay RR, Harshman
LC, Brandao R, Norton CK, Steinharter JA, Krajewski KM, Gao X,
Schutz FA, et al: Durable clinical benefit in metastatic renal cell
carcinoma patients who discontinue PD-1/PD-L1 therapy for
immune-related adverse events. Cancer Immunol Res. 6:402–408. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Skoulidis F, Goldberg ME, Greenawalt DM,
Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco
SE, Gay L, et al: STK11/LKB1 mutations and PD-1 inhibitor
resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov.
8:822–835. 2018. View Article : Google Scholar : PubMed/NCBI
|