Introduction
Anaplastic lymphoma kinase (ALK) gene
rearrangements are found in approximately 5% of non-small cell lung
cancer (NSCLC) patients, and are enriched in patients with
adenocarcinoma histology, patients with tumors of young onset, and
never or light-smokers (1,2). Several ALK tyrosine kinase inhibitors
(TKIs), such as crizotinib, alectinib, ceritinib, brigatinib, and
lorlatinib have been developed for ALK-positive NSCLC
(3–7), and crizotinib was the first
multi-targeted ALK-TKI to be approved. Despite initial dramatic
responses to crizotinib, the majority of patients show relapse
within 12 months because of the development of resistance (3). Only a few cases have shown long-lasting
response to crizotinib, especially over 5 years. Here we
experienced a very rare case of ALK-positive lung
adenocarcinoma with postoperative recurrence that maintained
complete response with crizotinib for over 5 years.
Case report
A 60-year-old male smoker with a right upper lobe
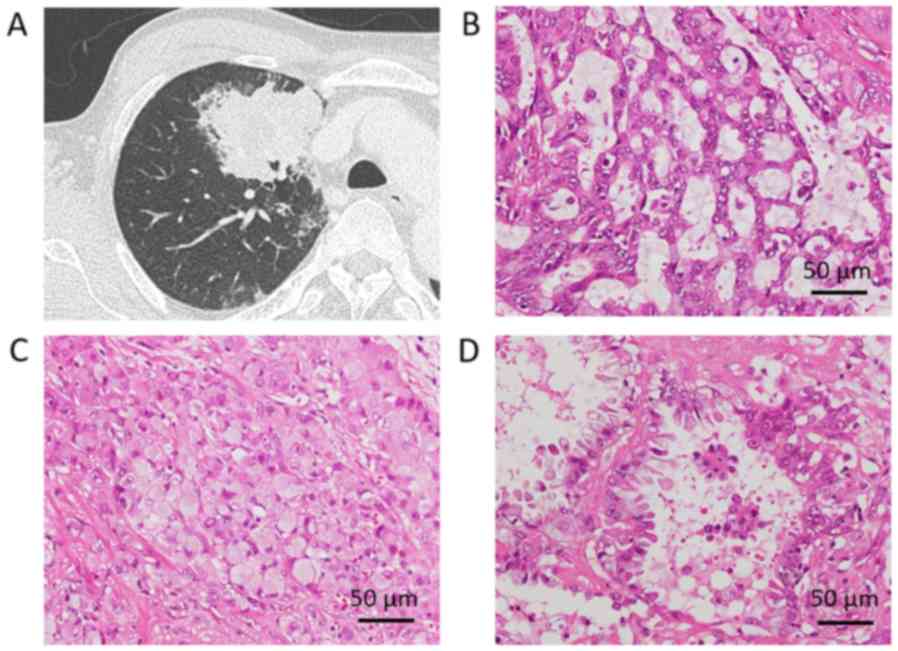
lung tumor was referred to our hospital for operation (Fig. 1A). The patient had a medical history
of controlled hypertension and hyperlipidemia. Transbronchial
biopsy showed histology of adenocarcinoma. Radical right upper
lobectomy with mediastinal lymph node dissection was performed.
Pathological examination revealed moderately differentiated
adenocarcinoma with acinar and solid component with cribriform
pattern (Fig. 1B-D). Micropapillary
component was identified within the acinar component and
signet-ring cells were present in the solid component. The tumor
was 7-cm in diameter and pleural invasion to superficial pleural
connective tissue, vessel invasion, and lymphatic invasion were
detected. Metastasis to hilar node was present, and the final
pathological stage was IIB according to the 7th edition of tumor,
node, and metastasis (TNM) classification. As postoperative
adjuvant therapy, the patient was administered three cycles of
carboplatin and S-1 (TS-1; Taiho Pharmaceutical, Tokyo, Japan)
followed by one year of tegafur-uracil (UFT; Taiho Pharmaceutical).
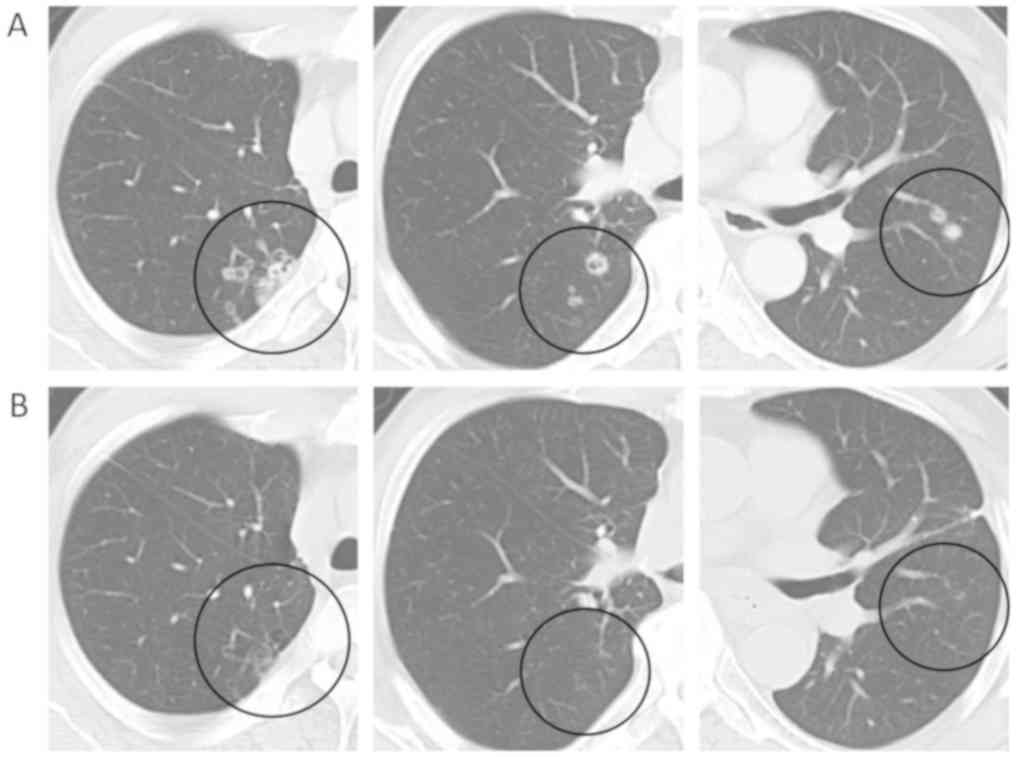
One year after the operation, multiple small nodules were detected
by computed tomography (CT) and follow-up CT scan showed that
nodules continued to grow (Fig. 2A).
At one year and 8 months after the operation, thoracoscopic
resection of a nodule in the right lower lobe was performed for
pathological diagnosis. Pathological examination of the lung nodule
revealed identical histology as the initial surgical specimen
(i.e., adenocarcinoma with solid tumor with acinar and
micropapillary components). Lung cancer recurrence was diagnosed,
and all other nodules detected by CT were also considered to be
recurrent lesions. Magnetic resonance imaging of brain and
18F-fluorodeoxyglucose positron emission tomography revealed there
was no other metastasis than multiple lung metastases.
Immunohistochemical analysis of the initial surgical specimen using
a commercial assay showed that tumor cells were positive for ALK
and fluorescence in situ hybridization confirmed the presence of
ALK gene rearrangement with a positive cell rate of 62%.
Analysis of the initial surgical specimen by next-generation
sequencing assay using FusionPlex (Archer, Boulder, CO, US)
revealed a variant type 2 of echinoderm microtubule-associated
protein-like 4 (EML4)-ALK rearrangement [exon 20 of EML4
fused to exon 20 of ALK (E20;A20)].
As the first-line treatment, crizotinib was
administered twice daily (250 mg) and the size of multiple nodules
remarkably decreased on follow-up CT after a month. Complete
response was confirmed after 4 months (Fig. 2B) and was maintained over 5 years
after the first administration of crizotinib. Grade 1 photopsia and
diarrhea were the only adverse events observed.
Discussion
We present here a case of ALK-rearranged lung
adenocarcinoma with postoperative multiple pulmonary metastases
that showed complete response to crizotinib over a period of 5
years. The majority of patients treated with crizotinib have a
relapse within 1 year (3). Clinical
trials of crizotinib revealed that progression-free survival (PFS)
was 10.9 months after first line treatment (3) and 7.7 months in patients who had
received one prior platinum-based regimen (8). Rangachari et al (9), reported two cases of advanced lung
adenocarcinoma with a PFS exceeding 5 years with crizotinib as
first-line treatment. To the best of our knowledge, the current
case is the third reported case of long-lasting PFS by crizotinib
treatment exceeding 5 years. In addition, the current case is the
first case depicting long-term complete response to crizotinib
after postoperative recurrence. The previous report by Rangachari
et al (9), does not include
clinical and pathological details of the two cases with
long-lasting PFS, and thus it is difficult to discuss the
clinicopathological tendencies of these cases.
Several variants of the EML4-ALK fusion have
been previously reported (10–12). The
most frequent variants are variant 1 (33%), in which exon 13 of
EML4 is fused to exon 20 of ALK (E13;A20); variant
3a/b (29%), in which exon 6a or 6b of EML4 is fused to exon
20 of ALK (E6a/b;A20); and variant 2 (9%), in which exon 20
of EML4 is fused to exon 20 of ALK (E20;A20)
(11). Other minor variants have
also been reported. Recent studies have suggested that the response
to crizotinib differs according to the ALK rearrangement
variant (13–18) (Table
I). Li et al (13),
reported that patients with variant 2 had a longer PFS compared
with patients with other variants. These clinical results are
supported by in vitro studies in which Ba/F3 cells
expressing variant 2 had higher sensitivity to crizotinib compared
with cells expressing other variants (15,19). The
results of these studies are consistent with our case, since our
patient also had variant 2 fusion and achieved long PFS with
crizotinib. However, such clinical differences in response or PFS
vary amongst reports (13–18). Because the previous clinical studies
were performed in small cohorts (Table
I), a definitive conclusion has not yet been established.
 | Table I.Different of efficacy of crizotinib
among ALK fusion variants. |
Table I.
Different of efficacy of crizotinib
among ALK fusion variants.
|
|
| Variant 1 | Variant 2 | Variant 3a/b |
|---|
|
|
|
|
|
|
|---|
| First author (Ref.
no.) | Total case | Case | ORR | PFS | Case | ORR | PFS | Case | ORR | PFS |
|---|
| Lei et al
(18) | 61 | 22 | 73% | 11.0 manon-v1/3a/b; n=21 ORR 81%, PFS 7.4
m | 18 | 56% | 10.9 m |
|
| Cha et al
(17) | 32 | 10 | 30% | x | 2 | 100% | x | 8 | 50% | x |
| Yoshida et al
(16) | 35 | 19 | 74% | 11.0 m | anon-v1; n=16 ORR 63%, PFS 4.2
m |
| Woo et al
(15) | 51 | anon-v3a/b; n=24, ORR 83%, 2-year
PFSR: 76% | 20 | 75% | 2-year |
|
|
|
|
|
|
|
|
|
|
| PFSR 26.4% |
| McLeer-Florin et
al (14) | 18 | v1/2; n=6, ORR 60%,
PFS 314 d | 8 | 63% | 192 d |
| Li et al
(13) | 60 | 14 | 46% | 10.7 m | 9 | 67% | 18.5 m | 20 | 65% | 7.9 m |
In the treatment of EGFR mutated lung cancer,
EGFR-TKIs sometimes maintain a good response for a long time. Lin
et al analyzed patients with EGFR mutation treated
with EGFR-TKIs and found that 14.6% of patients were 5-year
survivors (20). The absence of
extrathoracic metastasis was a significant factor associated with
prolonged overall survival (20). In
our case, the patient had multiple metastatic nodules, but these
were limited to pulmonary metastases. Thus, similar to EGFR
mutated lung cancer, absence of extrathoracic metastasis may also
be a factor related to long-lasting CR for patients with ALK
rearrangement.
In conclusion, here we presented a very rare case of
variant type 2 ALK-rearranged lung adenocarcinoma that
maintained complete response with crizotinib over 5 years. The
efficacy of crizotinib may vary among ALK fusion variants,
indicating that ALK variant type may represent an important factor
in guiding the treatment strategy for ALK-rearranged lung
adenocarcinoma. A large cohort analysis is required for further
study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contribution
TK designed the study. TK, TY, EY, SN, KiS, KT, RO,
AM, KeS wrote the manuscript. KT, TY, EY, SN, KT, RO, KiS, AM, and
KeS have contributed to the clinical management of the patient. AY
and JH analyzed pathological findings. YY performed the
next-generation sequencing assay. All authors critically reviewed
the manuscript and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval for this study was obtained from
Gunma University Hospital Ethics Committee. The patient provided
written informed consent.
Patient consent for publication
Written informed consent was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALK
|
anaplastic lymphoma kinase
|
|
NSCLC
|
non-small cell lung cancer
|
|
TKI
|
tyrosine kinase inhibitor
|
|
TNM
|
tumor, node, and metastasis
|
|
CT
|
computed tomography
|
|
EML4
|
echinoderm microtubule-associated
protein-like 4
|
|
PFS
|
progression-free survival
|
References
|
1
|
Soda M, Takada S, Takeuchi K, Choi YL,
Enomoto M, Ueno T, Haruta H, Hamada T, Yamashita Y, Ishikawa Y, et
al: A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad
Sci USA. 105:19893–19897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li T, Maus MK, Desai SJ, Beckett LA,
Stephens C, Huang E, Hsiang J, Zeger G, Danenberg KD, Astrow SH and
Gandara DR: Large-scale screening and molecular characterization of
EML4-ALK fusion variants in archival non-small-cell lung cancer
tumor specimens using quantitative reverse transcription polymerase
chain reaction assays. J Thorac Oncol. 9:18–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peters S, Camidge DR, Shaw AT, Gadgeel S,
Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, et al:
Alectinib versus crizotinib in untreated ALK-positive
non-small-cell lung cancer. N Engl J Med. 377:829–838. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soria JC, Tan DSW, Chiari R, Wu YL,
Paz-Ares L, Wolf J, Geater SL, Orlov S, Cortinovis D, Yu CJ, et al:
First-line ceritinib versus platinum-based chemotherapy in advanced
ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised,
open-label, phase 3 study. Lancet. 389:917–929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim DW, Tiseo M, Ahn MJ, Reckamp KL,
Hansen KH, Kim SW, Huber RM, West HL, Groen HJM, Hochmair MJ, et
al: Brigatinib in patients with crizotinib-refractory anaplastic
lymphoma kinase-positive non-small-cell lung cancer: A randomized,
multicenter phase II trial. J Clin Oncol. 35:2490–2498. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaw AT, Felip E, Bauer TM, Besse B,
Navarro A, Postel-Vinay S, Gainor JF, Johnson M, Dietrich J, James
LP, et al: Lorlatinib in non-small-cell lung cancer with ALK or
ROS1 rearrangement: An international, multicentre, open-label,
single-arm first-in-man phase 1 trial. Lancet Oncol. 18:1590–1599.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rangachari D, Le X, Shea M, Huberman MS,
VanderLaan PA, Kobayashi SS and Costa DB: Cases of ALK-rearranged
lung cancer with 5-year progression-free survival with crizotinib
as initial precision therapy. J Thorac Oncol. 12:e175–e177. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horn L and Pao W: EML4-ALK: Honing in on a
new target in non-small-cell lung cancer. J Clin Oncol.
27:4232–4235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sasaki T, Rodig SJ, Chirieac LR and Jänne
PA: The biology and treatment of EML4-ALK non-small cell lung
cancer. Eur J Cancer. 46:1773–1780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock
AB, Dagogo-Jack I, Jessop NA, Jiang GY, Le LP, Gowen K, et al:
Impact of EML4-ALK variant on resistance mechanisms and clinical
outcomes in ALK-positive lung cancer. J Clin Oncol. 36:1199–1206.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Zhang T, Zhang J, Li W, Yuan P, Xing
P, Zhang Z, Chuai S, Li J and Ying J: Response to crizotinib in
advanced ALK-rearranged non-small cell lung cancers with different
ALK-fusion variants. Lung Cancer. 118:128–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McLeer-Florin A, Duruisseaux M, Pinsolle
J, Dubourd S, Mondet J, Phillips Houlbracq M, Magnat N, Fauré J,
Chatagnon A, de Fraipont F, et al: ALK fusion variants detection by
targeted RNA-next generation sequencing and clinical responses to
crizotinib in ALK-positive non-small cell lung cancer. Lung Cancer.
116:15–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Woo CG, Seo S, Kim SW, Jang SJ, Park KS,
Song JY, Lee B, Richards MW, Bayliss R, Lee DH and Choi J:
Differential protein stability and clinical responses of EML4-ALK
fusion variants to various ALK inhibitors in advanced
ALK-rearranged non-small cell lung cancer. Ann Oncol. 28:791–797.
2017.PubMed/NCBI
|
|
16
|
Yoshida T, Oya Y, Tanaka K, Shimizu J,
Horio Y, Kuroda H, Sakao Y, Hida T and Yatabe Y: Differential
crizotinib response duration among ALK fusion variants in
ALK-positive non-small-cell lung cancer. J Clin Oncol.
34:3383–3389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cha YJ, Kim HR and Shim HS: Clinical
outcomes in ALK-rearranged lung adenocarcinomas according to ALK
fusion variants. J Transl Med. 14:2962016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lei YY, Yang JJ, Zhang XC, Zhong WZ, Zhou
Q, Tu HY, Tian HX, Guo WB, Yang LL, Yan HH, et al: Anaplastic
lymphoma kinase variants and the percentage of ALK-positive tumor
cells and the efficacy of crizotinib in advanced NSCLC. Clin Lung
Cancer. 17:223–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heuckmann JM, Balke-Want H, Malchers F,
Peifer M, Sos ML, Koker M, Meder L, Lovly CM, Heukamp LC, Pao W, et
al: Differential protein stability and ALK inhibitor sensitivity of
EML4-ALK fusion variants. Clin Cancer Res. 18:4682–4690. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin JJ, Cardarella S, Lydon CA, Dahlberg
SE, Jackman DM, Jänne PA and Johnson BE: Five-year survival in
EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs.
J Thorac Oncol. 11:556–565. 2016. View Article : Google Scholar : PubMed/NCBI
|