Introduction
Hepatocellular carcinoma (HCC) is the second most
common cause of cancer-related death worldwide (1). Several studies have reported the
clinical benefits of sorafenib in patients with advanced HCC, and
sorafenib is currently the only standard therapy in many countries
for patients with advanced HCC (2–4).
Furthermore, sorafenib improves survival in intermediate-stage HCC
patients not eligible for transarterial chemoembolization (TACE) or
who experienced progression after TACE (5–10). Ohki
et al reported that the therapeutic strategy of early
switching to sorafenib with TACE as maintenance therapy prolonged
progression-free survival compared with repeated conventional TACE
monotherapy in HCC patients unresponsive to TACE (9,10).
However, since some patients often become less responsive to
sorafenib, other second-line treatments are necessary. Regorafenib
has been established as a second-line treatment for patients who
experienced disease progression on sorafenib (11). However, administration of regorafenib
for HCC is confined to patients with good tolerability of sorafenib
≥400 mg, defined as having received sorafenib ≥400 mg/day for at
least 20 of the last 28 days of treatment, and Child-Pugh class A.
These criteria apply to only a small proportion of HCC patients in
clinical practice (12). If we can
predict the subset of HCC patients fulfilling these criteria prior
to initiation of sorafenib, it will influence the therapeutic
strategy. Although various previous reports about sorafenib showed
predictive factors for better prognosis in the advanced HCC with
extrahepatic metastasis (EHM) and macroscopic vascular invasion
(MVI), there is currently no information on the predictive factors
influencing the good tolerability of sorafenib in
intermediate-stage HCC patients in other wards advanced HCC without
EHM and MVI.
Recently, the indication for treatment HCC with
molecular targeting drug is expanding to intermediate stage. We
thought that it is necessary to select the patients who will
tolerate sorafenib prior to treatment in intermediate HCC.
Therefore, in this study, we reviewed the medical records of
TACE-refractory intermediate-stage HCC patients receiving sorafenib
at our institution and assessed the predictive baseline
characteristics for good tolerability to ≥400 mg sorafenib in order
to identify good candidates for second-line treatment with
regorafenib.
Patients and methods
Patients
From June 2009 to February 2018, there were 91
consecutive patients with advanced HCC who started sorafenib in our
institute. The inclusion criteria for treatment with sorafenib were
an Eastern Cooperative Oncology Group performance status (ECOG PS)
score of 2 or less, Child-Pugh class A or B, adequate hematologic
function (platelet count >50×103/µl and hemoglobin
>8.5 g/dl), adequate hepatic function (albumin >2.8 g/dl and
total bilirubin >3.0 mg/dl), alanine aminotransferase and
aspartate aminotransferase ≤5 times the upper limit of the normal
range, and adequate renal function (serum creatinine <1.5 times
the upper limit of the normal range according to the SHARP study)
(2). We targeted the object to only
TACE refractory intermediate stage HCC in this retrospective study.
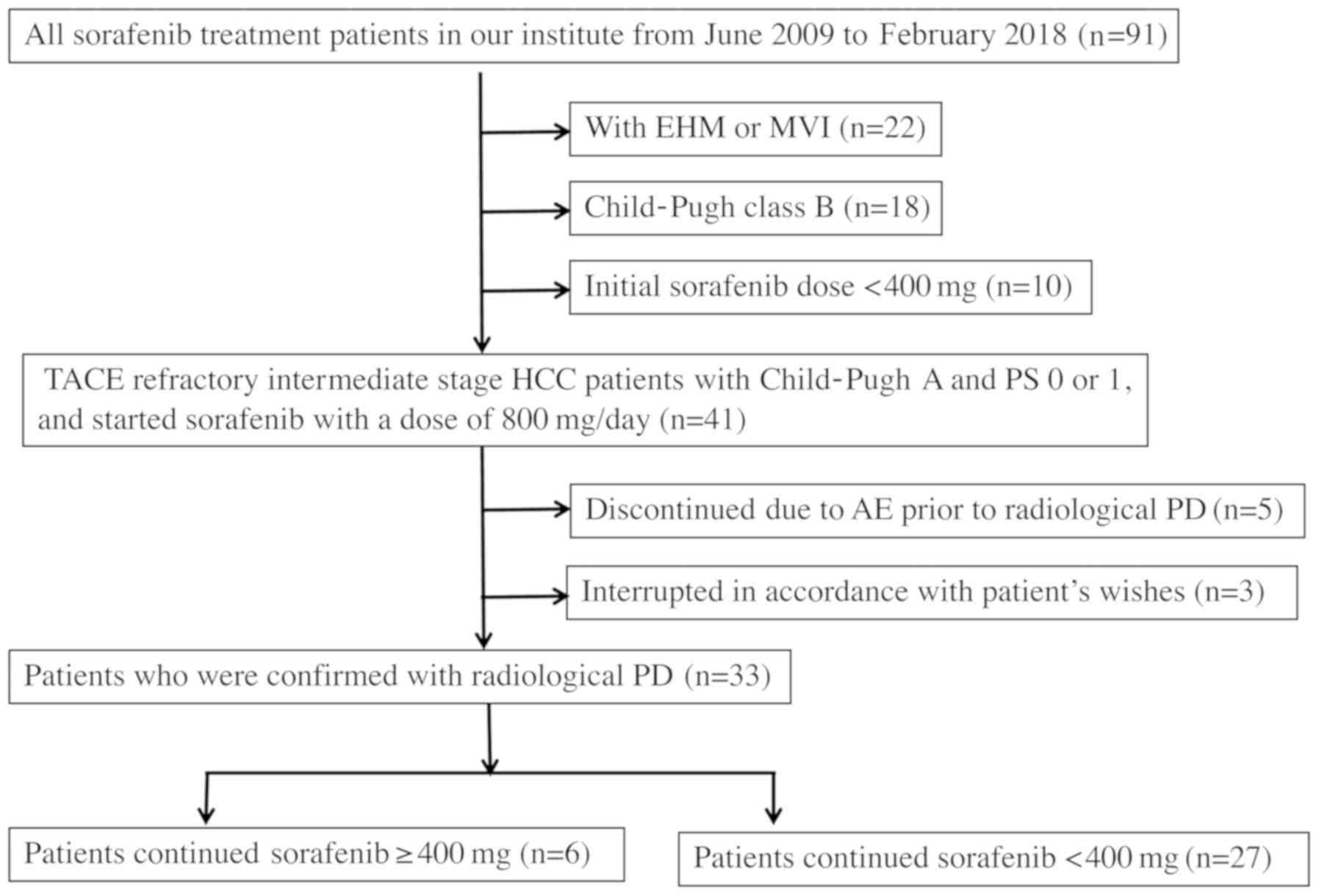
The flow chart for this study is showed in Fig. 1. The following patients were
excluded: Patients who had EHM or MVI (n=22), Child-Pugh B at the
start of sorafenib (n=18), patients who started sorafenib <400
mg for any reason (n=10), patients who discontinued sorafenib due
to sorafenib-related adverse events prior to radiological
progressive disease (PD) (n=5), patients who dropped out in
accordance with patient's wishes after start of treatment (n=2).
Remaining Thirty-three patients with TACE-refractory
intermediate-stage HCC who had confirmed PD were analyzed in this
retrospective study. These subjects were not candidates for surgery
or radiofrequency ablation. We compared baseline characteristic
parameters between patients who continued sorafenib ≥400 mg
(sorafenib ≥400 mg) and patients who could not continue ≥400 mg
(sorafenib <400 mg) in order to determine the predictive
baseline characteristics parameters for the ability to continue
sorafenib ≥400 mg.
Sorafenib treatment
The starting dose of sorafenib was 800 mg/day.
Before initiation of sorafenib treatment, patients were informed of
adverse events associated with sorafenib, including skin toxicity,
hypertension, diarrhea, and gastrointestinal disorders, which have
been reported in previous studies (13–15). The
efficacy and progression of sorafenib were assessed using the
modified Response Evaluation Criteria in Solid Tumors (mRECIST)
(13), and adverse effects were
assessed according to the Common Terminology Criteria (CTCAE) ver
4.0. All patients began using urea cream to prevent skin
toxicity.
Treatment interruptions and dose reductions (400 mg
once daily or 400 mg on alternate days) were permitted depending on
the severity and type of adverse events. When Grade 3 and more
adverse events was occurred sorafenib dose reduction or temporary
interruption was maintained until symptoms resolved to Grade 1 or 2
according to the guidelines provided by manufacturer. After dose
reduction or interruption, sorafenib dose was increased and
maintain ≥400 mg as much as possible. Patients continued sorafenib
treatment as long as possible, until adverse events were
intolerable or patients reached to PD. We defined sorafenib ≥400 mg
for patients could continue sorafenib 400 mg or more at least 80%
of the period from initiation of treatment to the final
observation, and sorafenib <400 mg for the rest.
The present study was conducted in accordance with
the Declaration of Helsinki, and the ethics committee of Hiroshima
Red Cross Hospital and Atomic-bomb Survivors Hospital institution
approved the study protocol. Written informed consent was obtained
from each participating patient. The study was conducted in
accordance with the Declaration of Helsinki, and the ethics
committee of our institution approved the study protocol.
Statistical analysis
Categorical variables were compared using the Fisher
exact test or χ2-test. Univariate survival analysis of
survival was performed using Kaplan-Meier survival curves with
log-rank survival comparison and 95% confidence intervals (95%
CIs). Cut-off levels for each categorical variable were determined
by preliminary testing, including receiver operating characteristic
(ROC) analysis. A logistic regression model was used to investigate
factors associated with continuation of sorafenib ≥400 mg.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed with EZR
(Saitama Medical Center, Jichi Medical University, Saitama, Japan),
which is a graphical user interface for R (The R Foundation for
Statistical Computing, Vienna, Austria). More precisely, it is a
modified version of R commander designed to add statistical
functions frequently used in biostatistics (16).
Results
Baseline characteristics
The baseline characteristics of our cohort are
summarized in Table I. Tumor staging
was based on the Tumor-Node-Metastasis staging system of the Liver
Cancer Study Group of Japan (17).
All 33 patients were classified as Child-Pugh A at initiation of
sorafenib treatment.
 | Table I.Baseline patient characteristics. |
Table I.
Baseline patient characteristics.
| Items | Value |
|---|
| Sex (male) | 26
(78.8)a |
| Age (year) | 76 (52–88) |
| BW (kg) | 61.5 (37–93) |
| BMI | 23.88
(14.9–30.1) |
| HBV/HCV/NBNC [n
(%)] | 3 (9)/21 (63)/9
(27)a |
| WBC (/µl) | 4300 (1700–8600) |
| RBC (/µl) | 406 (275–546) |
| Hb (g/dl) | 13.1 (9.6–16.6) |
| Ht (%) | 38.7 (29.6–46.3) |
| Plt
(×103/µl) | 98 (44–267) |
| PT activity (%) | 89.8
(54.8–111.4) |
| T-Bil (mg/dl) | 0.7 (0.3–2.6) |
| AST (IU/l) | 33 (16–76) |
| ALT (IU/l) | 31 (12–119) |
| γGTP (IU/l) | 45 (13–238) |
| BUN (mg/dl) | 14.5 (9.9–44) |
| Cr (mg/dl) | 0.84 (0.53–1.73) |
| ChE (U/l) | 204 (105–365) |
| Alb (g/dl) | 3.9 (3.2–5.0) |
| Tcho (mg/dl) | 153 (113–204) |
| TG (mg/dl) | 83 (45–199) |
| CRP (mg/dl) | 0.11 (0.0–0.58) |
| Child Pugh score
5 | 23 (70)a |
| ALBI grade 1 | 15 (46)a |
| MELD SCORE | 5.334
(2.851–8.982) |
| AFP (ng/ml) | 89 (2.2–94760) |
| DCP (mAu/ml) | 168 (11–9300) |
| Tumor number | 5 (1–25) |
| Tumor size (mm) | 22 (10–55) |
| Bilateral lesion [n
(%)] | 20 (64)a |
Sorafenib treatment
Among the 33 patients, 6 patients (18.1%) continued
sorafenib ≥400 mg, but 27 (71.9%) patients continued <400 mg.
While all of the patients had some lower than CTCAE Gr2 adverse
event during the treatment, they were within tolerable range by
supportive treatment. Since all 6 patients with sorafenib ≥400 mg
had Child Pugh A liver function and were considered eligible for
regorafenib, they switched the treatment sorafenib to regorafenib
and survived for a median 8 (6–12) months
after initiation of regorafenib.
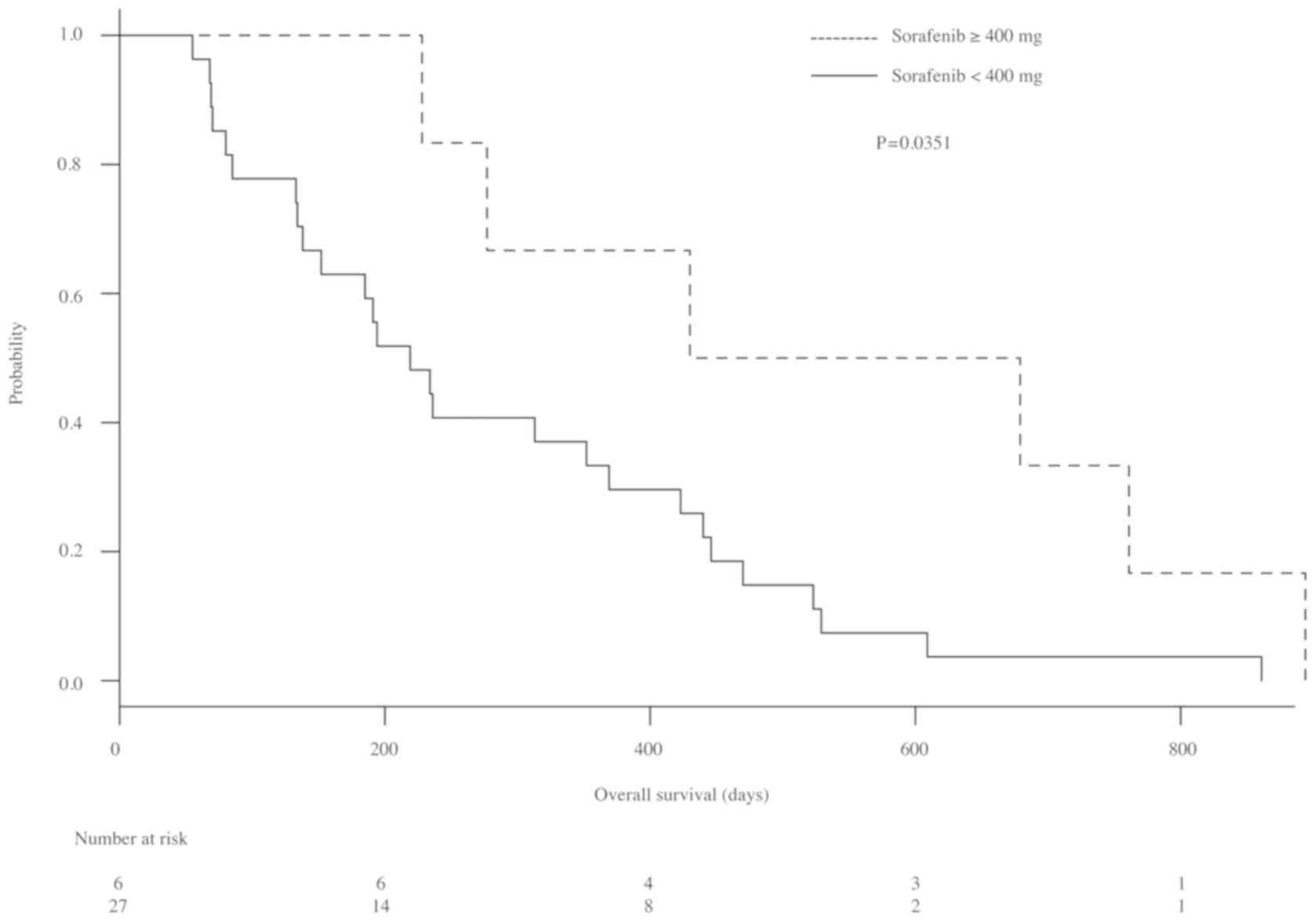
Comparison of OS based on each dose of sorafenib is
shown in Fig. 2. The median survival
of patients with sorafenib ≥400 and <400 mg were 554.5 (228–674)
days and 219 (134–369) days, respectively. The OS of patients with
≥400 mg sorafenib was significantly longer than that of patients
with <400 mg (P=0.0315).
Comparison of baseline characteristics and
predictive factors for continuation sorafenib dose ≥400 mg.
The baseline characteristics parameters of patients
with sorafenib ≥400 and <400 mg are described in Table II. There were significantly more
patients with age <75 years, with total cholesterol (T-Cho) ≥180
mg/dl (P=0.026) and with cholinesterase (ChE) ≥220 U/l (P=0.024) in
the patients who continued sorafenib ≥400 mg. Among other
parameters, BMI ≥24 kg/m2 (OR 6.871 (0.860–496.9),
P=0.062) and AFP levels <10 ng/ml (OR 9.305 (0.641–364.1),
P=0.085) had some tendency to influence continuation of sorafenib
≥400 mg, but these tendencies were not significant. Table III shows the univariate and
multivariate logistic regression analyses of baseline predictive
factors for continuation of sorafenib ≥400 mg in TACE-refractory
intermediate-stage HCC. In a univariate analysis for factors
influencing continuation of sorafenib ≥400 mg, age <75 years,
T-cho ≥180 mg/dl and ChE ≥220 U/l were significant factors, and a
multivariate analysis of these factors confirmed ChE ≥220 U/l as a
prognostic factor significantly predicting continuation of
sorafenib ≥400 mg [hazard ratio: 11.9, 95% CI: 1.190–118.0;
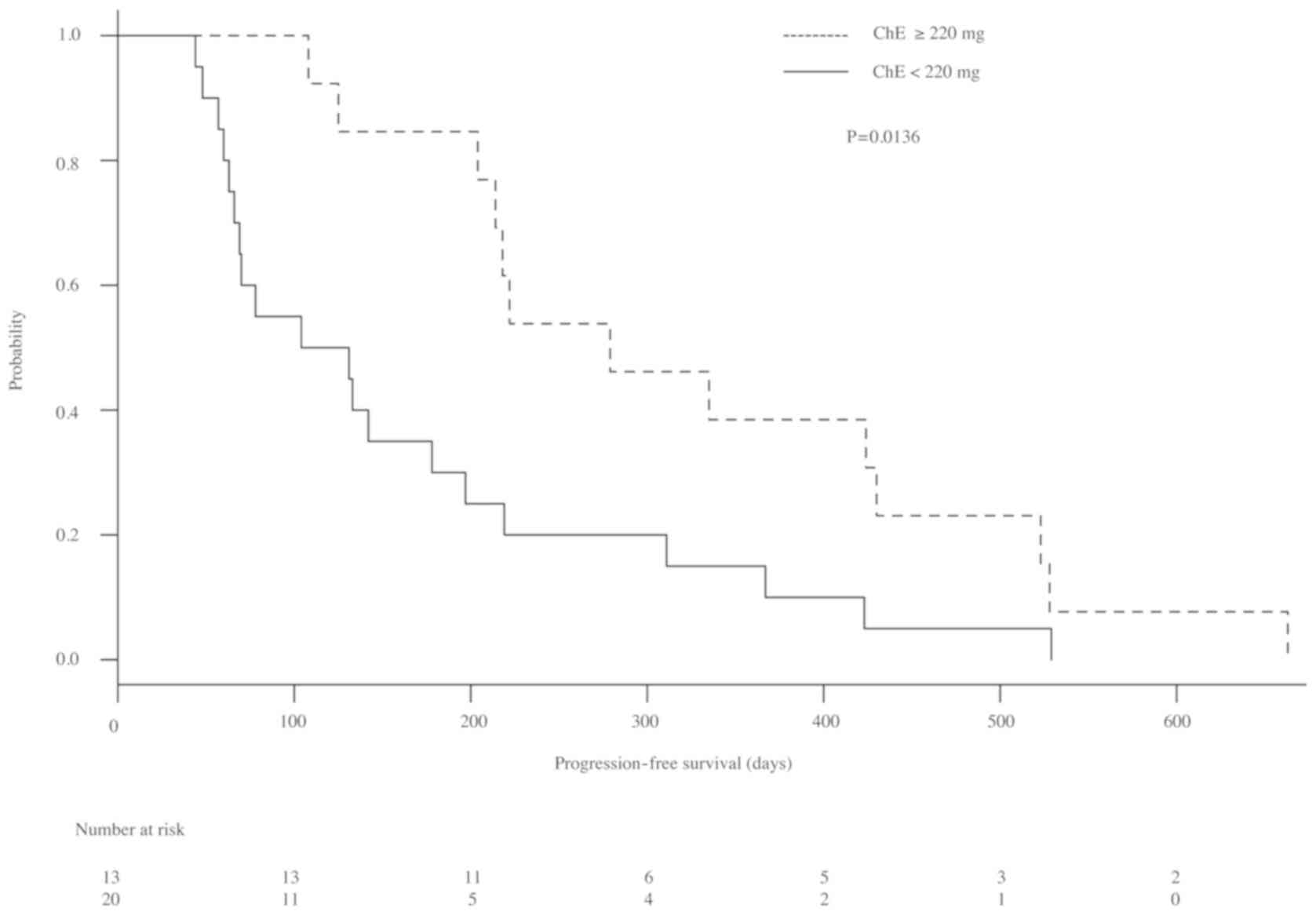
P=0.004]. The Kaplan-Meier survival curves for progression-free
survival (PFS) and OS with each parameter are shown in Figs. 3 and 4. Median PFS in patients with ChE levels
≥220 U/l and those with ChE levels <220 U/l were 279 (204–403)
days and 117.5 (63–197) days, respectively. Furthermore, PFS was
significantly longer in patients with ChE levels ≥220 U/l than in
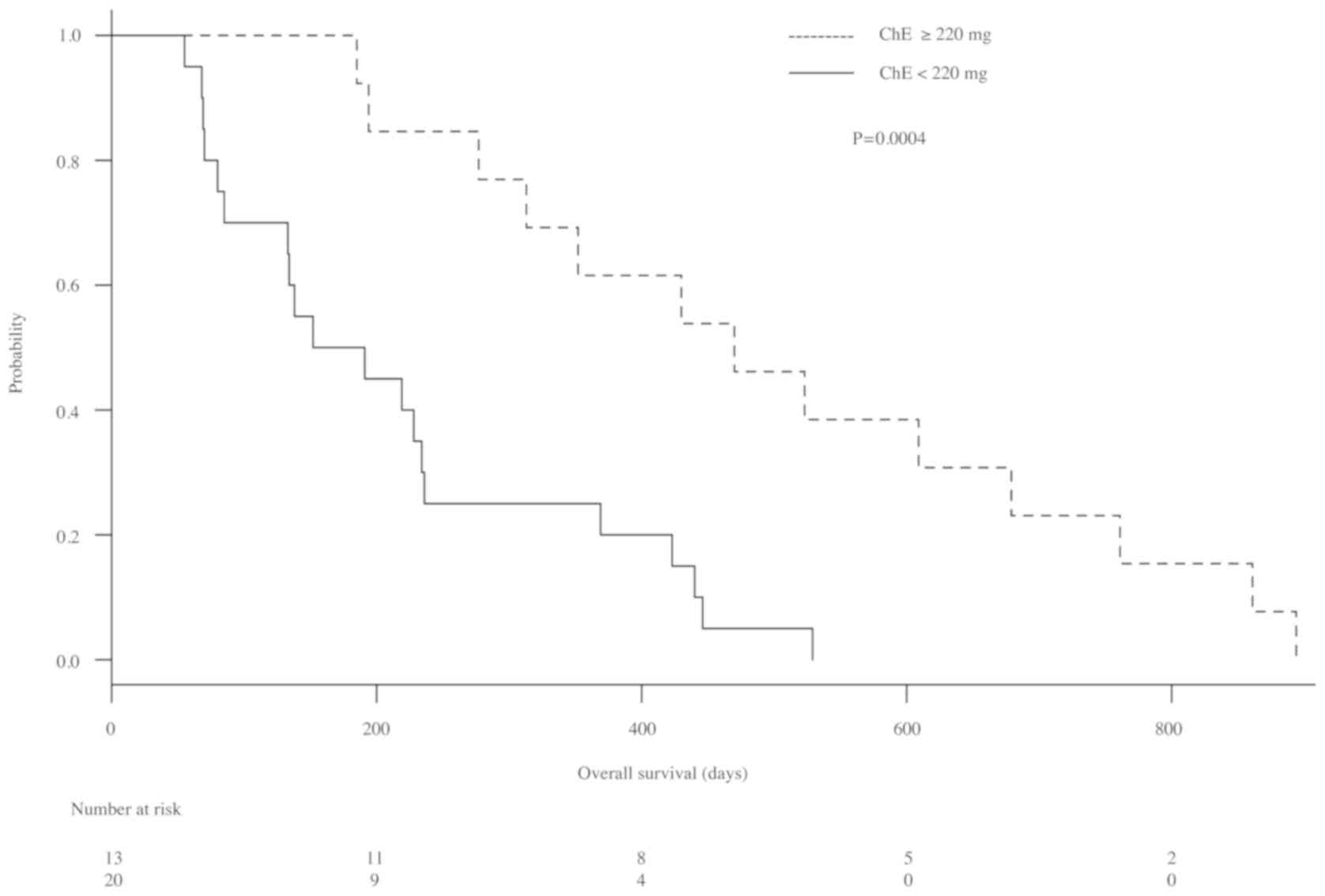
patients with ChE levels <220 U/l (P=0.0136; Fig. 3). Furthermore, median OS of patients
with ChE levels ≥220 U/l and those with ChE levels <220 U/l were
470 (277–679) days and 171.5 (80–236) days, respectively. The OS of
patients with ChE ≥220 U/l was significantly longer than that of
patients with ChE <220 U/l (P=0.0004; Fig. 4).
 | Table II.The baseline characteristics of
patients undergoing sorafenib ≥400 and <400 mg treatment. |
Table II.
The baseline characteristics of
patients undergoing sorafenib ≥400 and <400 mg treatment.
| Characteristic | ≥400 mg (n=6) [n
(%)] | <400 mg (n=27) [n
(%)] | P-value |
|---|
| Sex |
|
| 0.38 |
| Male | 6 (100) | 20 (74.1) |
|
|
Female | 0 (0) | 7 (25.9) |
|
| Age |
|
| 0.021 |
| <75
year | 6 (100) | 17 (63.0) |
|
| ≥75
year | 0 (0) | 10 (37.0) |
|
| BW |
|
| 0.159 |
| ≥70
kg | 4 (66.7) | 8 (29.6) |
|
| <70
kg | 2 (33.3) | 19 (70.4) |
|
| BMI |
|
| 0.085 |
|
≥24 | 5 (83.3) | 11 (40.7) |
|
|
<24 | 1 (16.7) | 16 (59.3) |
|
| HCV |
|
| 0.643 |
|
HCV | 3 (50.0) | 18 (66.6) |
|
|
Non-HCV | 3 (50.0) | 9 (33.4) |
|
| HBV |
|
| 0.464 |
|
HBV | 1 (16.7) | 2 (7.4) |
|
|
Non-HBV | 5 (83.3) | 25 (92.6) |
|
| NBNC |
|
| 1.000 |
|
NBNC | 2 (33.3) | 7 (25.9) |
|
|
HCV+HBV | 4 (66.7) | 20 (74.1) |
|
| WBC |
|
| 0.616 |
|
≥3000/µl | 2 (33.3) | 6 (22.2) |
|
|
<3000/µl | 4 (66.7) | 21 (77.8) |
|
| Hb |
|
| 0.153 |
| <13
g/dl | 1 (16.7) | 15 (55.6) |
|
| ≥13
g/dl | 5 (83.3) | 12 (44.4) |
|
| Ht |
|
| 0.375 |
|
<40% | 2 (33.3) | 16 (59.3) |
|
|
≥40% | 4 (66.7) | 11 (40.7) |
|
| Plt |
|
| 0.216 |
|
<70×103/µl | 2 (33.3) | 24 (88.9) |
|
|
≥70×103/µl | 4 (66.7) | 3 (11.1) |
|
| T-bil |
|
| 0.309 |
| <1.0
mg/dl | 3 (50.0) | 21 (77.8) |
|
| ≥1.0
mg/dl | 3 (50.0) | 6 (22.2) |
|
| AST |
|
| 0.364 |
| <40
IU/l | 5 (83.3) | 15 (55.6) |
|
| ≥40
IU/l | 1 (16.7) | 12 (44.4) |
|
| ALT |
|
| 0.216 |
| <50
IU/l | 4 (66.7) | 24 (88.9) |
|
| ≥50
IU/l | 2 (33.3) | 2 (11.1) |
|
| γGTP |
|
| 0.658 |
| <50
IU/l | 3 (50.0) | 17 (63.0) |
|
| ≥50
IU/l | 3 (50.0) | 10 (37.0) |
|
| Alb |
|
| 0.616 |
| <4.0
g/dl | 2 (33.3) | 6 (22.2) |
|
| ≥4.0
g/dl | 4 (66.7) | 21 (77.8) |
|
| ChE |
|
| 0.024 |
| ≥220
U/l | 5 (83.3) | 8 (29.6) |
|
| <220
IU/l | 1 (16.7) | 19 (70.4) |
|
| BUN |
|
| 0.308 |
| ≥21
mg/dl | 0 (0.0) | 7 (25.9) |
|
| <21
mg/dl | 6 (100) | 20 (74.1) |
|
| Cr |
|
| 0.375 |
| <0.8
mg/dl | 4 (66.7) | 11 (40.7) |
|
| ≥0.8
mg/dl | 2 (33.3) | 16 (59.3) |
|
| CRP |
|
| 0.309 |
| <0.2
mg/dl | 3 (50.0) | 21 (77.8) |
|
| ≥0.2
mg/dl | 3 (50.0) | 6 (22.2) |
|
| T-cho |
|
| 0.026 |
| ≥180
mg/dl | 4 (66.7) | 4 (14.8) |
|
| <180
mg/dl | 2 (33.3) | 23 (85.2) |
|
| Child Pugh
score |
|
| 0.640 |
| 5 | 5(83.3) | 18(66.6) |
|
|
>5 | 1 (16.7) | 9 (33.3) |
|
| ALBI |
|
| 0.375 |
| 1 | 4(66.7) | 11(40.7) |
|
|
>1 | 2 (33.3) | 16 (59.3) |
|
| MELD score |
|
| 1 |
|
<4 | 1 (16.7) | 10 (37.0) |
|
| ≥4 | 5 (83.3) | 17 (63.0) |
|
| AFP |
|
| 0.062 |
| <10
ng/ml | 1 (16.7) | 18 (66.7) |
|
| ≥10
ng/ml | 5 (83.3) | 9 (33.3) |
|
| DCP |
|
| 0.373 |
| <50
mAu/ml | 2 (33.3) | 6 (22.2) |
|
| ≥50
mAu/ml | 4 (66.7) | 21 (77.8) |
|
| Tumor number |
|
| 0.159 |
|
<5 | 2 (33.3) | 19 (70.4) |
|
| ≥5 | 4 (66.7) | 8 (29.6) |
|
| Tumor size |
|
| 0.64 |
| >30
mm | 1 (16.7) | 9 (33.3) |
|
| ≤30
mm | 5 (83.3) | 18 (66.7) |
|
| Bilateral
lesion |
|
|
|
|
Bilateral | 3 (50.0) | 17 (63.0) | 0.659 |
|
One-side | 3 (50.0) | 10 (37.0) |
|
| Prior TACE |
|
| 1 |
| <3
times | 5 (83.3) | 21 (77.8) |
|
| ≥3
times | 1 (16.7) | 6 (22.2) |
|
 | Table III.Factors influencing continuation of
sorafenib ≥400 mg. |
Table III.
Factors influencing continuation of
sorafenib ≥400 mg.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Sex
(male/female) | inf | 0.313-inf | 0.301 |
|
|
|
| Age <75/≥75
(years) | 0 | 0.000–0.643 | 0.021 |
|
|
|
| BW ≥70/<70
(kg) | 4.502 | 0.524–59.44 | 0.159 |
|
|
|
| BMI ≥24/<24 | 6.871 | 0.641–364.1 | 0.085 |
|
|
|
| HCV/non-HCV | 0.643 | 0.056–4.609 | 0.511 |
|
|
|
| WBC ≥3000/<3000
(µl) | 0.582 | 0.063–7.916 | 0.616 |
|
|
|
| Hb <13/≥13
(g/dl) | 5.937 | 0.555–314.2 | 0.175 |
|
|
|
| Ht
<40/≥40(%) | 2.43 | 0.238–19.30 | 0.418 |
|
|
|
| Plt
<70×103/≥70×103 (µl) | 2.815 | 0.022- 4.09 | 0.216 |
|
|
|
| T-bil <1.0/≥1.0
(mg/dl) | 3.345 | 0.355–32.27 | 0.309 |
|
|
|
| AST <40/≥ 40
(IU/l) | 0.259 | 0.005- 2.79 | 0.364 |
|
|
|
| ALT <50/≥50
(IU/l) | 3.783 | 0.245–46.43 | 0.216 |
|
|
|
| γGTP <50≥ 50
(IU/l) | 1.672 | 0.187–15.05 | 0.658 |
|
|
|
| Alb <4.0/≥4.0
(g/dl) | 1.717 | 0.126–15.94 | 0.616 |
|
|
|
| ChE ≥220/<220
(U/l) | 38 | 1.003–588.0 | 0.024 | 11.9 | 1.190–118.0 | 0.004 |
| BUN ≥21<21
(mg/dl) | 0 | 0.000–3.189 | 0.301 |
|
|
|
| Cr <0.8/≥0.8
(mg/dl) | 0.355 | 0.027–2.986 | 0.374 |
|
|
|
| CRP <0.2/≥0.2
(mg/dl) | 3.345 | 0.354–32.28 | 0.309 |
|
|
|
| T-cho ≥180/<180
(mg/dl) | 10.31 | 1.085–151.5 | 0.026 |
|
|
|
| Child Pugh score
5/>5 | 0.409 | 0.007–4.535 | 0.640 |
|
|
|
| ALBI 1/>1 | 0.355 | 0.027–2.986 | 0.375 |
|
|
|
| MELD score
<4/≥4 | 0.635 | 0.039–39.29 | 1 |
|
|
|
| AFP <10/≥10
(ng/ml) | 9.305 | 0.860–496.9 | 0.062 |
|
|
|
| DCP <50/≥50
(mAu/ml) | 0.582 | 0.063–7.916 | 0.616 |
|
|
|
| Tumor number
<5/≥5 | 1.5 | 0.524–59.45 | 0.159 |
|
|
|
| Tumor size
>30/≤30 (mm) | 0.714 | 0.008–4.536 | 0.64 |
|
|
|
| Bilateral
lesion/non-bilateral lesion | 0.598 | 0.067–5.353 | 0.659 |
|
|
|
| Prior TACE <3
times/≥3 times | 0.707 | 0.013–8.437 | 1 |
|
|
|
Discussion
Recently, the indication for treatment of HCC with
molecular targeting drug is expanding to intermediate stage. We
thought that it is necessary to select the patients who will
tolerate the molecular targeting drug prior to treatment. We
revealed that patients with TACE-refractory HCC who had high ChE
levels prior initiation of sorafenib would be more likely to be
able to continue sorafenib ≥400 mg, which means that they would be
eligible for second-line treatment with regorafenib. Takeda et
al reported that lower serum ChE level is a significant
predictor of poor prognosis and severe liver damage in advanced HCC
patients with sorafenib (18). These
findings showed our result prove our result was reasonable
evidence. However, the object of their study included advanced
stage HCC such as extra hepatic metastasis and vascular invasion
case. Therefore, we focused on only intermediate stage. To the best
of our knowledge, this is the first study to investigate the
factors associated with continuation of second-line sorafenib
focused to only intermediate-stage TACE-refractory HCC in other
wards without advanced HCC without EHM and MVI.
Currently, most treatment guidelines for advanced
HCC, which use BCLC staging, indicate sorafenib as the standard
systemic therapy (16–22). Furthermore, the European guidelines
also recommend sorafenib for patients with intermediate-stage HCC
(BCLC stage B) who have failed at least two cycles of TACE or with
progression following TACE (2–10). The
Japanese guidelines base their treatment recommendations on other
factors, recommend sorafenib as the first-line treatment for
patients with EHM and/or MVI and for TACE-refractory patients
classified as Child-Pugh A (23).
Since sorafenib is recommended for TACE-refractory patients, we
also treat TACE-refractory patients with advanced HCC, including
intermediate-stage, with sorafenib. However, many patients in this
study could not continue sorafenib treatment because of adverse
events. Some previous studies have reported that sorafenib
discontinuation may cause HCC flares, and patients who could
continue sorafenib had better prognosis than patients who
discontinued sorafenib (14,15,24–26).
Whereas our study also showed that patients who continued sorafenib
≥400 mg had good liver function and were candidates for regorafenib
or other treatment; therefore, we analyzed predictive factors for
continuation of sorafenib ≥400 mg.
Most of the previous studies, objects for these
studies were included EHM and MVI, found that candidates for
second-line sorafenib who failed sorafenib had better prognosis
than non-candidates. This can be explained by previous studies.
Uchikawa et al revealed that absence of MVI and serum
albumin >3.5 g were predictive factors for candidates (14). Ogasawara et al showed that a
lower Child-Pugh score and a better ECOG PS were predictors of
eligibility for second-line therapy, such as sorafenib ≥400 mg
(12). However, these studies
included patients with advanced-stage HCC, and therefore these
predictive factors may be specific to that patient cohort. In
contrast, most TACE-refractory patients in whom sorafenib treatment
is indicated in the real world have intermediate-stage disease
without MVI and EHM, and the predictive factors of candidates for
second-line sorafenib treatment in these patients have not been
defined. Therefore, we limited our subjects in this study to
patients with intermediate-stage HCC. In this study, in the
univariate analysis for factors influencing continuation of
sorafenib ≥400 mg found only age <75 years, T-cho levels ≥180
mg/dL, and ChE levels ≥220 U/l to be significant factors. Among
other parameters, BMI ≥24 kg/m2 and AFP levels <10
ng/ml had some tendency to influence continuation of sorafenib ≥400
mg, but these tendencies were not significant. No significant
differences were observed in other parameters, including Child-Pugh
score, MELD score, and ALBI grade. Eventually, ChE levels ≥220 U/l
was only significant factor in multivariate analysis.
The patients with high levels of ChE did not have
severe obesity or fatty liver disease; therefore, we thought that
high ChE indicated lack of cirrhosis and good remnant liver
function. This idea is similar to those proposed in previous
studies (12,14). Takeda et al reported that
pretreatment ChE level is a reliable prognostic marker for advanced
HCC in the sorafenib era (18). The
cut-off level in their study was 140 U/l, whereas the cut-off level
in our study was 220 U/l. We performed preliminary testing for
various cut-off levels, including 140 U/l, 205 U/l (median of our
cohorts) by refereeing to Takeda's report, similar results were
obtained in each parameter. Of these cut-off levels, the ChE level
of 220 U/l had most significant odds ratio in our study cohort.
Additionally, on performing ROC analysis, the cut-off level of ChE
was 220 IU/ml (specificity: 0.692, sensitivity: 0.714, area under
the curve, 0.6264). Therefore, we decided to use a ChE cut-off
level of 220 U/l. We considered that since patients with baseline
ChE levels ≥220 U/l had better remnant liver function, they could
continue receiving sorafenib ≥400 mg.
The present study has some limitations. First, this
study was performed retrospectively and was a single institute
study. Second, its sample size was small, since there are few in
intermediate stage HCC treated with only sorafenib at that time.
Third, all of the included patients were Japanese, who are well
known to experience adverse events due to sorafenib. Finally, the
subject of this study was limited only PD case to analyze the
indication of second line treatment. However, good response case
such as CR should be occurred in real clinical setting, while this
study was not included CR case. Whereas we presented baseline high
value of ChE in intermediate-stage HCC predicts the ability to
continue sorafenib ≥400 mg, it is uncertain that high value of ChE
patients will be the better prognosis of intermediate HCC in real
clinical setting. Therefore, we consider that larger sample size
and prospective additional analyses are needed in order to clarify
whether intermediate HCC patients with high value of ChE levels
will show a good prognosis.
In conclusion, we are the first to determine that
continuation of sorafenib is associated with better prognosis in
patients with TACE-refractory intermediate-stage HCC. We suggest
that it is important to select appropriate therapy beyond
second-line treatment in this population. This is particularly
important since patients with high baseline levels of ChE could
continue sorafenib ≥400 mg, leading them to be candidates for
second-line treatment and to have a better prognosis.
Acknowledgements
The authors would like to thank and Associate
Professor Hiroshi Aikata (Department of Gastroenterology and
Metabolism, Hiroshima University Hospital) and Professor Kazuaki
Chayama (Department of Gastroenterology and Metabolism, Hiroshima
University Hospital) for their valuable comments.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST was responsible for the concept and design of the
study and for data interpretation and wrote this manuscript. TF and
NM assisted with data interpretation and article preparation. KT
assisted with data interpretation and article preparation and
supervised the project.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Hiroshima Red Cross Hospital and Atomic-bomb
Survivors' Hospital, as per the Declaration of Helsinki. Written
informed consent was obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Lu R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the asia-pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikeda M, Mitsunaga S, Shimizu S, Ohno I,
Takahashi H, Okuyama H, Kuwahara A, Kondo S, Morizane C, Ueno H, et
al: Efficacy of sorafenib in patients with hepatocellular carcinoma
refractory to transcatheter arterial chemoembolization. J
Gastroenterol. 49:932–940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ogasawara S, Chiba T, Ooka Y, Kanogawa N,
Motoyama T, Suzuki E, Tawada A, Kanai F, Yoshikawa M and Yokosuka
O: Efficacy of sorafenib in intermediate-stage hepatocellular
carcinoma patients refractory to transarterial chemoembolization.
Oncology. 87:330–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arizumi T, Ueshima K, Minami T, Kono M,
Chishina H, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, et al:
Effectiveness of sorafenib in patients with transcatheter arterial
chemoembolization (TACE) refractory and intermediate-stage
hepatocellular carcinoma. Liver Cancer. 4:253–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kudo M, Imanaka K, Chida N, Nakachi K, Tak
WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al: Phase
III study of sorafenib after transarterial chemoembolization in
Japanese and Korean patients with unresectable hepatocellular
carcinoma. Eur J Cancer. 47:2117–2127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lencioni R, Llovet JM, Han G, Tak WY, Yang
J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, et al: Sorafenib
or placebo plus TACE with doxorubicin-eluting beads for
intermediate stage HCC: The SPACE trial. J Hepatol. 64:1090–1098.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohki T, Sato K, Yamagami M, Ito D, Yamada
T, Kawanishi K, Kojima K, Seki M, Toda N and Tagawa K: Efficacy of
transcatheter arterial chemoembolization followed by sorafenib for
intermediate/advanced hepatocellular carcinoma in patients in
Japan: A retrospective analysis. Clin Drug Investig. 35:751–759.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohki T, Sato K, Yamagami M, Ito D, Yamada
T, Kawanishi K, Kojima K, Seki M, Toda N and Tagawa K: Erratum to:
Efficacy of transcatheter arterial chemoembolization followed by
sorafenib for intermediate/advanced hepatocellular carcinoma in
patients in Japan: A retrospective analysis. Clin Drug Investig.
36:93–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ogasawara S, Chiba T, Ooka Y, Suzuki E,
Maeda T, Yokoyama M, Wakamatsu T, Inoue M, Saito T, Kobayashi K, et
al: Characteristics of patients with sorafenib-treated advanced
hepatocellular carcinoma eligible for second-line treatment. Invest
New Drugs. 36:332–339. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uchikawa S, Kawaoka T, Aikata H, Kodama K,
Nishida Y, Inagaki Y, Hatooka M, Morio K, Nakahara T, Murakami E,
et al: Clinical outcomes of sorafenib treatment failure for
advanced hepatocellular carcinoma and candidates for regorafenib
treatment in real-world practice. Hepatol Res. 48:814–820. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuzuya T, Ishigami M, Ishizu Y, Honda T,
Hayashi K, Ishikawa T, Nakano I, Hirooka Y and Goto H: Prognostic
factors associated with postprogression survival in advanced
hepatocellular carcinoma patients treated with sorafenib not
eligible for second-line regorafenib treatment. Oncology. 95:91–99.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanda Y: Investigation of freely-available
easy-to use software ‘EZR’ for medial statistics. Bone Marrow
Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
The general rules for the clinical and
pathological study of primary liver cancer study group of japan.
Liver cancer study group of Japan. Jpn J Surg. 19:98–129. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeda H, Nishikawa H, Iguchi E, Ohara Y,
Sakamoto A, Hatamaru K, Henmi S, Saito S, Nasu A, Komekado H, et
al: Impact of pretreatment cholinesterase level in unresectable
advanced hepatocellular carcinoma patients treated with sorafenib.
Mol Clin Oncol. 1:241–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
European association for the study of the
liver electronic address, . simpleEasloffice@easloffice.eu;
European association for the study of the liver: EASL clinical
practice guideline: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brux J and Shernman M; American
association for the study of liver disease, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heimbach JK, Kulil LM, Finn RS, Sirlin CB,
Abecassis MM, Roberts LR, Zhu AX, Murad MH and Marrero JA: AASLD
guidelines for treatment of hepatocellular carcinoma. Hepatology.
67:358–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Verslype C, Rosmorduc O and Rougier P;
ESMO Guidelines Working Group, : Hepatocellular carcinoma:
ESMO-ESDO clinical practice guideline for diagnosis, treatment and
follow-up. Ann Oncol. 23 (Suppl 7):vii41–vii48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kudo M, Matsui O, Izumi N, Iijima H,
Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, et
al: JSH consensus-based clinical practice guidelines for the
management of hepatocellular carcinoma: 2014 update by the liver
cancer study group of Japan. Liver Cancer. 3:458–468. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terashima T, Yamashita T, Horii R, Arai K,
Kawaguchi K, Kitamura K, Yamashita T, Sakai Y, Mizukoshi E, Honda M
and Kaneko S: Potential efficacy of therapies targeting
intrahepatic lesions after sorafenib treatment of patients with
hepatocellular carcinoma. BMC Cancer. 31:3382016. View Article : Google Scholar
|
|
25
|
Wada Y, Takami Y, Tateishi M, Ryu T,
Mikagi K and Saitsu H: The efficacy of continued sorafenib
treatment after radiologic confirmation of progressive disease in
patients with advanced hepatocellular carcinoma. PLoS One.
11:e01464562016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu SR, Zhang YQ, Hu BS, He X, Huang JW,
Zhan MX, Lu LG and Li JP: Sorafenib continuation after first
disease progression could reduce disease flares and provide
survival benefits in patients with hepatocellular carcinoma: A
pilot retrospective study. Asian Pac J Cancer Prev. 15:3151–3156.
2014. View Article : Google Scholar : PubMed/NCBI
|