Introduction
Epithelial ovarian cancer (EOC) can occur at any age
but is most commonly diagnosed in postmenopausal females, as the
peak incidence is between the ages of 50 and 60 years. Standard EOC
management involves primary surgery, including total abdominal
hysterectomy, bilateral salpingo-oophorectomy, pelvic/para-aortic
lymph node (LN) dissection, omentectomy and tumor debulking,
followed by taxane/platinum-based adjuvant chemotherapy. Recently,
women tend to give birth to their first child at an older age, and
the diagnosis of EOC before childbearing has become more frequent.
Because approximately 14% of all EOC cases occur in women aged
under 40 years, some patients may choose fertility preservation
(1). Fertility-sparing surgery
(FSS), with preservation of the contralateral ovary and uterus, for
women of reproductive age has been indicated for patients with
stage I EOC (2). It is estimated
that 7–8% of all stage I EOC patients are younger than 35 years of
age (3).
According to the recent National Comprehensive
Cancer Network (NCCN) guidelines, patients with unilateral stage
(IA or IC) EOC based on comprehensive surgical staging can undergo
FSS, regardless of grade (G) and histology (4). The present clinical guidelines of the
European Society of Medical Oncology (ESMO) state that young women
with stage IA or IC and favorable histological characteristics,
such as non-clear cell carcinoma (CCC) and G1 or G2 tumors, are
subject to FSS only following complete surgical staging that
includes lymphadenectomy (5).
However, regarding patients with high risk prognostic factors such
as G3/CCC or stage IC subtypes, the safety of FSS is controversial.
In the present study, we investigated the recurrence rates,
survival, reproductive and obstetrical outcomes for women with
stage I EOC treated with FSS.
Patients and methods
Study design
After obtaining the institutional review board
approval of each of the seven participating institutions belonging
to the Tohoku Gynecologic Cancer Unit (TGCU), the medical records
of 29 patients with stage I EOC who had undergone FSS between 2000
and 2010 were retrospectively analyzed.
Participants
Additional criteria included that all patients were
aged ≤40 years at the time of initial diagnosis, strongly desired
to retain their fertility, and were informed about the possible
risks and benefits of FSS. Exclusion criteria were non-epithelial
histological type tumors (germ cell tumors and sex-cord stromal
tumors), borderline malignant tumor, unclassified adenocarcinoma,
and advanced EOC (≥stage II). All patients underwent FSS, such as
unilateral oophorectomy or ovarian cystectomy with conservation of
the uterus and contralateral ovary. Pathological diagnosis for
histologic cell type, tumor differentiation and disease staging
were performed in each institution. Staging was determined
according to the International Federation of Gynecology and
Obstetrics (FIGO) classification (2014). Centralized pathological
review by a pathologist was performed at Fukushima Medical
University Hospital to confirm tumor histology type and histologic
differentiation. Following completion of primary treatment,
patients were examined every 1–3 months during the first 2 years,
every 3–6 months during the next 3 years and yearly thereafter.
Recurrences were diagnosed during regular follow-up visits and
confirmed on computed tomographic and/or magnetic resonance imaging
scans.
All patients had provided written informed consent
for surgery. The present study was approved by the Research Ethics
Committee of each of the seven participating institutions belonging
to the TGCU: Fukushima Medical University School of Medicine
(Fukushima, Japan), Tohoku University Graduate School of Medicine
(Sendai, Japan), Miyagi Cancer Center (Natori, Japan), Yamagata
University School of Medicine (Yamagata, Japan), Iwate Medical
University School of Medicine (Morioka, Japan), Akita University
School of Medicine (Akita, Japan) and Hirosaki University School of
Medicine (Hirosaki, Japan).
Statistical analysis
Overall survival (OS) and relapse-free survival
(RFS) were evaluated as clinical outcomes. Survival distributions
were calculated using the Kaplan-Meier method, and statistical
significance was determined using the log-rank test. Values of
P<0.05 were considered statistically significant. Statistical
analysis of data was performed using SPSS 25 (IBM Corp., Armonk,
NY, USA).
Results
Clinicopathological
characteristics
A total of 29 patients with stage I EOC who were
treated with FSS were enrolled in the present study. The
clinicopathological characteristics of all patients are shown in
Table I. The mean age was 27.2 years
(range, 12 to 39 years), and the numbers of patients with stage IA
and stage IC EOC were 14 and 15, respectively. The most frequent
histological type was mucinous (16 cases, 55.2%), followed by
serous (five, 17.2%) and endometrioid (five, 17.2%). Tumor
differentiation was 13 (44.8%), 10 (34.5%) and 6 (20.7%) for G1, G2
and G3/CCC, respectively. Adjuvant chemotherapy was given to 19
(65.5%) patients with stage IA (7/14, 50%) and stage IC (12/15,
80%) EOC (data not shown). Eighteen patients underwent
platinum-based chemotherapy, and one patient underwent oral
etoposide. After a median follow-up of 60.6 months (range, 6–135
months), the clinical outcomes were as follows: 26 patients (89.7%)
had no evidence of disease (NED); one (3.4%) patient was alive with
disease (AWD); and two patients (6.9%) were dead of disease (DOD).
Twenty-one patients did not experience a change in menstruation
status after treatment, and two patients had amenorrhea. After FSS,
five patients achieved seven pregnancies, resulting in the birth of
six healthy children. Among the five patients, four had received
platinum-based chemotherapy, and their babies showed no congenital
malformations. As for primary symptoms, abdominal symptoms
accounted for over 80% of all symptoms followed by atypical genital
bleeding (10.3%).
 | Table I.Clinicopathlogical characterstics of
patients treated with FSS. |
Table I.
Clinicopathlogical characterstics of
patients treated with FSS.
| Mean age, years
(range) | 27.2 (12–39) |
| Surgical procedures,
n (%) |
|
|
Cystectomy | 1 (3.5) |
| USO | 10 (34.5) |
| USO,
OMT | 3 (10.3) |
| USO,
OMT, LA | 15 (51.7) |
| Tumor histology, n
(%) |
|
|
Mucinous | 16 (55.2) |
|
Serous | 5 (17.2) |
|
Endometrioid | 5 (17.2) |
| Clear
cell | 3 (10.4) |
| Grade,
an |
|
| G1 | 13 |
| G2 | 10 |
| G3 | 3 |
| FIGO stage, n
(%) |
|
| IA | 14 (48.3) |
| G1 | 6 |
| G2 | 5 |
| G3 | 2 |
| Clear cell | 1 |
|
IC1 | 6 (20.7) |
| G1 | 3 |
| G2 | 3 |
| G3 | 0 |
| Clear cell | 0 |
|
IC3 | 9 (31.0) |
| G1 | 4 |
| G2 | 2 |
| G3 | 1 |
| Clear
cell | 2 |
| Adjuvant
chemotherapy, n (%) |
|
|
Yes | 19 (65.5) |
| No | 10 (34.5) |
| Recurrence, n
(%) |
|
| No | 24 (82.3) |
|
Yes | 5 (17.2) |
| Clinical outcome, n
(%) |
|
|
NED | 26 (89.7) |
|
AWD | 1 (3.4) |
|
DOD | 2 (6.9) |
| Menstruation,
n |
|
|
Regular | 21 |
|
Irregular | 1 |
|
Amenorrhea | 2 |
|
Unknown | 5 |
| Reproductive
outcome, bn |
|
|
Parity | 7 |
| Primary symptom,
cn (%) |
|
|
Birth | 6 |
|
Abdominal symptom | 24 (82.8) |
|
Atypical genital bleeding | 3 (10.3) |
| Medical
examination | 2 (6.9) |
|
Unknown | 1 (3.4) |
Clinical outcome of FSS in patients
with stage I EOC
Recurrence was identified during the follow-up
period in five patients (17.2%) whose clinical details are
described in Table II. The
recurrence sites were: the pelvis n=2; pelvic LN n=1;
cervical LN n=1; and pelvic LN and peritoneum n=1.
Median duration from primary treatment to recurrence was 43.8
months (range, 17–55 months). After all five patients with
recurrences were treated as outlined in Table II, two patients had NED, one patient
was AWD, and two patients were DOD at 23 and 71 months after
diagnosis. The estimated survival rate of all patients was 95.7% at
five years and 89.3% at ten years.
 | Table II.Clinical details of relapsed
patients. |
Table II.
Clinical details of relapsed
patients.
| Case | Age | Stage | Histology | Grade | Adjuvant
chemotherapy | Site of
recurrence | RFS (mo) | Salvage
recurrence | Status | OS (mo) |
|---|
| 1 | 33 | IC3 | Serous | 1 | Yes | Pelvis | 40 | Radical
surgery | NED | 119 |
| 2 | 36 | IC3 | Clear cell |
| Yes | Pelvic LN | 55 | Radical
surgery | NED | 107 |
| 3 | 27 | IC3 | Serous | 3 | Yes | Pelvis | 54 | Radical
surgery | AWD | 104 |
| 4 | 19 | IC3 | Mucinous | 2 | No | Cervical LN | 17 | CCRT | DOD | 23 |
| 5 | 21 | IA | Serous | 1 | Yes | Pelvic LN
Peritoneum | 53 | Chemotherapy | DOD | 71 |
We compared the OS and RFS for histological
differentiation and FIGO stage. All patients were classified into
two subgroups from the point of view of prognostic factors. The
first subgroup was G1/G2 vs. G3/CCC for histological
differentiation and the second subgroup was IA/IC1 vs. 1C3 for
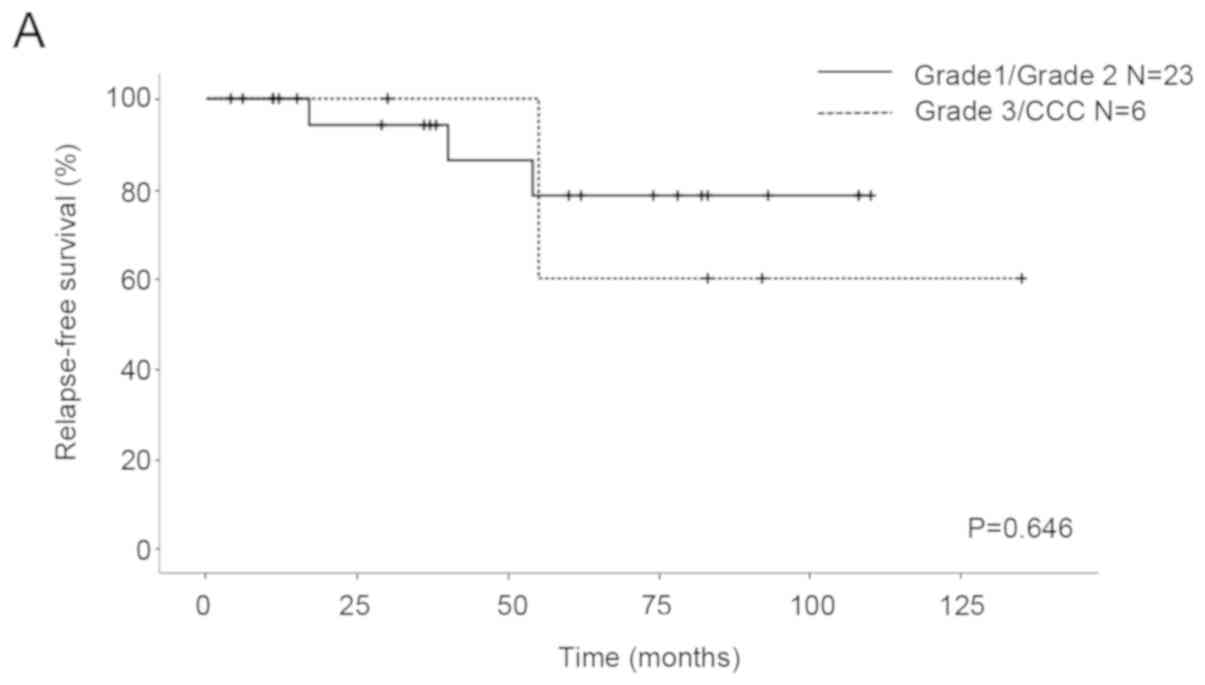
stage. As for the patients with G1/G2 and G3/CCC, the five-year RFS
rates were 78.4 and 60%, respectively, and the five-year OS rates
were 94.1 and 100%, respectively. No significant differences in RFS
and OS were seen between the patients (RFS P=0.646, OS P=0.356;
Fig. 1). With regard to the patients
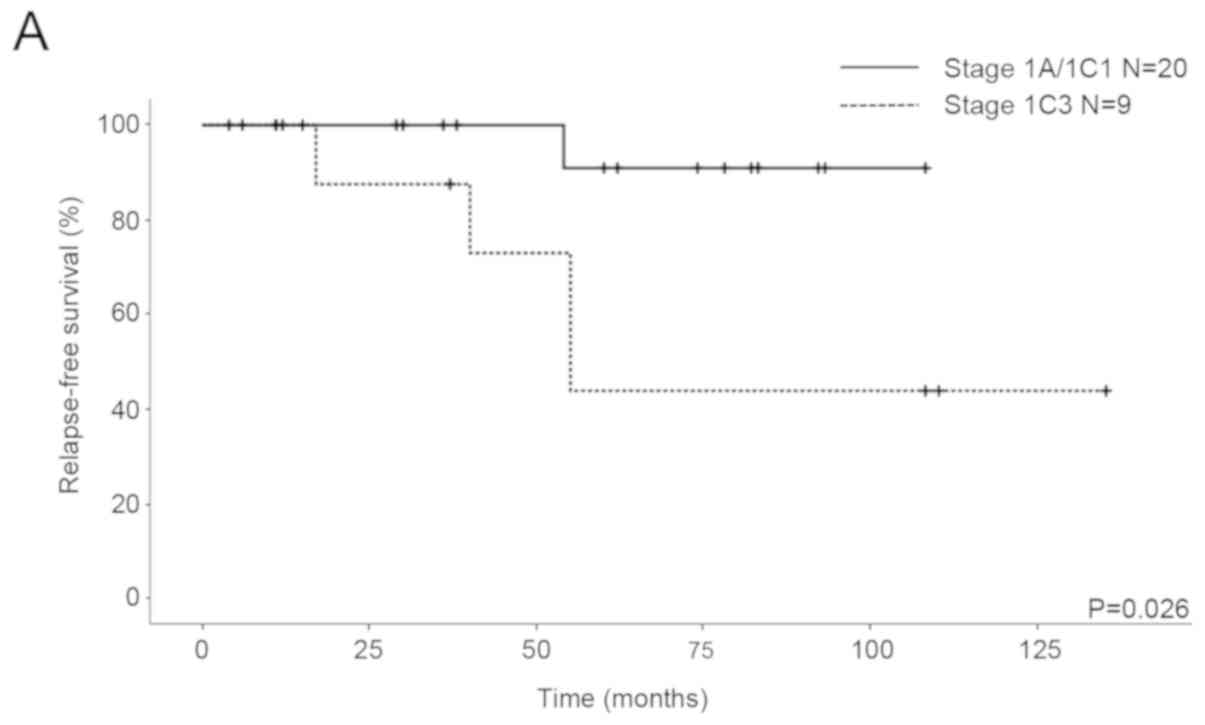
with stage IA/IC1 and 1C3 EOC, the five-year RFS rates were 90.9
and 43.8%, respectively, and the five-year OS rates were 100 and
87.5%, respectively. Significant differences in RFS were seen
between the patients (P=0.026; Fig.
2A), although there was no significant difference in OS
(P=0.712; Fig. 2B).
Discussion
The final goal of FSS is curable, safe and good
reproductive and obstetrical outcomes in cases of early EOC in
young women who desire childbearing. In our series, after a median
follow-up of 60.6 months, the recurrence rate among the 29 stage I
EOC patients who underwent FSS was 13% (5 of 29), which is similar
to the majority of trials focusing on early-stage EOC, treated with
either a radical approach or FSS, falling between the reported 4
and 15% (6). Schlaerth et al
(7), reported that the five-year
survival data for 123 patients who underwent surgical treatment for
stage I EOC for fertility-preservation revealed no significant
differences in five-year RFS (84% vs. 78%) or OS (84% vs. 82%) when
compared to standard surgery. In addition, a recent study reported
that FSS was not associated with increased risk of death in young
women with stage I EOC compared with conventional surgery (8). The present study revealed a five-year
RFS rate of 73.1% (data not shown) and an OS rate of 95.7%, which
are consistent with the results of previous reports (7,9).
There have been many studies that confirm the safety
of FSS for stage I EOC patients. Histologic tumor grade is one of
the most important risk factors of recurrence of EOC. In a recent
comprehensive literature review of 1,150 patients who underwent
FSS, 21 teams reported that there were 139 relapsed patients. The
recurrence rate in patients with stage IA/IC of G1/G2 tumors
(7–11%) was lower than that of patients with G3 tumors (23–29%)
(6). G3 tumor is an independent risk
factor and is associated with distant relapse and lower OS
(6). In addition, it has been
reported that although FSS can be safe and appropriate for patients
with G1/G2 tumors, it is not recommended for patients with G3
tumors (10).
CCC has a great risk of recurrence and poor
prognosis in comparison with other pathological types (serous,
endometrioid, and mucinous) of EOC because of a relative resistance
to first line platinum-based chemotherapy (11). According to the American College of
Obstetricians and Gynecologists (ACOG) and ESMO guidelines, CCC is
classified as an unfavorable histological type for FSS (5,12). In a
recent study, G3 and CCC were the only independent risk factors for
the survival of stage I EOC patients of reproductive age (13). It has been noted that the time to
give pregnancy permission after FSS should be considered carefully,
because the majority of reported recurrences of CCC occurred at
extraovarian locations within the first two years following initial
surgical staging (14). On the other
hand, the selection criteria for FSS could include patients with
stage IA CCC (15,16). There was no significant difference in
the RFS and OS between the EOC patients with G1/G2 and those with
G3/CCC in the current study (Fig.
1), and histological grading did not show prognostic factors.
The reason for this could be that the number of subjects in our
study was too small to evaluate the appropriateness of FSS for
patients with poor differentiation and unfavorable histology.
Therefore, FSS should be considered with caution in G3/CCC
patients.
Staging is also an important factor for prognosis of
EOC patients who undergo FSS. A previous study suggested that
patients with stage IA EOC have survival approaching 94%, whereas
it is approximately 84% for patients with IC EOC (17). FIGO staging represents a change of
stage IC if cyst rupture is noted preoperatively or
postoperatively. Although in patients with intraoperative rupture,
survival was not different from patients whose tumors had intact
capsules (85 and 78%, respectively), it was significantly different
from patients with preoperative rupture (59%) (18). Recently, the 2014 FIGO staging system
modified stage IC and subdivided it into three new clinically and
prognostically more relevant subgroups (19). Although no significant survival
differences in patients have been observed between stage IC1 and
stage IA, the survival of stage IC2/3 has been reported to be worse
than that of stage IA and IC1 (20,21). In
a systematic review, the recurrence rate of stage IC2/3 (23%) after
FSS was significantly higher than that of stage IC1 (12%) (14).
Although we were unable to investigate preoperative
rupture in the current study because patients with IC2 were not
included in the study population, cytology positive as stage IC3
indicated a high risk of recurrence in the patients who underwent
FSS (Fig. 2A). Probably due to a
small sample size, the recurrence rate in the IC3 and G3/CCC
patients was very high (67%, 2 of 3; Table II). However, there was no
significant difference in OS (Fig.
2B). Although the recurrence rate of stage IC3 (4/9) was more
than that of stage IA/IC1 (1/20), the mortality rates were similar
because three patients with recurrence in stage IC3 were curative.
Further studies with larger sample sizes are needed to identify the
differences in OS.
According to previous studies, recurrence in the
remaining ovary is likely to be successfully treated with surgery
and chemotherapy, and does not affect the long-term survival of FSS
patients (14,22,23).
However, patients who had relapse in LNs or PC, which is typical of
clear cell histology, had a poor prognosis (24). Compared with G1/G2 tumors, G3 tumors
give rise to a higher rate of extraovarian recurrences (6,14). In
the current study, the recurrence sites were the pelvis
(n=2), LN and peritoneum (n=1) and LN (n=2),
and there were no patients showing recurrence exclusively in the
residual ovary (Table II). The
extraovarian recurrence rate of the G1/G2 tumors (3/23, 13%) was
lower than that of the G3/CCC tumors (2/6, 33.3%), and this result
was similar to that of a previous report (G1; 9%, G2; 11.2%, G3;
23.5%, CCC; 15.3%) (25). The
extraovarian recurrence rate in patients with positive cytology
(4/9, 44.4%) also showed a higher proportion than that in patients
with negative cytology (1/20, 5%) in the present study.
The reproductive outcome was considered favorable in
our series. Most patients had regular menstruation after surgery
and chemotherapy. Four patients, who underwent chemotherapy, gave
birth to six infants without congenital anomalies. Recent studies
have described good reproductive outcomes after chemotherapy for
malignant tumors (10,13). The usefulness of a wedge biopsy of
the normal-appearing contralateral ovary in FSS is unknown. In
recent data, an incidence of microscopic metastasis in the opposite
normal looking ovaries was reported to be approximately 0–5%
(10,26). However, none of the patients who
underwent FSS had microscopic metastases in their macroscopically
normal ovaries detected by routine biopsies (27,28). In
another series, micrometastasis in an ovary with a grossly normal
appearance was very rare (3 of 118, 2.5%) (29). The survival of patients with an
isolated ovarian recurrence is better than that of patients with
other types of recurrence (22). In
the present study, among the five patients who had recurrence, none
experienced said recurrence on the contralateral ovary. As wedge
biopsy can cause mechanical infertility or ovarian failure, if the
opposite ovary is of normal size, shape, and position, surgical
evaluation should not be routinely performed.
There were some limitations in the present study.
The first and biggest limitation is the small sample size, compared
to those of other multicenter research studies. Survival evaluation
of detailed classifications, including histological tumor grade,
histology and staging, could not be performed. A larger sample size
would likely provide more definitive results. Secondly, our study
was not a randomized controlled trial, but a retrospective study,
and did not have sufficient power to provide a definitive
conclusion. Thirdly, although obstetric outcome is as important as
oncological safety for patients receiving FSS, we unfortunately
could not present data on reproductive outcome because some young
patients who were married could not be followed up on for adequate
periods and several unmarried patients were included in this study.
Fourthly, we were unable to obtain the total number and information
of all EOC patients in each institution. Therefore, we could not
compare FSS and radical surgery for stage I EOC. Finally,
lymphadenectomy was not performed for some patients because they
had often undergone surgery for a presumed benign tumor or
emergency. LN evaluation is recommended in the surgical treatment
of early stage EOC according to the FIGO criteria, in order to
perform accurate clinical staging and to select an adequate
adjuvant systemic chemotherapy.
In conclusion, our data confirmed that FSS could be
considered in the treatment of fertile women with stage IA and IC1
EOC and has a sufficient reproductive and obstetrical outcome.
However, we were unable to confirm the safety of FSS for patients
with stage IC3 EOC. Since the increased risk of stage I EOC in
patients with positive cytology is mainly related to a higher
incidence of extraovarian spread rather than to a higher relapse
rate in the preserved ovary, these patients should be carefully
informed about their prognosis when FSS is chosen. Our results are
consistent with those of previous studies and we believe that these
data can help treat early EOC in young women who wish to bear
children following treatment. In future, prospective controlled
studies should be conducted to confirm these findings.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Tohoku
Gynecologic Cancer Unit (Morioka, Japan).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request
Authors' contributions
TW drafted the manuscript and analyzed the data. SSo
analyzed the data. YK supervised the centralized pathological
review. HN, HT, SSh, NY, HY, TO, SN, TS, MK, TB, DS, NS, YT, MF, YY
and KF contributed to the study design and data collection. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of each of the seven participating institutions
belonging to the TGCU: Fukushima Medical University School of
Medicine (Fukushima, Japan), Tohoku University Graduate School of
Medicine (Sendai, Japan), Miyagi Cancer Center (Natori, Japan),
Yamagata University School of Medicine (Yamagata, Japan), Iwate
Medical University School of Medicine (Morioka, Japan), Akita
University School of Medicine (Akita, Japan) and Hirosaki
University School of Medicine (Hirosaki, Japan). All patients had
provided written informed consent for surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nasioudis D, Chapman-Davis E, Frey MK,
Witkin SS and Holcomb K: Could fertility-sparing surgery be
considered for women with early stage ovarian clear cell carcinoma?
J Gynecol Oncol. 28:e712017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yin J, Wang Y, Shan Y, Li Y, Jin Y and Pan
L: Pregnancy and oncologic outcomes of early stage low grade
epithelial ovarian cancer after fertility sparing surgery: A
retrospective study in one tertiary hospital of China. J Ovarian
Res. 12:442019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eftekhar M, Pourmasumi S and Karimi-Zarchi
M: Preservation of ovarian function during chemotherapy and
radiotherapy in young women with malignancies. Iran J Reprod Med.
12:377–382. 2014.PubMed/NCBI
|
|
4
|
Morgan RJ Jr, Armstrong DK, Alvarez RD,
Bakkum-Gamez JN, Behbakht K, Chen LM, Copeland L, Crispens MA,
DeRosa M, Dorigo O, et al: Ovarian cancer, version 1.2016, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
14:1134–1163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ledermann JA, Raja FA, Fotopoulou C,
Gonzalez-Martin A, Colombo N and Sessa C; ESMO Guidelines Working
Group, : Newly diagnosed and relapsed epithelial ovarian carcinoma:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24 (Suppl 6):vi24–vi32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fruscio R, Corso S, Ceppi L, Garavaglia D,
Garbi A, Floriani I, Franchi D, Cantù MG, Bonazzi CM, Milani R, et
al: Conservative management of early-stage epithelial ovarian
cancer: Results of a large retrospective series. Ann Oncol.
24:138–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schlaerth AC, Chi DS, Poynor EA, Barakat
RR and Brown CL: Long-term survival after fertility-sparing surgery
for epithelial ovarian cancer. Int J Gynecol Cancer. 19:1199–1204.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Melamed A, Rizzo AE, Nitecki R, Gockley
AA, Bregar AJ, Schorge JO, Del Carmen MG and Rauh-Hain JA:
All-cause mortality after fertility-sparing surgery for stage I
epithelial ovarian cancer. Obstet Gynecol. 130:71–79. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ditto A, Martinelli F, Lorusso D, Haeusler
E, Carcangiu M and Raspagliesi F: Fertility sparing surgery in
early stage epithelial ovarian cancer. J Gynecol Oncol. 25:320–327.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JY, Kim DY, Suh DS, Kim JH, Kim YM,
Kim YT and Nam JH: Outcomes of fertility-sparing surgery for
invasive epithelial ovarian cancer: Oncologic safety and
reproductive outcomes. Gynecol Oncol. 110:345–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugiyama T, Kamura T, Kigawa J, Terakawa
N, Kikuchi Y, Kita T, Suzuki M, Sato I and Taguchi K: Clinical
characteristics of clear cell carcinoma of the ovary: A distinct
histologic type with poor prognosis and resistance to
platinum-based chemotherapy. Cancer. 88:2584–2589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
American College of Obstetricians and
Gynecologists: ACOG practice bulletin. Management of adnexal
masses. Obstet Gynecol. 110:201–214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang X, Yang J, Yu M, Xie W, Cao D, Wu M,
Pan L, Huang H, You Y and Shen K: Oncofertility in patients with
stage I epithelial ovarian cancer: Fertility-sparing surgery in
young women of reproductive age. World J Surg Oncol. 15:1542017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bentivegna E, Gouy S, Maulard A, Pautier
P, Leary A, Colombo N and Morice P: Fertility-sparing surgery in
epithelial ovarian cancer: A systematic review of oncological
issues. Ann Oncol. 27:1994–2004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satoh T, Hatae M, Watanabe Y, Yaegashi N,
Ishiko O, Kodama S, Yamaguchi S, Ochiai K, Takano M, Yokota H, et
al: Outcomes of fertility-sparing surgery for stage I epithelial
ovarian cancer: A proposal for patient selection. J Clin Oncol.
28:1727–1732. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kajiyama H, Shibata K, Mizuno M, Hosono S,
Kawai M, Nagasaka T and Kikkawa F: Fertility-sparing surgery in
patients with clear-cell carcinoma of the ovary: Is it possible?
Hum Reprod. 26:3297–3302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmed FY, Wiltshaw E, A'Hern RP, Nicol B,
Shepherd J, Blake P, Fisher C and Gore ME: Natural history and
prognosis of untreated stage I epithelial ovarian carcinoma. J Clin
Oncol. 14:2968–2975. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sjövall K, Nilsson B and Einhorn N:
Different types of rupture of the tumor capsule and the impact on
survival in early ovarian carcinoma. Int J Gynecol Cancer.
4:333–336. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prat J; FIGO Committee on Gynecologic
Oncology, : Staging classification for cancer of the ovary,
fallopian tube, and peritoneum. Int J Gynaecol Obstet. 124:1–5.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosendahl M, Høgdall CK and Mosgaard BJ:
Restaging and survival analysis of 4036 ovarian cancer patients
according to the 2013 FIGO classification for ovarian, fallopian
tube, and primary peritoneal cancer. Int J Gynecol Cancer.
26:680–687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suh DH, Kim TH, Kim JW, Kim SY, Kim HS,
Lee TS, Chung HH, Kim YB, Park NH and Song YS: Improvements to the
FIGO staging for ovarian cancer: Reconsideration of lymphatic
spread and intraoperative tumor rupture. J Gynecol Oncol.
24:352–358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zapardiel I, Diestro MD and Aletti G:
Conservative treatment of early stage ovarian cancer: Oncological
and fertility outcomes. Eur J Surg Oncol. 40:387–393. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marpeau O, Schilder J, Zafrani Y, Uzan C,
Gouy S, Lhommé C and Morice P: Prognosis of patients who relapse
after fertility-sparing surgery in epithelial ovarian cancer. Ann
Surg Oncol. 15:478–483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Groen RS, Gershenson DM and Fader AN:
Updates and emerging therapies for rare epithelial ovarian cancers:
One size no longer fits all. Gynecol Oncol. 136:373–383. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bentivegna E, Fruscio R, Roussin S, Ceppi
L, Satoh T, Kajiyama H, Uzan C, Colombo N, Gouy S and Morice P:
Long-term follow-up of patients with an isolated ovarian recurrence
after conservative treatment of epithelial ovarian cancer: Review
of the results of an international multicenter study comprising 545
patients. Fertil Steril. 104:1319–1324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morice P, Wicart-Poque F, Rey A, El-Hassan
J, Pautier P, Lhommé C, de Crevosier R, Haie-Meder C, Duvillard P
and Castaigne D: Results of conservative treatment in epithelial
ovarian carcinoma. Cancer. 92:2412–2418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morice P, Camatte S, El Hassan J, Pautier
P, Duvillard P and Castaigne D: Clinical outcomes and fertility
after conservative treatment of ovarian borderline tumors. Fertil
Steril. 75:92–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zanetta G, Chiari S, Rota S, Bratina G,
Maneo A, Torri V and Mangioni C: Conservative surgery for stage I
ovarian carcinoma in women of childbearing age. Br J Obstet
Gynaecol. 104:1030–1035. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Benjamin I, Morgan MA and Rubin SC: Occult
bilateral involvement in stage I epithelial ovarian cancer. Gynecol
Oncol. 72:288–291. 1999. View Article : Google Scholar : PubMed/NCBI
|