Introduction
Colorectal carcinoma (CRC) is a common malignancy in
both sexes and a freFquent cause of cancer-related death.
Approximately 20% of patients with newly diagnosed CRC will have
synchronous metastatic disease (1),
and 15% will have resectable liver metastasis (2-4). Resection of
metastases limited to the liver coupled with resection of the
primary tumor is associated with significant improvement in
survival (5,6).
Chemotherapy after the hepatectomy may improve the
survival of patients with resectable CRC liver metastasis (7). However, the optimal duration, timing,
and regimen for this improvement have not been established.
Furthermore, preoperative chemotherapy bridging to surgical
intervention in primary unresectable CRC liver metastasis has been
shown to be beneficial (8,9). The response to preoperative
chemotherapy is useful to predict the prognosis in initially
unresectable and resectable disease (10). However, the prognostic benefit of
chemotherapy before hepatectomy in patients with CRC and resectable
or marginally resectable liver metastases remains unclear. It has
been reported that the prognosis of patients with synchronous and
metachronous CRC liver metastasis is different (11,12).
Therefore, we focused on CRC with synchronous liver metastasis and
investigated whether preoperative chemotherapy improved the
surgical curability in this patient population by assessing
survival time and recurrence.
Patients and methods
Patients
This retrospective study involved 106 patients
treated at three Japanese hospitals. We investigated patients with
CRC and resectable or marginally resectable synchronous metastases
treated at Hokkaido University Hospital, Hokkaido Cancer Center,
and Hokkaido Hospital, Japan Community Health Care Organization
(all in Hokaido, Japan) between April 2006 and August 2017.
Patients were excluded if they had any other distant metastases at
the first treatment (surgery or prior chemotherapy). Simultaneous
or metachronous resection of the primary lesion and liver
metastasis; surgical approach (open or laparoscopic); and the
duration, timing, and regimen of chemotherapy (including adjuvant
therapy) were decided at the discretion of the attending surgeon
and medical oncologist in each case. We retrospectively collected
and analyzed information about all eligible patients from their
medical charts.
Overall survival (OS) was defined as the interval
between the day of the first treatment and the day of death from
any cause or the last follow-up. We compared the OS of 64 patients
who received neoadjuvant chemotherapy (NAC) with that of 42
patients who did not. To assess the prognostic course after
surgery, we also compared OS, relapse-free survival (RFS), and
survival after recurrence (SAR) between 43 patients who responded
to preoperative chemotherapy (responders) and 21 who did not
(non-responders).
Liver metastases were classified into three
subgroups, H1, H2, and H3, according to their extent. H1 comprised
patients with fewer than three liver metastases with a maximum
diameter <5 cm, whereas H3 comprised those with more than four
metastases with a maximum diameter >5 cm. The H2 subgroup
comprised patients who were excluded from subgroups H1 and
H3(13). Clinical responses to the
preoperative chemotherapy were evaluated on the basis of the
revised Response Evaluation Criteria in Solid Tumors (RECIST)
guidelines, version 1.1(14).
The Ethics Committees of Hokkaido University
Hospital and all participating hospitals approved this study as an
exempt human subject research (no. 017-0399) and informed consent
was obtained from all patients by the opt-out method, in accordance
with the guidelines of the Japanese Ministry of Health, Labor and
Welfare (Tokyo, Japan).
Statistical analysis
Summary statistics for continuous data are described
as mean and 95% confidence intervals (CI). All statistical tests
were performed with an α level of 0.05 (two-sided). The Chi-square
test and Student's t-test were performed for categorical and
continuous data, respectively. Survival curves and median follow-up
times were estimated by the Kaplan-Meier method, and the survival
curves of each group were compared using log-rank tests. Survival
was compared in the NAC group and the non-NAC group, and then
prognostic comparison between responders and non-responders in the
NAC group was conducted. In the latter comparison, the survival
curves of the non-NAC group were used for reference. To confirm the
consistency of the primary results, Cox's proportional hazards
regression was also performed to estimate the hazard ratios (HRs)
while adjusting for baseline covariants. Multivariate regression
analysis was conducted with selected values according to the
pretreatment condition (primary site, primary tumor
differentiation, age, sex, primary tumor depth, lymph node
metastasis, number and size of liver metastasis, and lymph and
venous vessel invasion in the primary lesion) and surgical strategy
(simultaneous resection and preoperative chemotherapy). All
statistical analyses were performed using JMP Pro, version 13.0.0
(SAS Institute, Inc.).
Results
Patient characteristics
Patient characteristics are shown in Table I. There was no difference in the
site, histological type, tumor depth, and lymph node metastasis of
the primary lesion between patients in the NAC group and those in
the non-NAC group. There were more patients with H1 liver
metastases in the non-NAC group (74%) than in the NAC group (34%).
In the NAC group, preoperative chemotherapy was administered for
5.7 months, during which 50 patients (78%) received a single
regimen and 54 patients (84%) received oxaliplatin. After
evaluating the clinical response to chemotherapy in the NAC group,
complete response, partial response, stable disease, and
progressive disease were observed in 1 (2%), 42 (67%), 7 (11%), and
14 (21%) patients, respectively. Simultaneous resection of the
primary lesion and liver metastasis was performed in 4 patients
(6%) in the NAC group and 21 (50%) patients in the non-NAC group
(P=0.0001). There were no differences between the two groups
in terms of the type of procedure, histological margins, and
administration of adjuvant chemotherapy. The duration of adjuvant
chemotherapy was longer in the NAC group than in the non-NAC group
(P=0.02), and irinotecan and bevacizumab were more commonly
used in the NAC group (P=0.002).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variables | Non-NAC (n=42) | NAC (n=64) | P-value |
|---|
| Age (years) | 64.0 (61.1-66.9) | 61.2 (58.9-63.5) | 0.14 |
| Sex | | | 0.63 |
|
Male | 19 (45%) | 26 (41%) | |
|
Female | 23 (55%) | 38 (59%) | |
| Tumor location
(primary tumor) | | | 0.74 |
|
Right | 11 (26%) | 15 (23%) | |
|
Left | 31 (74%) | 49 (77%) | |
|
Histologya | | | 0.72 |
|
Differentiated
adenocarcinoma | 6 (14%) | 9 (14%) | |
|
Other | 35 (83%) | 53 (84%) | |
| Tumor invasion to
serosa or adjacent structures (primary tumor)a | | | 0.86 |
|
No | 34 (81%) | 46 (72%) | |
|
Yes | 8 (19%) | 17 (27%) | |
| Lymph node
metastasisa | | | 0.61 |
|
Negative | 4 (10%) | 8 (13%) | |
|
Positive | 34 (81%) | 49 (77%) | |
| Liver metastasis
(pretreatment) | | | 0.0003 |
|
H1 | 9 (21%) | 22 (34%) | |
|
H2 | 31 (74%) | 30 (47%) | |
|
H3 | 2 (5%) | 12 (19%) | |
| Liver metastasis
(solitary and <5 cm) | | | 0.01 |
|
Yes | 12 (29%) | 6 (9%) | |
|
No | 30 (71%) | 58 (91%) | |
| Lymph vessel
invasion (primary tumor)a | | | 0.42 |
|
Negative | 12 (29%) | 23 (36%) | |
|
Positive | 28 (67%) | 38 (59%) | |
| Venous invasion
(primary tumor)a | | | 0.71 |
|
Negative | 7 (17%) | 9 (14%) | |
|
Positive | 33 (79%) | 52 (81%) | |
| Primary
resection | | | 0.0001 |
|
Simultaneous | 21 (50%) | 4 (6%) | |
|
Metachronous | 21 (50%) | 60 (94%) | |
| Clinical
responseb | | | - |
|
CR | - | 1 (2%) | |
|
PR | - | 42 (66%) | |
|
SD | - | 7 (11%) | |
|
PD | - | 14 (22%) | |
| Procedure | | | 0.84 |
|
Partial | 25 (60%) | 35 (55%) | |
|
Segmentectomy | 9 (21%) | 14 (22%) | |
|
Lobectomy | 8 (19%) | 15 (23%) | |
| With ablation | | | 0.34 |
|
Yes | 3 (7%) | 2 (3%) | |
|
No | 39 (93%) | 62 (97%) | |
| Histological
margina (liver
specimens) | | | 0.56 |
|
Negative | 33 (79%) | 46 (72%) | |
|
Positive | 7 (17%) | 16 (25%) | |
| Adjuvant
chemotherapy | | | 0.13 |
|
Yes | 25 (60%) | 47 (73%) | |
|
No | 17 (40%) | 17 (27%) | |
| Waiting period
(months) | 0.9 (-2.7-4.6) | 3.02
(0.21-5.84) | 0.81 |
| Duration
(months) | 7.54
(5.89-9.19) | 5.11
(3.93-6.29) | 0.02 |
| Oxaliplatin | | | 0.50 |
|
Yes | 13 (52%) | 20 (43%) | |
|
No | 12 (48%) | 27 (57%) | |
| 5-FU only | | | 0.19 |
|
Yes | 13 (52%) | 17 (36%) | |
|
No | 12 (48%) | 30 (64%) | |
| Irinotecan | | | 0.002 |
|
Yes | 0 | | 15 (32%) |
|
No | 25 (100%) | 32 (68%) | |
| Bevacizumab | | | 0.04 |
|
Yes | 1 (4%) | 11 (23%) | |
|
No | 24 (96%) | 36 (77%) | |
| Re-hepatectomy
after recurrencea | | | 0.73 |
|
Yes | 11 (44%) | 19 (43%) | |
|
No | 16 (64%) | 25 (57%) | |
Overall survival
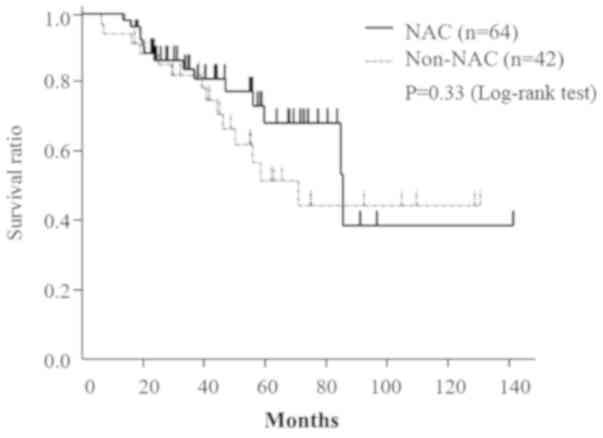
The median follow-up period was 41 months (2.5-141.2
months). There were 1 and 2 patients lost to follow-up (because
they moved away) in the NAC and non-NAC groups, respectively. The
median survival and 5-year OS rates in the NAC and non-NAC groups
were 86.0 and 71.6 months and 75.1 and 54.3%, respectively
(P=0.33; Fig. 1).
Risk factors for poor prognosis
Univariate regression analysis identified lymph
vessel invasion in the primary lesion [P=0.002; odds ratio
(OR), 4.62; 95% CI, 1.61-19.4] and female sex (P=0.03; OR,
2.25; 95% CI, 1.06-4.91) as positively associated with poor
prognosis, while NAC status was not associated with poor prognosis
(P=0.79; OR, 0.93; 95% CI, 0.56-1.49).
Multivariate regression analysis showed that
undiffe-rentiated adenocarcinoma (P=0.02; OR, 3.84; 95% CI,
1.16-12.1), a primary tumor invasion to the serosa or adjacent
structures (P=0.01; OR, 4.99; 95% CI, 1.39-19.9), and
simultaneous resection (P=0.009; OR, 5.39; 95% CI,
1.50-20.3) were associated with a poor prognosis. Not receiving
preoperative chemotherapy was not associated with a poor prognosis
(P=0.28; Table II).
 | Table IIRisk factors for shorter
survival. |
Table II
Risk factors for shorter
survival.
| | Multivariate
analysis |
|---|
| Risk factor | HR | 95% CI | P-value |
|---|
| Primary site | | | 0.84 |
|
Left | 1.00 | - | |
|
Right | 0.89 | 0.29-2.54 | |
| Differentiated
adenocarcinoma | | | 0.02 |
|
Yes | 1.00 | - | |
|
No | 3.84 | 1.16-12.1 | |
| Age (years) | | | 0.28 |
|
<50 | 1.00 | - | |
|
≥50 | 2.30 | 0.54-16.3 | |
| Sex | | | 0.63 |
|
Male | 1.00 | - | |
|
Female | 1.29 | 0.44-3.82 | |
| Tumor invasion to
serosa or adjacent structures (primary tumor) | | | 0.01 |
|
No | 1.00 | - | |
|
Yes | 4.99 | 1.39-19.9 | |
|
Lymph node
metastasis | | | 0.96 |
|
Negative | 1.00 | - | |
|
Positive | 1.03 | 0.27-5.11 | |
| Liver
metastasis | | | 0.07 |
|
H1 | 1.00 | - | |
|
H2 | 1.02 | 0.32-3.25 | |
|
H3 | 3.76 | 0.89-16.1 | |
| Liver metastasis
(solitary and <5 cm) | | | 0.20 |
|
Yes | 1.00 | - | |
|
No | 2.82 | 0.58-21.0 | |
| Lymph vessel
invasion (primary tumor) | | | 0.07 |
|
Negative | 1.00 | - | |
|
Positive | 3.11 | 0.89-14.8 | |
| Venous invasion
(primary tumor) | | | 0.75 |
|
Negative | 1.00 | - | |
|
Positive | 1.25 | 0.33-6.28 | |
| Simultaneous
resection | | | 0.009 |
|
No | 1.00 | - | |
|
Yes | 5.39 | 1.50-20.3 | |
| NAC | | | 0.28 |
|
No | 1.00 | - | |
|
Yes | 0.57 | 0.21-1.59 | |
Analysis of OS, RFS, and SAR according
to H subgroups
Since there were differences between the NAC and
non-NAC groups with respect to liver metastasis (subgroup H), we
stratified the patients into two groups, H1 and H2/H3, and analyzed
the OS, RFS, and SAR in each. There were no survival differences
between the NAC and non-NAC groups between the H1 (P=0.53)
and H2/H3 patients (P=0.16). Moreover, RFS and SAR in the
NAC and non-NAC groups were not significantly different between the
H1 (P=0.36, 0.45) and H2/H3 patients (P=0.26, 0.24;
Fig. 2).
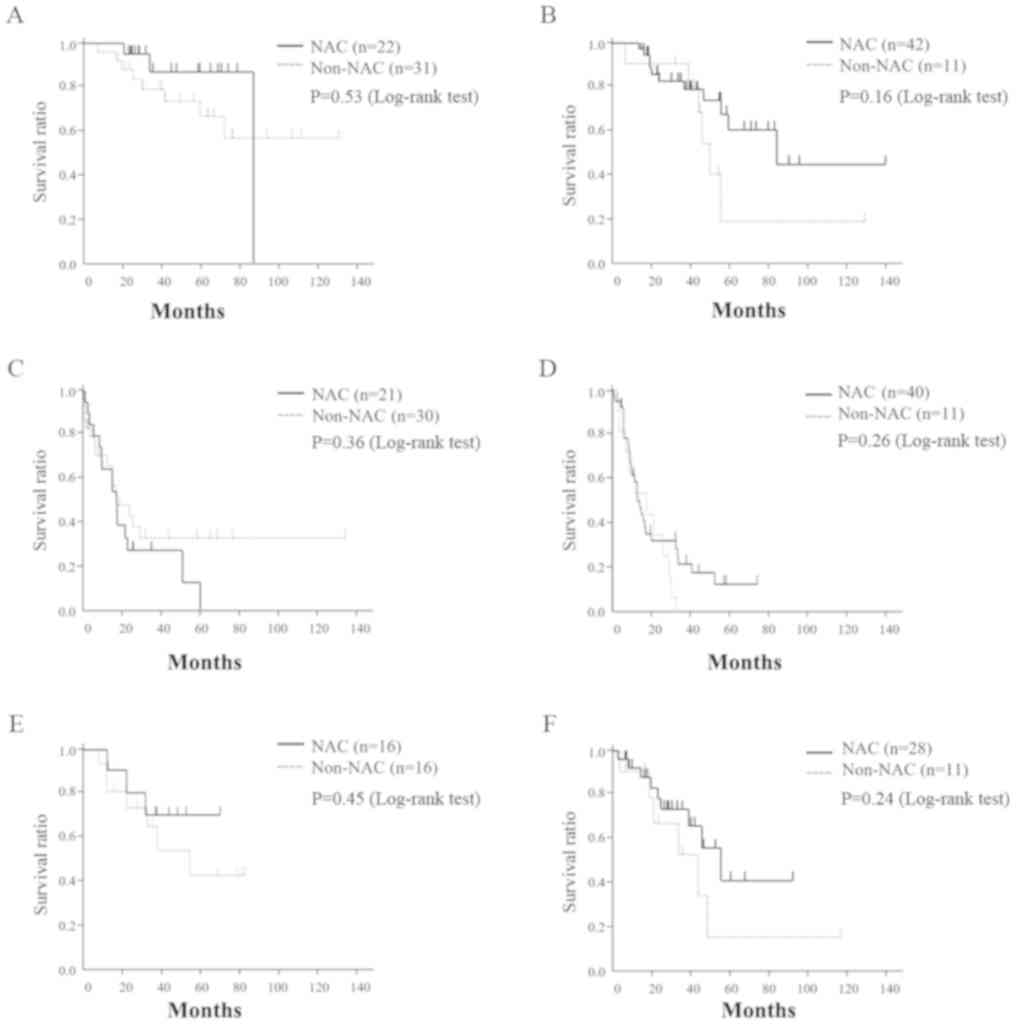 | Figure 2.Association between preoperative
chemotherapy status and overall, relapse-free and after recurrence
survival (subgroup analysis). (A) OS in H1; (B) OS in H2, 3; (C)
RFS in H1; (D) RFS in H2, 3; (E) SAR in H1; (F) SAR in H2 and H3.
NAC, neoadjuvant chemotherapy; RFS, relapse-free survival; SAR,
survival after recurrence; OS, overall survival; H1, patients with
fewer than 3 liver metastases with a maximum diameter <5 cm; H3,
patients with more than 4 metastases with a maximum diameter >5
cm; H2, patients excluded from subgroups H1 and H3. |
Prognosis of the patients who
responded to preoperative chemotherapy
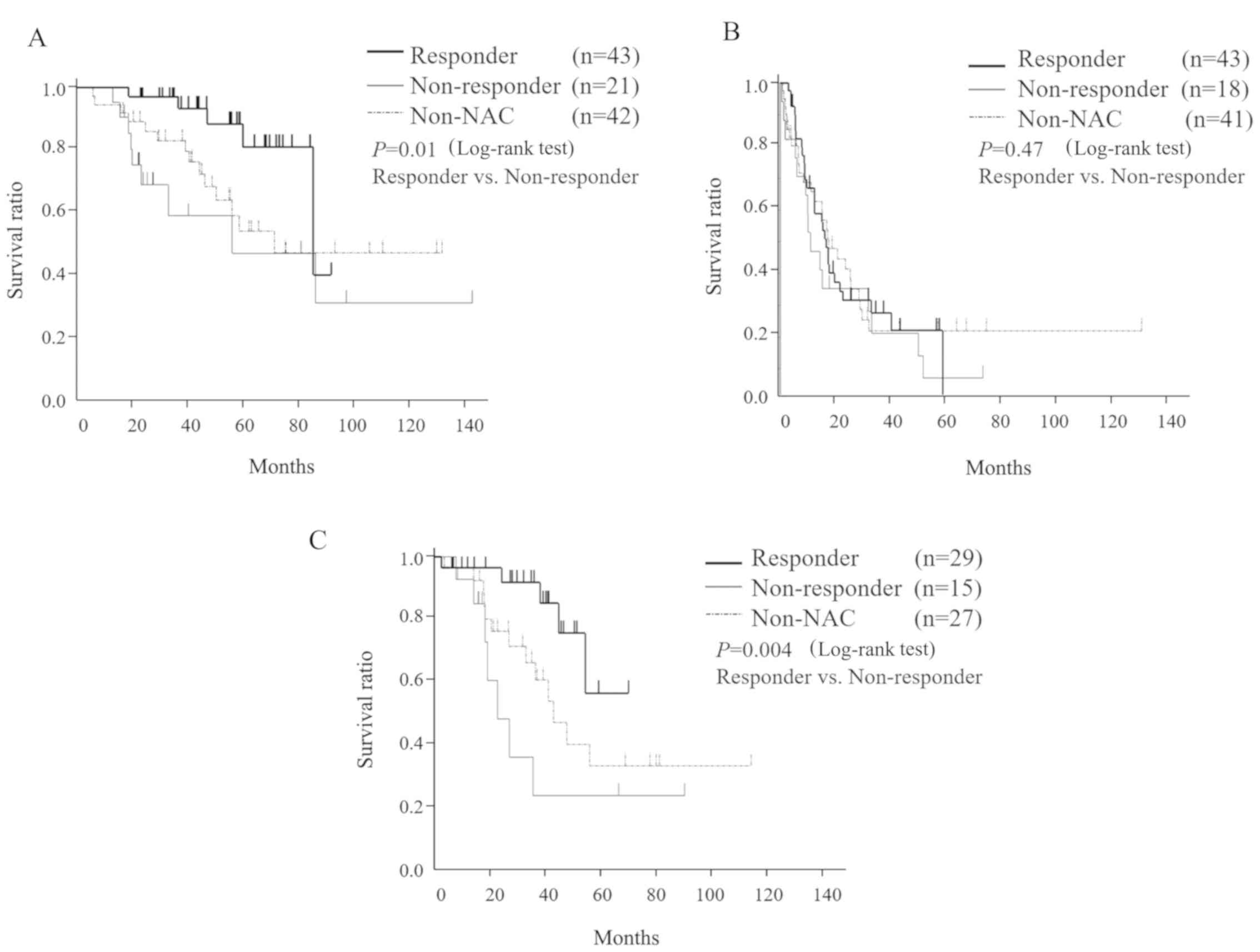
To assess the prognosis of responders (n=43) and
non-responders (n=21), we compared their OS, RFS, and SAR.
Anti-epidermal growth factor receptor (EGFR) therapy was more
commonly administered to the responders (25 patients, 58%) than the
non-responders (10 patients, 47%). There was no difference in the
characteristics of the primary lesion, type and timing of
procedure, histological margins, and adjuvant chemotherapy between
the two groups (Table III). The
median OS was significantly longer in responders (85 months) than
in non-responders (56 months). However, the median RFS in
responders and non-responders was not significantly different at
16.4 and 10.7 months, respectively. In addition, the median SAR was
significantly longer in responders (over 70 months) than in
non-responders (24 months). OS, RFS, and SAR in the non-NAC group
were 71.6, 17.2, and 44 months, respectively (Fig. 3). There was no difference in the
characteristics of the first recurrence site and surgical
intervention or chemotherapy administered after recurrence
(Table III).
 | Table IIIDifferences in patient
characteristics between responders and non-responders to
chemotherapy. |
Table III
Differences in patient
characteristics between responders and non-responders to
chemotherapy.
| Patient
characteristic | Non-responders
(n=21) | Responders
(n=43) | P-value |
|---|
| Age (years) | 62.0
(58.3-65.7) | 60.8
(58.3-63.4) | 0.60 |
| Sex | | | 0.18 |
|
Male | 11 (52%) | 15 (35%) | |
|
Female | 10 (48%) | 28 (65%) | |
| Tumor location | | | 0.05 |
|
Right | 8 (38%) | 7 (16%) | |
|
Left | 13 (62%) | 36 (84%) | |
|
Histologya | | | 0.25 |
|
Differentiated | 5 (24%) | 4 (9%) | |
|
Other | 16 (76%) | 37 (86%) | |
| Tumor invasion to
serosa or adjacent structures (primary tumor)a | | | 0.12 |
|
No | 11 (52%) | 34 (79%) | |
|
Yes | 10 (48%) | 7 (16%) | |
| Lymph node
metastasisa | | | 0.90 |
|
Negative | 3 (14%) | 5 (12%) | |
|
Positive | 17 (81%) | 32 (74%) | |
| Liver metastasis
(pretreatment) | | | 0.22 |
|
H1 | 8 (38%) | 14 (33%) | |
|
H2 | 7 (33%) | 23 (53%) | |
|
H3 | 6 (29%) | 6 (14%) | |
| Lymph vessel
invasion (primary tumor)a | | | 0.47 |
|
Negative | 6 (29%) | 17 (40%) | |
|
Positive | 14 (67%) | 24 (56%) | |
| Venous invasion
(primary tumor)a | | | 0.53 |
|
Negative | 2 (10%) | 7 (16%) | |
|
Positive | 18 (86%) | 34 (79%) | |
| Primary
resection | | | 0.44 |
|
Simultaneous | 19 (90%) | 41 (95%) | |
|
Metachronous | 2 (10%) | 2 (5%) | |
|
Neoadjuvant
period (months) | 5.35
(3.44-7.25) | 5.03
(3.97-6.10) | 0.77 |
| Regimen number | | | 0.58 |
|
1 | 14 (67%) | 36 (84%) | |
|
2 | 6 (29%) | 4 (9%) | |
|
3 | 1 (5%) | 3 (7%) | |
| Oxaliplatin | | | 0.83 |
|
Yes | 18 (86%) | 36 (84%) | |
|
No | 3 (14%) | 7 (16%) | |
| 5-FU only | | | 0.97 |
|
Yes | 2 (10%) | 4 (9%) | |
|
No | 19 (90%) | 39 (91%) | |
| Irinotecan | | | 0.40 |
|
Yes | 8 (38%) | 12 (28%) | |
|
No | 13 (62%) | 31 (72%) | |
| Bevacizumab | | | 0.42 |
|
Yes | 10 (48%) | 25 (58%) | |
|
No | 11 (52%) | 18 (42%) | |
|
Cetuximab/Panitumumab | | | 0.02 |
|
Yes | 3 (14%) | 18 (42%) | |
|
No | 18 (86%) | 25 (58%) | |
| New lesion after
NAC (extra hepatic) | 3 (14%) | 0 | 0.01 |
| Procedure | | | 0.44 |
|
Partial
resection | 9 (43%) | 26 (60%) | |
|
Segmentectomy | 5 (24%) | 9 (21%) | |
|
Lobectomy | 7 (33%) | 8 (19%) | |
| With ablation | | | 0.59 |
|
Yes | 1 (5%) | 1 (2%) | |
|
No | 20 (95%) | 42 (98%) | |
| Histological
margina (liver
specimens) | | | 0.05 |
|
Negative | 11 (52%) | 35 (81%) | |
|
Positive | 9 (43%) | 7 (16%) | |
| Hepatic
recurrence | | | 0.94 |
|
Yes | 11 (73%) | 21 (72%) | |
|
No | 4 (27%) | 8 (28%) | |
| Pulmonary
recurrence | | | 0.69 |
|
Yes | 5 (33%) | 8 (28%) | |
|
No | 10 (67%) | 21 (72%) | |
| Peritoneal
recurrence | | | 0.46 |
|
Yes | 0 | 1 (3%) | |
|
No | 15 (100%) | 28 (97%) | |
| Re-hepatectomy
after recurrence | | | 0.34 |
|
Yes | 5 (33%) | 14 (48%) | |
|
No | 10 (67%) | 15 (52%) | |
| Pulmonary resection
after recurrence | | | 0.76 |
|
Yes | 2 (13%) | 3 (10%) | |
|
No | 13 (87%) | 26 (90%) | |
Discussion
We showed that preoperative chemotherapy in patients
with CRC and synchronous liver metastasis did not prolong survival.
Moreover, on the subgroup analysis of patients based on their
response to chemotherapy, we notably demonstrated that even in
responders it did not prolong RFS although it did prolong OS and
SAR. This means that preoperative chemotherapy does not improve the
curative potential of surgery but does improve the post-recurrence
prognosis for responders. In other words, the response to the
preoperative chemotherapy merely predicted the response to the
chemotherapy after recurrence. Empirically, preoperative
chemotherapy is often administered to allow an observational period
to determine whether new lesions will appear soon after the first
treatment. However, on the basis of the findings of the current
study, this approach is not logical because there were no
differences in the time to relapse after the hepatectomy whether
chemotherapy was administered before the hepatectomy or not.
Therefore, surgery should be recommended for patients with CRC and
synchronous liver metastasis when the attending surgeon determines
the tumor to be resectable. Additionally, when we compared the poor
prognostic factors using a Cox regression analysis in the NAC or
non-NAC groups independently, the hepatic factor after neoadjuvant
chemotherapy along with the T factor in the primary site were
independent prognostic factors in the NAC group (Table SI), whereas being female, the
absence of adjuvant chemotherapy, and the lymph vessel invasions in
the primary site were independent factors in the non-NAC group
(Table SII). It is significant that
the H factor after neoadjuvant therapy was one of the independent
prognostic factors in the NAC group because it reflected the
response to chemotherapy. In fact, 21 and 4% of the cases had down-
and up-stages of the H factor, respectively. In that mean, in more
cases, NAC improved the resectability. In marginal cases,
resectability differs depending on the attending surgeon (15), and NAC may improve the opportunity
for safe resections. However, in the present study, NAC did not
improve recurrent free survival even in the responders, although it
improved the survival time by prolonging survival after recurrence.
Therefore, waiting periods in considering recurrence after
resection might not be needed in easily resectable cases. It is not
beneficial to introduce watching and neoadjuvant chemotherapy in
cases in which the hepatic metastasis can be safely resected.
Therefore, we suggested that NAC should not be routinely offered to
patients with resectable liver metastasis before hepatectomy.
The present's study findings are in accordance with
those of the European Organisation for Research and Treatment of
Cancer Intergroup trial 40983, a large, European randomized
controlled trial in which preoperative folinic
acid-fluorouracil-oxaliplatin (FOLFOX) therapy was not found to be
beneficial (16,17). However, it is possible that some
biases may have detracted from the prognostic analyses in the
present study. The patients in the NAC group had more advanced
hepatic metastatic stages before the treatment, and there were more
solitary tumors ≤5 cm in the patients in the non-NAC group. It has
been reported that such patients who undergo metastasectomy have a
better prognosis without the need for chemotherapy (18). More patients in the non-NAC group
underwent simultaneous resection of the primary and metastatic
tumors, possibly because of easier resectability. Nevertheless,
previous studies have shown no difference in recurrence and
survival between patients undergoing simultaneous and metachronous
liver resection (19,20). In an attempt to adjust for these
biases, we assessed the risk factors for a poor prognosis.
Multivariate regression analysis of the pretreatment condition and
surgical strategy also revealed that preoperative chemotherapy did
not prolong OS. Moreover, there were no prognostic differences
between the H1 and H2/H3 subgroups of the NAC and non-NAC groups.
Unfortunately, we lacked the efficient appropriate data about on
the RAS and B-RAF mutations. However, regarding the K-RAS exon 2,
we had the data on 17 cases in the non-NAC group and 55 cases in
the NAC group. In these 72 cases, the overall survival between the
K-RAS wild-type genes and mutations showed no differences (Fig. S1). The Cox regression also showed
that both the NAC and K-RAS mutations were not prognostic factors
regarding the overall survival (Table
SIII).
Regarding the association between the clinical
response and PFS, Yoshita et al reported a positive
correlation between the clinical response to preoperative
chemotherapy and prolonged PFS (21), in contrast to our report, using
RECIST and computed tomography morphologic criteria (22,23). In
their report, the median PFS was 4.6 months longer in responders
than in non-responders. However, almost all patients in both groups
ultimately developed recurrence within 30 months (21). Thus, preoperative chemotherapy
delayed recurrence slightly in responders but did not improve the
recurrence rate. Therefore, we still recommend surgery as the
initial treatment in patients with CRC and resectable liver
metastasis. We additionally analyzed the prognostic impact of
adjuvant and neoadjuvant usage of bevacizumab and
cetuximab/panitumumab, respectively. None of the neoadjuvant or
adjuvant usages of anti-tumoral medicines influenced PFS. Instead,
T, N, and lymph vessel invasion in the primary site along with
historically positive margins had poor prognosis in terms of
recurrence (Table SIV). However,
the number cases of each usage of the agent was limited. Therefore,
further investigations with more cases are needed.
In addition to the small sample size and
retrospective design, the greatest limitation of this study is that
the preoperative chemotherapy regimens were not standardized,
although the majority contained oxaliplatin. There is no
established regimen in the adjuvant or neoadjuvant setting for
resectable CRC liver metastasis (7,12,18).
However, for conversion therapy of unresectable metastasis, both
oxaliplatin and irinotecan with anti-vascular endothelial growth
factor receptor or anti-EGFR antibodies have been reported to be
effective in decreasing the size or number of metastatic tumors
(8,9). If the aim is to improve tumor
resectability of marginally resectable tumors, administering
preoperative chemotherapy and selecting the regimen according to
the concept of conversion therapy can be a rational approach. Tumor
response reaches a plateau within eight weeks of chemotherapy
(9,24), and six cycles or more of FOLFOX
increase postoperative morbidity due to sinusoidal injury (25). Therefore, four to six cycles of
preoperative chemotherapy are suitable for downsizing marginally
unresectable tumors to enable safe resection. Although this study
provides significant and clinically relevant findings regarding the
implications of preoperative chemotherapy, prospective studies with
standardized patient characteristics and chemotherapy regimens are
needed to validate our results.
In conclusion, we found that preoperative
chemotherapy in patients with CRC and synchronous liver metastasis
did not prolong survival or improve surgical curability.
Preoperative chemotherapy is a useful predictor, as response to the
chemotherapy before the surgery reflects the response to the
chemotherapy after the recurrence. However, preoperative
chemotherapy did not prevent recurrence, even in responders.
Therefore, hepatectomy in patients with CRC and synchronous liver
metastasis should be recommended if the tumor is determined to be
resectable, and preoperative chemotherapy should not be routinely
offered to patients with resectable liver metastasis before their
hepatectomy.
Supplementary Material
Association between K-RAS status and
overall survival in K-RAS available cohort.
Risk factors for shorter survival in
NAC group.
Risk factors for shorter survival in
Non-NAC group.
Risk factors for shorter survival in
K-RAS available cohort.
Risk factors for shorter progression
free survival in NAC group.
Acknowledgements
The authors would like to thank Dr Futoshi Kawamata,
Dr Hirofumi Kamachi, Dr Tatsuya Orimo, Dr Kenji Wakayama, Dr Shingo
Shimada, Dr Akihisa Nagatsu, Dr Takanori Ohata, Dr Yosuke Ohno, Dr
Susumu Shibasaki, Dr Hideki Kawamura (Department of
Gastroenterological Surgery 1, Graduate School of Medicine,
Hokkaido University), Dr Tomonori Hamada (Department of
Gastroenterological Surgery, Hokkaido Cancer Center), and Dr
Kazuaki Nakanishi (Department of Surgery, Hokkaido Hospital, Japan
Community Healthcare Organization) for help with data collection
and useful discussions.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NI, TK, HY, SH, YM, TS, YT, KK, HI, TY and AT
substantially contributed to the conception and design;
acquisition, analysis, and interpretation of data; drafting of the
article or revising it critically for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committees of Hokkaido University
Hospital and all participating hospitals approved this study as an
exempt human subject research (no. 017-0399) and informed consent
was obtained from all patients by the opt-out method, in accordance
with the guidelines of the Japanese Ministry of Health, Labor and
Welfare (Tokyo, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cook AD, Single R and McCahill LE:
Surgical resection of primary tumors in patients who present with
stage IV colorectal cancer: An analysis of surveillance,
epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol.
12:637–645. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nordlinger B, Van Cutsem E, Rougier P,
Köhne CH, Ychou M, Sobrero A, Adam R, Arvidsson D, Carrato A,
Georgoulias V, et al: Does chemotherapy prior to liver resection
increase the potential for cure in patients with metastatic
colorectal cancer? A report from the European Colorectal Metastases
Treatment Group. Eur J Cancer. 43:2037–2045. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Van den Eynde M and Hendlisz A: Treatment
of colorectal liver metastases: A review. Rev Recent Clin Trials.
4:56–62. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Poston GJ, Figueras J, Giuliante F, Nuzzo
G, Sobrero AF, Gigot JF, Nordlinger B, Adam R, Gruenberger T, Choti
MA, et al: Urgent need for a new staging system in advanced
colorectal cancer. J Clin Oncol. 26:4828–4833. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Abdalla EK, Vauthey JN, Ellis LM, Ellis V,
Pollock R, Broglio KR, Hess K and Curley SA: Recurrence and
outcomes following hepatic resection, radiofrequency ablation, and
combined resection/ablation for colorectal liver metastases. Ann
Surg. 239:818–825; discussion 825-827. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Oba M, Hasegawa K, Shindoh J, Yamashita S,
Sakamoto Y, Makuuchi M and Kokudo N: Survival benefit of repeat
resection of successive recurrences after the initial hepatic
resection for colorectal liver metastases. Surgery. 159:632–640.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mitry E, Fields AL, Bleiberg H, Labianca
R, Portier G, Tu D, Nitti D, Torri V, Elias D, O'Callaghan C, et
al: Adjuvant chemotherapy after potentially curative resection of
metastases from colorectal cancer: A pooled analysis of two
randomized trials. J Clin Oncol. 26:4906–4911. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bokemeyer C, Van Cutsem E, Rougier P,
Ciardiello F, Heeger S, Schlichting M, Celik I and Köhne CH:
Addition of cetuximab to chemotherapy as first-line treatment for
KRAS wild-type metastatic colorectal cancer: Pooled analysis of the
CRYSTAL and OPUS randomized clinical trials. Eur J Cancer.
48:1466–1475. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Folprecht G, Gruenberger T, Bechstein WO,
Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher
J, Weitz J, et al: Tumour response and secondary resectability of
colorectal liver metastases following neoadjuvant chemotherapy with
cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol.
11:38–47. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shin SJ, Ahn JB, Choi JS, Choi GH, Lee KY,
Baik SH, Min BS, Hur H, Roh JK and Kim NK: Implications of clinical
risk score to predict outcomes of liver-confined metastasis of
colorectal cancer. Surg Oncol. 21:e125–e130. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lambert LA, Colacchio TA and Barth RJ Jr:
Interval hepatic resection of colorectal metastases improves
patient selection. Arch Surg. 135:473–479; discussion 479-480.
2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nishioka Y, Moriyama J, Matoba S,
Kuroyanagi H, Hashimoto M and Shindoh J: Prognostic impact of
adjuvant chemotherapy after hepatic resection for synchronous and
early metachronous colorectal liver metastases. Dig Surg.
35:187–195. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Japanese Society for Cancer of the Colon
and Rectum: Japanese Classification of Colorectal Carcinoma. 9th
edition. Kanehara & Co. Ltd., Tokyo, p16, 2018.
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Takatsuki M, Tokunaga S, Uchida S, Sakoda
M, Shirabe K, Beppu T, Emi Y, Oki E, Ueno S, Eguchi S, et al:
Evaluation of resectability after neoadjuvant chemotherapy for
primary non-resectable colorectal liver metastases: A multicenter
study. Eur J Surg Oncol. 42:184–189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nordlinger B, Sorbye H, Glimelius B,
Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole
ET, Finch-Jones M, et al: Perioperative chemotherapy with FOLFOX4
and surgery versus surgery alone for resectable liver metastases
from colorectal cancer (EORTC Intergroup trial 40983): A randomised
controlled trial. Lancet. 371:1007–1016. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nordlinger B, Sorbye H, Glimelius B,
Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole
ET, Finch-Jones M, et al: Perioperative FOLFOX4 chemotherapy and
surgery versus surgery alone for resectable liver metastases from
colorectal cancer (EORTC 40983): Long-term results of a randomised,
controlled, phase 3 trial. Lancet Oncol. 14:1208–1215.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hasegawa K, Saiura A, Takayama T, Miyagawa
S, Yamamoto J, Ijichi M, Teruya M, Yoshimi F, Kawasaki S, Koyama H,
et al: Adjuvant oral uracil-tegafur with leucovorin for colorectal
cancer liver metastases: A randomized controlled trial. PLoS One.
11(e0162400)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
de Haas RJ, Adam R, Wicherts DA, Azoulay
D, Bismuth H, Vibert E, Salloum C, Perdigao F, Benkabbou A and
Castaing D: Comparison of simultaneous or delayed liver surgery for
limited synchronous colorectal metastases. Br J Surg. 97:1279–1289.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Ali SM, Pawlik TM, Rodriguez-Bigas MA,
Monson JRT, Chang GJ and Larson DW: Timing of surgical resection
for curative colorectal cancer with liver metastasis. Ann Surg
Oncol. 25:32–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoshita H, Hosokawa A, Ueda A, Ando T,
Kajiura S, Kato H, Kawabe H, Tomizawa G, Horikawa N, Yabuhita K, et
al: Predictive value of optimal morphologic response to first-line
chemotherapy in patients with colorectal liver metastases.
Digestion. 89:43–48. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chun YS, Vauthey JN, Boonsirikamchai P,
Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H,
Charnsangavej C and Loyer EM: Association of computed tomography
morphologic criteria with pathologic response and survival in
patients treated with bevacizumab for colorectal liver metastases.
JAMA. 302:2338–2344. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shindoh J, Loyer EM, Kopetz S,
Boonsirikamchai P, Maru DM, Chun YS, Zimmitti G, Curley SA,
Charnsangavej C, Aloia TA and Vauthey JN: Optimal morphologic
response to preoperative chemotherapy: An alternate outcome end
point before resection of hepatic colorectal metastases. J Clin
Oncol. 30:4566–4572. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Haller DG: Safety of oxaliplatin in the
treatment of colorectal cancer. Oncology (Williston Park).
14:15–20. 2000.
|
|
25
|
Nakano H, Oussoultzoglou E, Rosso E,
Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P and Jaeck D:
Sinusoidal injury increases morbidity after major hepatectomy in
patients with colorectal liver metastases receiving preoperative
chemotherapy. Ann Surg. 247:118–124. 2008.PubMed/NCBI View Article : Google Scholar
|