Introduction
While screening using abdominal ultrasonographic
examination has been widely used for early-stage renal cell
carcinoma (RCC), up to approximately 20-30% of RCC patients have
metastases at initial presentation (1). The introduction of targeted agents has
enabled physicians to improve prognosis over the past decade
compared with that for patients treated with cytokine therapy
(2). Furthermore, previous research
has demonstrated that the prognosis in the later period of the era
of targeted therapy was better than that in the initial period
(3).
Aging is associated with an increased risk of
developing various malignant neoplasms, including RCC (4). RCC is most frequently detected between
the ages of 60 and 70 years, and more than 25% of newly diagnosed
RCC patients are older than 75(5).
Greater care should be taken when planning the therapeutic strategy
for older patients regardless of systemic therapy or surgical
treatment because they have potential comorbidities. It is also
very important to clarify the background and prognosis for mRCC
patients, who are more elderly. Therefore, we investigated the
characteristics before treatment and the outcomes of targeted
therapy for older patients with mRCC and compared the results with
those for a younger patients.
Patients and methods
Two hundred and seventy-seven patients with
metastatic renal cell carcinoma (mRCC) who were treated with
tyrosine kinase inhibitor (TKI) as the first-line therapy at our
institute and other hospitals in Hiroshima Prefecture in Japan from
January 2008 to May 2018 were retrospectively investigated by
reviewing clinicopathological data. Ethical approval was given by
the Ethical Committee of Hiroshima University (Hiroshima, Japan)
(Allowance notification number: E-45), and after that, it was given
by the committee at each collaborative institute. In accordance
with the previous study (6) patients
aged 75 years or older were classified into the older-aged group,
and the others were classified into the younger-aged group.
Clinical and pathological data including age, sex, histological
finding, metastasis status, comorbidities, selection of and severe
adverse events of first-line agent, prior nephrectomy, Karnofsky
performance status, and international mRCC database consortium
(IMDC) risk were collected for all patients, and the distribution
of these parameters for each group was compared. The overall
survival (OS) rate of each group was analyzed by further
classification in accordance with their first-line agent and the
period in which targeted therapy was started.
Statistical analysis
Differences in the distribution of variables among
groups were analyzed using a Chi-square test for categorical
variables and a Mann-Whitney U test for continuous variables. Tumor
responses were determined using an investigator assessment based on
the response evaluation criteria in solid tumors (RECIST) version
1.1. The OS rate was determined using the Kaplan-Meier method, and
differences between groups were analyzed using log-rank testing.
All statistical analyses were conducted using Statview 5.0 software
(Abacus Concepts, Inc.). P<0.05 was considered statistically
significant.
Results
This study cohort consisted of 277 cases of targeted
therapy for mRCC. A total of 55 cases (19.9%) were classified into
the older-aged group, and the other 222 cases (80.1%) were
classified into the younger-aged group. The characteristics of the
cases are listed in Table I. The
rates of cases categorized as poor risk on the basis of IMDC risk
criteria and of those treated with sorafenib were significantly
higher in the older-aged group than in the younger-aged group while
those treated with sunitinib were significantly lower. Median age
in the older- and younger-aged groups was 78 and 63 years. The rate
of patients with cardiovascular diseases and malignant diseases
including hypertension, ischemic heart disease, heart failure, and
arrhythmia besides RCC in the older-aged group was significantly
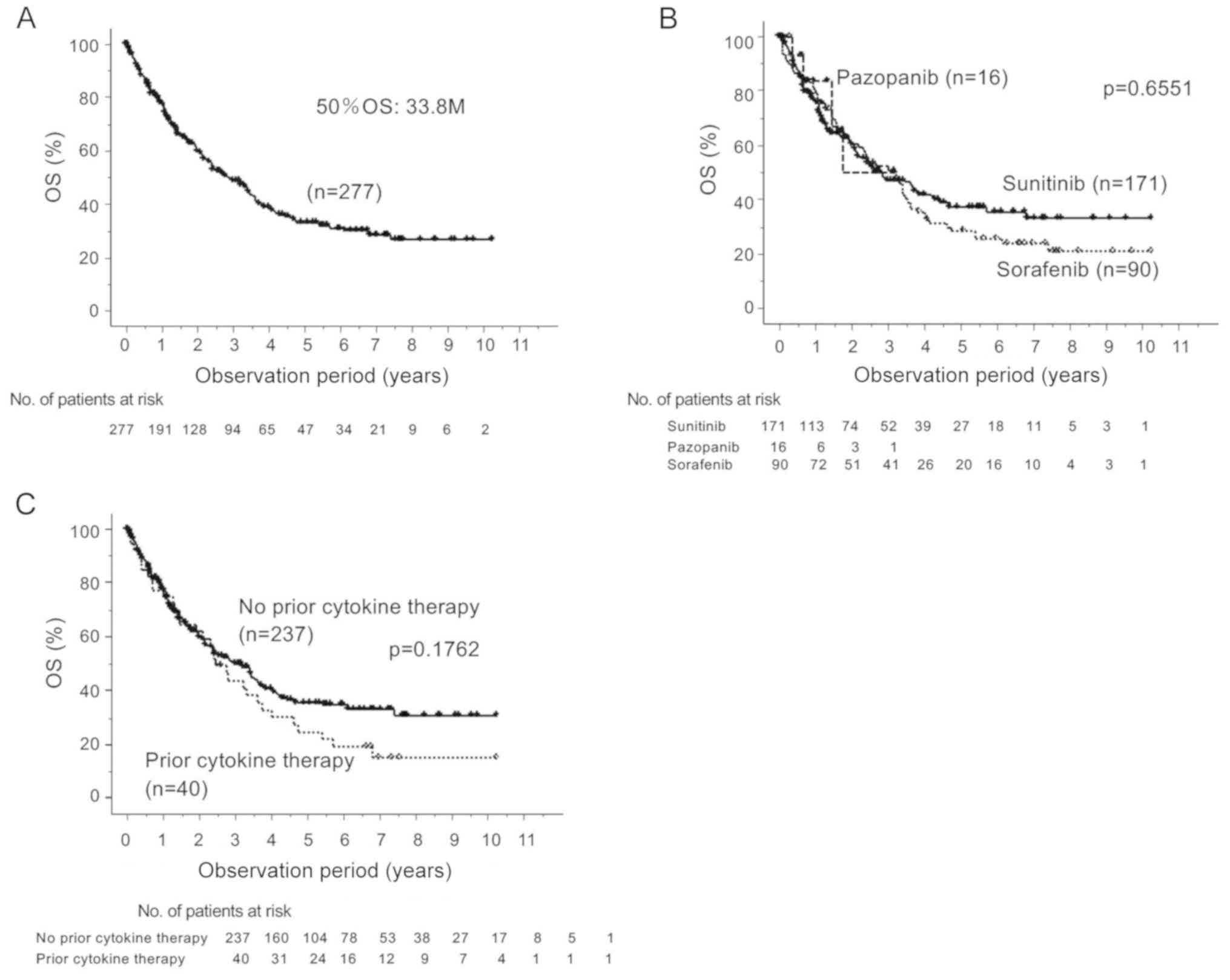
higher than that in the younger-aged group (Table II). The 50% OS rate for the entire
cohort was 33.8 months, and there was no significant difference in
the rate regardless of the choice of first-line agent or the
existence of prior cytokine therapy (Fig. 1). The rate of cases in which
first-line therapy was stopped because of adverse events was
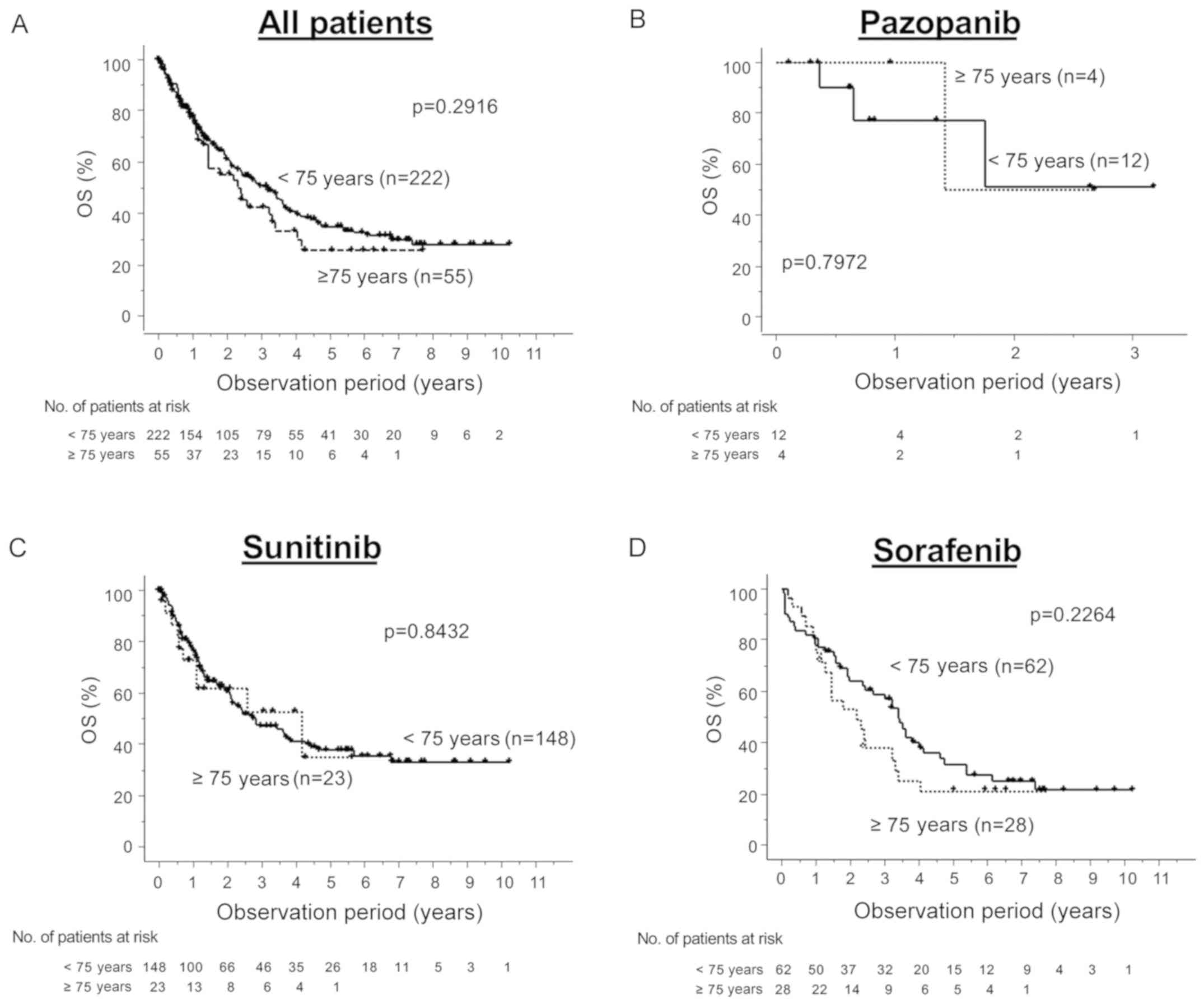
significantly higher in the higher-aged group (Table III). There was no significant
difference in the OS rate between the higher- and younger-aged
groups regardless of the choice of first-line agent (Fig. 2). Table
IV compares the characteristics of the patients on the basis of
the period in which they started first-line TKI: Either 2008-2010
or 2011-2018. In the latter phase, the rates of patients treated
with first-line sunitinib or pazopanib and/or subsequent nivolumab
were significantly higher than those in the earlier phase while the
rates of patients treated with sorafenib and/or prior cytokine
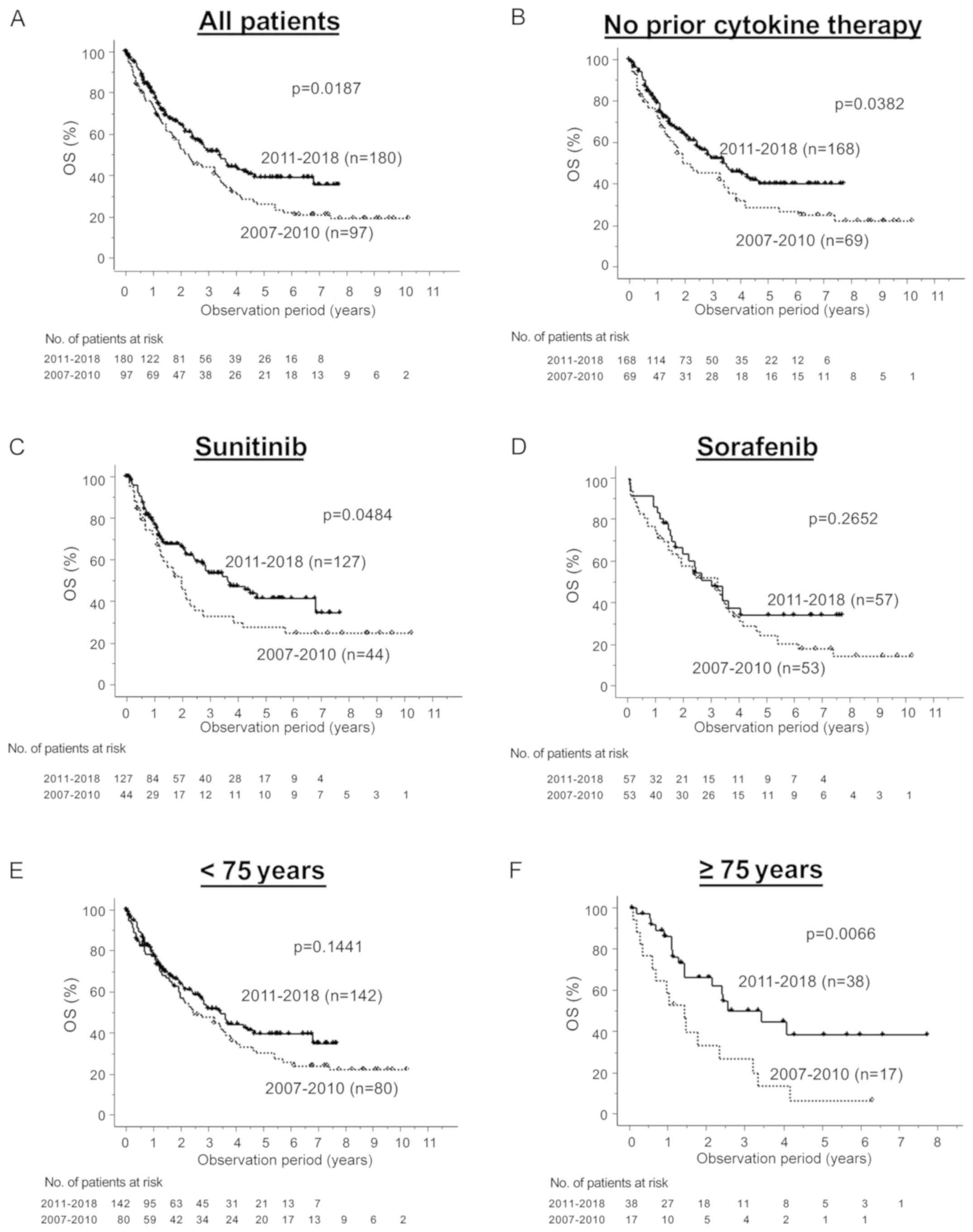
therapy were significantly lower. For all patients, for those
treated with sunitinib as a first-line therapy and for those
without prior cytokine therapy, there was a significant improvement
in the OS rate for patients starting therapy after 2011 compared
with the rate for those starting it before 2010 (Fig. 3A-C). The 50% OS rate in patients
starting targeted therapy before 2010 and after 2011 was
respectively 17.1 and 38.6 months for the older-aged group
(P=0.0066), while there was no significant difference in the rate
for the younger-aged one (P=0.1441, 50% OS; 35.9 vs. 30.5 months)
(Fig. 3E and F).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variables | <75 years (n=222)
n (%) | ≥75 years (n=55) n
(%) | P-value | Total (n=277) n
(%) |
|---|
| Sex | | | 0.3430 | |
|
Male | 182 (82.0) | 42 (76.4) | | 224 (80.9) |
|
Female | 40 (18.0) | 13 (23.6) | | 53 (19.1) |
| Pathology | | | 0.4251 | |
|
Clear | 170 (76.6) | 46 (83.6) | | 216 (78.0) |
|
Non-clear | 22 (9.9) | 5 (9.1) | | 27 (9.7) |
|
Unknown | 30 (13.5) | 4 (7.3) | | 34 (12.3) |
| Karnofsky PS | | | 0.4460 | |
|
≥80% | 203 (91.4) | 52 (94.5) | | 255 (92.1) |
|
<80% | 19 (8.6) | 3 (5.5) | | 22 (7.9) |
| IMDC risk | | | 0.0067 | |
|
Favorable | 46 (20.7) | 7 (12.7) | | 53 (19.1) |
|
Intermediate | 118 (53.2) | 42 (76.4) | | 160 (57.8) |
|
Poor | 58 (26.1) | 6 (10.9) | | 64 (23.1) |
| Metastatic site | | | | |
|
Lung | 149 (67.1) | 37 (67.3) | 0.9825 | 186 (67.2) |
|
Lymph
node | 71 (32.0) | 12 (21.8) | 0.1407 | 83 (30.0) |
|
Liver | 26 (11.7) | 9 (16.4) | 0.3526 | 35 (12.6) |
|
Bone | 59 (26.6) | 13 (23.6) | 0.6563 | 72 (26.0) |
|
Adrenal
gland | 23 (10.4) | 6 (10.9) | 0.9053 | 29 (10.5) |
|
Ipsilateral
kidney | 17 (7.7) | 4 (7.3) | 0.9231 | 21 (7.6) |
|
≥2
organs | 107 (48.2) | 25 (45.5) | 0.7153 | 132 (47.7) |
| Prior
nephrectomy | | | 0.5194 | |
|
Radical | 102 (45.9) | 30 (54.5) | | 132 (47.7) |
|
Cytoreductive | 76 (34.2) | 16 (29.1) | | 92 (33.2) |
|
None | 44 (19.8) | 9 (16.4) | | 53 (19.1) |
| First-line
agent | | | 0.0027 | |
|
Sunitinib | 148 (66.7) | 23 (41.8) | | 171 (61.7) |
|
Pazopanib | 12 (5.4) | 4 (7.3) | | 16 (5.8) |
|
Sorafenib | 62 (27.9) | 28 (50.9) | | 90 (32.5) |
| Prior cytokine
therapy | | | 0.1901 | |
|
(-) | 193 (86.9) | 44 (80.0) | | 237 (85.6) |
|
(+) | 29 (13.1) | 11 (20.0) | | 40 (14.4) |
| Subsequent
nivolumab therapy | | | 0.9331 | |
|
(-) | 201 (90.5) | 50 (90.9) | | 251 (90.6) |
|
(+) | 21 (9.5) | 5 (9.1) | | 26 (9.4) |
 | Table IIComparison of comorbidities. |
Table II
Comparison of comorbidities.
| Variables | <75 years
(n=222) n (%) | ≥75 years (n=55) n
(%) | P-value | Total (n=277) n
(%) |
|---|
| Hypertension | 46 (20.7) | 19 (34.5) | 0.0303 | 65 (23.5) |
| Ischemic heart
disease/heart failure | 6 (2.7) | 6 (10.9) | 0.0074 | 12 (4.3) |
| Arrhythmia | 6 (2.7) | 7 (12.7) | 0.0016 | 13 (4.7) |
| Vascular
disease | 3 (1.4) | 3 (5.5) | 0.0613 | 6 (2.2) |
| Cerebrovascular
disease | 5 (2.5) | 3 (5.5) | 0.2043 | 8 (2.9) |
| Respiratory
disease | 8 (3.6) | 2 (3.6) | 0.9907 | 10 (3.6) |
| Renal
dysfunction | 8 (3.6) | 5 (9.1) | 0.0850 | 13 (4.7) |
| Other malignant
neoplasm | 16 (7.2) | 11 (20.0) | 0.0042 | 27 (9.7) |
 | Table IIIDiscontinuation of first-line agents
caused by adverse events. |
Table III
Discontinuation of first-line agents
caused by adverse events.
| Variables | <75 years
(n=222) n (%) | ≥75 years (n=55) n
(%) | P-value | Total (n=277) n
(%) |
|---|
| Cardiovascular
event | 5 (2.3) | 3 (5.5) | 0.2043 | 8 (2.9) |
| Respiratory
event | 1 (0.5) | 0 (0) | 0.6180 | 1 (0.4) |
| Gastrointestinal
event | 10 (4.5) | 3 (5.5) | 0.7655 | 13 (4.7) |
| Bone marrow
suppression | 27 (12.2) | 6 (10.9) | 0.7973 | 33 (11.9) |
| Dermatological
toxicity | 17 (7.7) | 4 (7.3) | 0.9231 | 20 (7.2) |
| Endocrinological
event | 1 (0.5) | 1 (1.8) | 0.2835 | 2 (0.7) |
| Liver/renal
dysfunction | 9 (4.1) | 2 (3.6) | 0.8871 | 9 (3.3) |
| Neurological
event | 2 (0.9) | 2 (3.6) | 0.1279 | 4 (1.4) |
| General
fatigue | 0 (0) | 2 (3.6) | 0.0044 | 2 (0.7) |
| Patient's
desire | 9 (4.1) | 5 (9.1) | 0.1269 | 5 (1.8) |
| Total | 65 (29.3) | 24 (43.6) | 0.0412 | 89 (32.1) |
 | Table IVCharacteristics of patients comparing
at timing starting first-line TKI. |
Table IV
Characteristics of patients comparing
at timing starting first-line TKI.
| Variables | 2007-2010 (n=97) n
(%) | 2011-2018 (n=180) n
(%) | P-value | Total (n=277) n
(%) |
|---|
| Age (years) | | | 0.4755 | |
|
≥75 | 17 (17.5) | 38 (21.1) | | 55 (19.9) |
|
<75 | 80 (82.5) | 142 (78.9) | | 222 (80.1) |
| Sex | | | 0.0750 | |
|
Male | 84 (86.6) | 140 (77.8) | | 224 (80.9) |
|
Female | 13 (13.4) | 40 (22.2) | | 53 (19.1) |
| Pathology | | | 0.0190 | |
|
Clear | 71 (73.2) | 145 (80.6) | | 216 (78.0) |
|
Non-clear | 7 (7.2) | 20 (11.1) | | 27 (9.7) |
|
Unknown | 19 (19.6) | 15 (8.3) | | 34 (12.3) |
| Karnofsky PS | | | 0.5460 | |
|
≥80% | 88 (90.7) | 167 (92.8) | | 255 (92.1) |
|
<80% | 9 (9.3) | 13 (7.2) | | 22 (7.9) |
| IMDC risk | | | 0.8991 | |
|
Favorable | 20 (20.6) | 33 (18.3) | | 53 (19.1) |
|
Intermediate | 55 (56.7) | 105 (58.3) | | 160 (57.8) |
|
Poor | 22 (22.7) | 42 (23.3) | | 64 (23.1) |
| Metastatic
site | | | | |
|
Lung | 65 (67.0) | 121 (67.2) | 0.9714 | 186 (67.2) |
|
Lymph
node | 28 (28.9) | 55 (30.6) | 0.7697 | 83 (30.0) |
|
Liver | 15 (15.5) | 20 (11.1) | 0.2983 | 35 (12.6) |
|
Bone | 29 (29.9) | 43 (23.9) | 0.2768 | 72 (26.0) |
|
Adrenal
gland | 7 (7.2) | 22 (12.2) | 0.1943 | 29 (10.5) |
|
Ipsilateral
kidney | 6 (6.2) | 15 (8.3) | 0.5194 | 21 (7.6) |
|
≥2
organs | 49 (50.5) | 83 (46.1) | 0.4839 | 132 (47.7) |
| Prior
nephrectomy | | | 0.4416 | |
|
Radical | 51 (52.6) | 81 (45.0) | | 132 (47.7) |
|
Cytoreductive | 28 (28.9) | 64 (35.6) | | 92 (33.2) |
|
None | 18 (18.6) | 35 (19.4) | | 53 (19.1) |
| 1st-line agent | | | <0.0001 | |
|
Sunitinib | 44 (45.4) | 127 (70.6) | | 171 (61.7) |
|
Pazopanib | 0 (0) | 16 (8.9) | | 16 (5.8) |
|
Sorafenib | 53 (54.6) | 37 (20.6) | | 90 (32.5) |
| Prior cytokine
therapy | | | <0.0001 | |
|
(-) | 69 (71.1) | 168 (93.3) | | 237 (85.6) |
|
(+) | 28 (28.9) | 12 (6.7) | | 40 (14.4) |
| Subsequent
nivolumab therapy | | | <0.0001 | |
|
(-) | 97(100) | 154 (85.6) | | 251 (90.6) |
|
(+) | 0 (0) | 26 (14.4) | | 26 (9.4) |
Discussion
In the present study, we assessed the improvement in
the prognosis for patients with mRCC in the earlier and later
phases of the era of targeted therapy for all patients and for
patients without prior cytokine therapy. The results showed that
the prognosis for older patients improved after the introduction of
targeted therapy. To the best of our knowledge, this study is the
first to demonstrate an improvement in the prognosis for older
patients during the later period of targeted therapy compared with
that in the initial one.
Recently, several randomized control trials have
demonstrated the efficacy of immune checkpoint inhibitors (ICIs)
and combination regimens including ICIs for mRCC patients (7-10),
and as such, many patients with mRCC are being treated with ICI.
However, in the CheckMate 025 trials, nivolumab, one of the most
common ICIs for mRCC, was reported to be less effective in older
patients than in younger ones (7).
Moreover, when patients with mRCC receive ICI therapy,
immune-related adverse events (irAEs) are inevitable. Physicians
need to assess whether patients can overcome such irAEs. These
considerations means that TKI is likely to remain one of the
standard options for older patients with mRCC.
We can see an improvement in the OS rate in the
entire cohort. We can also see some improvement even in the
younger-aged group, but it is not statistically significant. Thus,
we hypothesize that this improvement represents outcomes particular
to the older-aged group. It is relatively easy to manage younger
patients who receive targeted therapy because many of them are
generally healthy despite having metastatic RCC. In contrast, many
older patients have additional comorbidities (e.g., cardiovascular
or respiratory disease), which can prevent them from continuing to
receive targeted therapy. Many physicians have now mastered the
management of patients who receive targeted therapy in terms of
agent choice, agent dose, and adverse events. Therefore, we can see
significant improvement in the OS rate, especially for older
patients who have more comorbidities.
There are two possible explanations as to why the
prognosis for older patients has improved more than that for
younger ones. The first is the changes in and increased number of
therapeutic options for both first- and second-line therapies. The
non-inferiority of pazopanib compared with sunitinib as a
first-line option and the improvement in progression-free survival
due to axitinib therapy compared with sorafenib therapy along with
the superior OS rate due to nivolumab therapy as second-line
options for mRCC have been demonstrated by randomized controlled
trials (7,8,11).
Moreover, these therapies have been recommended as viable
first-line and second-line options in several published guidelines
(12,13). On the basis of this evidence, they
are now covered by the national health insurance system in Japan.
In particular, since pazopanib is reported to be relatively
tolerable for patients (14), it
seems poised to become a viable option for older patients with
severe comorbidities. This increase in the number of treatment
options enables physicians to choose the best one for each patient
in accordance with their characteristics. However, in this study,
pazopanib was administered to only four patients in the higher-aged
group, so its effect may be limited to the cohort of this study. As
more than half of the patients in the initial period received
sorafenib as first-line therapy, the change in the rate of
sorafenib as a first-line option might provide some insight into
the improvement in the OS rate.
The other possible explanation for the improved
prognosis for older patients is optimal management of a therapeutic
schedule personalized for each patient. Several previous studies
have demonstrated the efficacy and safety of various agents [e.g.,
sunitinib (6,15-18)
and sorafenib (19)] for older
patients, and another study reported that an alternative schedule
of sunitinib treatment provided a greater improvement in the OS
rate compared with the standard schedule (20). Since sunitinib was prescribed on the
basis of a personalized schedule in the later period, the data from
our present study for patients treated with sunitinib is consistent
with those reports (Fig. 3C).
The limitation of the present work is that it is a
small retrospective study based on real-world data. Physicians were
assumed to have taken into account the comorbidity of patients and
the tolerability of each agent when the therapeutic option was
chosen. Therefore, some patient-selection bias due to the exclusion
of patients with very severe comorbidities from targeted therapy is
entirely possible. The older-aged group in this study consisted of
19.9% of the patients, which is a higher rate than in the previous
study (21). There might be some
differences between countries in terms of the age distribution of
patients with mRCC who undergo systemic therapy. In addition, we
have no data of clinical classification based on the data of some
special molecules identified by gene analysis. A prospective
observation study using a greater amount of data is required to
further clarify the characteristics and the prognosis for older
patients with mRCC.
We have demonstrated that the prognosis for older
patients has improved compared with that when targeted therapy was
first introduced. Our data clearly indicate the benefit to older
patients of the recent rapid introduction of new agents as
therapeutic options for mRCC. It is thus expected that the
continuing increase of therapeutic options (including ICIs as well
as targeted agents) will lead to further improvement in the
prognosis for patients with mRCC in the coming years. At the same
time, we expect that a more personalized therapeutic strategy will
be established for older patients that is based on key molecules
identified through gene sequencing in addition to conventional
predictive factors for prognosis and agent efficacy (22,23) as
older patients tend to have more comorbidities than younger
ones.
Acknowledgements
In addition to the authors, the following
investigators participated in the study: Dr. Hideo Iwamoto, Kure
Medical Center; Dr. Ryo Tasaka, Hiroshima General Hospital; Dr.
Koichi Shoji and Dr. Ryoken Yamanaka, Higashi-Hiroshima Medical
Center; Shinji Matsuzaki, Fukuyama Medical Center; Dr. Yoshinori
Nakano, Onomichi General Hospital; and Dr. Yuki Kohada, Hiroshima
Prefectural Hospital.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
JT, SI, TH and AM conceived and designed this study.
DM, KM, YH, MKat, MKaj, MS, SM, HM and SF acquired the data. JT, SI
and TH analyzed the data. JT drafted the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Hiroshima University (Allowance notification no. E-45)
(Hiroshima, Japan). The requirement of informed consent was waived
by the Institutional Review Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pal SK, Nelson RA and Vogelzang N:
Disease-specific survival in de novo metastatic renal cell
carcinoma in the cytokine and targeted therapy era. PLoS One.
8(e63341)2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pal SK, Ghate SR, Li N, Swallow E, Peeples
M, Zichlin ML, Perez JR, Agarwal N and Vogelzang NJ: Real-world
survival outcomes and prognostic factors among patients receiving
first targeted therapy for advanced renal cell carcinoma: A
SEER-medicare database analysis. Clin Genitourin Cancer.
15:e573–e582. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Janssen-Heijnen ML, Gondos A, Bray F,
Hakulinen T, Brewster DH, Brenner H and Coebergh JW: Clinical
relevance of conditional survival of cancer patients in Europe:
Age-specific analyses of 13 cancers. J Clin Oncol. 28:2520–2528.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pal SK, Vanderwalde A, Hurria A and Figlin
RA: Systemic therapies for metastatic renal cell carcinoma in older
adults. Drugs Aging. 28:635–649. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miyake H, Aki R, Matsushita Y, Tamura K,
Motoyama D, Ito T, Sugiyama T and Otsuka A: Significance of age in
Japanese patients receiving sunitinib as first-line systemic
therapy for metastatic renal cell carcinoma: Comparative assessment
of efficacy and safety between patients aged <75 and ≥75 years.
Anticancer Res. 38:3593–3599. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Motzer RJ, Penkov K, Haanen J, Rini B,
Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S,
Uemura M, et al: Avelumab plus axitinib versus sunitinib for
advanced renal-cell carcinoma. N Engl J Med. 380:1103–1115.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Motzer RJ, Escudier B, Tomczak P, Hutson
TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J,
Hariharan S, et al: Axitinib versus sorafenib as second-line
treatment for advanced renal cell carcinoma: Overall survival
analysis and updated results from a randomised phase 3 trial.
Lancet Oncol. 14:552–562. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ljungberg B, Albiges L, Abu-Ghanem Y,
Bensalah K, Dabestani S, Fernández-Pello S, Giles RH, Hofmann F,
Hora M, Kuczyk MA, et al: European Association of urology
guidelines on renal cell carcinoma: The 2019 Update. Eur Urol.
75:799–810. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
NCCN clinical practice guidelines in
oncology (NCCN guidelines) Kidney Cancer. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
|
|
14
|
Escudier B, Porta C, Bono P, Powles T,
Eisen T, Sternberg CN, Gschwend JE, De Giorgi U, Parikh O, Hawkins
R, et al: Randomized, controlled, double-blind, cross-over trial
assessing treatment preference for pazopanib versus sunitinib in
patients with metastatic renal cell carcinoma: PISCES Study. J Clin
Oncol. 32:1412–1418. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
De Giorgi U, Scarpi E, Sacco C, Aieta M,
Lo Re G, Sava T, Masini C, De Vincenzo F, Baldazzi V, Camerini A,
et al: Standard vs. adapted sunitinib regimen in elderly patients
with metastatic renal cell cancer: Results from a large
retrospective analysis. Clin Genitourin Cancer. 12:182–189.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hutson TE, Bukowski RM, Rini BI, Gore ME,
Larkin JM, Figlin RA, Barrios CH, Escudier B, Lin X, Fly K, et al:
Efficacy and safety of sunitinib in elderly patients with
metastatic renal cell carcinoma. Br J Cancer. 110:1125–1132.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Brunello A, Basso U, Sacco C, Sava T, De
Vivo R, Camerini A, Barile C, Roma A, Maruzzo M, Falci C, et al:
Safety and activity of sunitinib in elderly patients (≥70 years)
with metastatic renal cell carcinoma: A multicenter study. Ann
Oncol. 24:336–342. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Poprach A, Lakomy R, Bortlicek Z, Melichar
B, Pavlik T, Slaby O, Vyzula R, Svoboda M, Kiss I, Studentova H, et
al: Efficacy of sunitinib in elderly patients with metastatic renal
cell carcinoma: Data from real-world clinical practice. Drugs
Aging. 33:655–663. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dutcher JP, Tannir N, Bellmunt J and
Escudier B: Experience with sorafenib and the elderly patient. Med
Oncol. 27:1359–1370. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Atkinson BJ, Kalra S, Wang X, Bathala T,
Corn P, Tannir NM and Jonasch E: Clinical outcomes for patients
with metastatic renal cell carcinoma treated with alternative
sunitinib schedules. J Urol. 191:611–618. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Khambati HK, Choueiri TK, Kollmannsberger
CK, North S, Bjarnason GA, Vaishampayan UN, Wood L, Knox JJ, Tan
MH, MacKenzie MJ, et al: Efficacy of targeted therapy for
metastatic renal cell carcinoma in the elderly patient population.
Clin Genitourin Cancer. 12:354–358. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Teishima J, Inoue S, Hayashi T and
Matsubara A: Current status of prognostic factors in patients with
metastatic renal cell carcinoma. Int J Urol. 26:608–617.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
De Giorgi U, Procopio G, Giannarelli D,
Sabbatini R, Bearz A, Buti S, Basso U, Mitterer M, Ortega C, Bidoli
P, et al: Association of systemic inflammation index and body mass
index with survival in patients with renal cell cancer treated with
nivolumab. Clin Cancer Res. 25:3839–3846. 2019.PubMed/NCBI View Article : Google Scholar
|