Introduction
Breast cancer treatment has gradually evolved to
reduce side effects without compromising treatment outcomes
(1,2). Treatment-related side effects
negatively affect quality of life, delay treatment, and compromise
results. The typical side effects include cardiac toxicity,
dermatitis, and lymphedema by multimodal treatments using surgery,
adjuvant chemotherapy and radiotherapy (RT) (3-7).
Among them, the incidence of breast cancer-related lymphedema
(BCRL) after treatment ranges from 14 to 40% (8), caused principally by axillary lymph
node dissection (ALND) and adjuvant RT, particularly irradiation of
the axillary level I and II (8-10).
Sentinel lymph node biopsy has thus become standard treatment for
clinically node-negative patients and has become an alternative for
complete ALND. RT has also evolved from application of
three-dimensional conformal RT (3D-CRT) to intensity-modulated RT
(IMRT) or volumetric modulated arc therapy (VMAT) to achieve better
conformity with the treatment target and reduce unnecessary
irradiation of normal organs (11-22).
VMAT has the potential for reduced treatment time compared with
IMRT and the clinical use of VMAT is increasing gradually (23). When planning VMAT, dose-volume
constraints should be applied to reduce or minimize irradiation of
normal tissue. If axillary constraints are imposed in VMAT plan,
the axillary dose can be less than that of 3D-CRT. However, it is
unclear whether incidental axillary irradiation differs in VMAT
without axillary constraint, as with 3D-CRT. In the present study,
we investigated the difference in incidental irradiation dose on
the axilla and dosimetric characteristics between the two
aforementioned plans.
Materials and methods
Ethics approval
This study was approved by The Institutional Review
Board of Soonchunhyang University Cheonan Hospital (approval no.
SCHCA 2019-11-015-001) (Chungnam, Republic of Korea). Due to the
retrospective nature, the requirement to obtain informed consent of
the patients was waived by the board. All the procedures in this
study were in accordance with the Declaration of Helsinki. Patients
who participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Stimulation of radiotherapy
We investigated 20 patients with left breast cancer
who received breast-conserving surgery followed by postoperative RT
in 2015 and 2016. Eighteen patients (90%) were T1 stage and two
patients (10%) were Tis stage. There were no axillary metastases in
all patients. All patients underwent a free-breathing simulation
computed tomography scan using the Philips Brilliance Big Bore
(Philips Medical Systems) with 3 mm slice thickness. The patients
lay supine on a no-tilting breast board (CIVCO Medical Solutions)
without tilting angle, with the arms above the head for appropriate
exposure of their breasts and axillae.
Contouring and planning
The clinical target volumes (CTVs) and axillary
level I-III were delineated on the basis of the Radiation Therapy
Oncology Group contouring guidelines for breast cancer (24). The superior CTV border was the
sternal notch or 1 cm above the breast tissue, and the inferior
border was 2 cm below the inframammary fold. The medial CTV border
was the mid-sternum line and the lateral border was the
mid-axillary line or a line 2 cm lateral to breast tissue. To
generate the planning target volume (PTV), the CTV was expanded by
1 cm in all directions, and then 3 mm of skin was trimmed from the
anterior border of the body surface. Axillary levels I-III were
contoured separately, based on the positions of the pectoralis
major, pectoralis minor, and intercostal muscles as well as the
ribs. The organs at risk (OARs) included the ipsilateral and
contralateral lung, heart, and spinal cord. All VMAT and 3D-CRT
plans were performed using a radiation treatment planning system
(Eclipse ver. 8.9; Varian Medical Systems Inc.) and 6-MV photon.
Each 3D-CRT plan consisted of four portals with two optimal
tangential angles. The physical wedges in the x- and y-axis were
applied once at each portal to improve the dose distribution. Each
VMAT plan was performed using the same algorithm with the same
energy as for 3D-CRT. The beam arrangement was optimized between
290 and 180 to conform maximally to individual PTVs and afford the
OARs the best possible preservation. A ring structure around the
PTV was created to obtain the appropriate treatment target
conformity. During optimization of VMAT plan, the normal tissue
objective was conducted dose-volume constraints for the heart and
ipsilateral lung, but not the axilla to set similar conditions
between 3D-CRT and VMAT. The prescription dose was 50 Gy in 25
fractions, and the plans were normalized such that ≥95% of the PTV
received 100% of the prescription dose.
Statistical analysis
We recorded all doses to the PTVs, OARs and axillary
level I-III. Additionally, the integral dose (ID) was
reconstructed. The ID was calculated using the simplified formula
ID=Vbody (liters) x Dmean in body,
outside PTV (Gy) (25). Plan
data were analyzed using the Mann-Whitney test with the SPSS 18.0
(SPSS, Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The median age of the patients was 47 years (range:
36-66 years). Invasive ductal carcinoma was confirmed in 17
patients (85.0%). Table I shows the
patient characteristics.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Valuesa |
|---|
| Age, year | 47 (36-66) |
| T stage | |
|
Tis | 2 (10.0) |
|
T1 | 18 (90.0) |
| Pathology | |
|
DCIS | 2 (10.0) |
|
IDC | 17 (85.0) |
|
ILC | 1 (5.0) |
| Volumes of
structures | |
|
Body | 19,180.5
(13,984.4-27,079.2) |
|
PTV | 446.3
(102.9-1,174.6) |
|
Heart | 605.9
(516.0-968.7) |
|
Spinal
cord | 50.4
(36.9-66.9) |
|
Ipsilateral
lung | 927.8
(627.3-1,324.5) |
|
Total
lung | 2,162.3
(1,573.9-2,996.6) |
| Axilla | |
|
Level I | 63.6
(35.7-106.0) |
|
Level
II | 20.7
(13.3-41.8) |
|
Level
III | 13.5
(9.3-24.3) |
Doses of PTV and OARs
VMAT afforded ≥90% PTV coverage more reliably than
did 3D-CRT. The V45Gy of VMAT was 99.7±0.2%, vs.
99.2±0.9% for 3D-CRT (P=0.002). However, the mean PTV dose did not
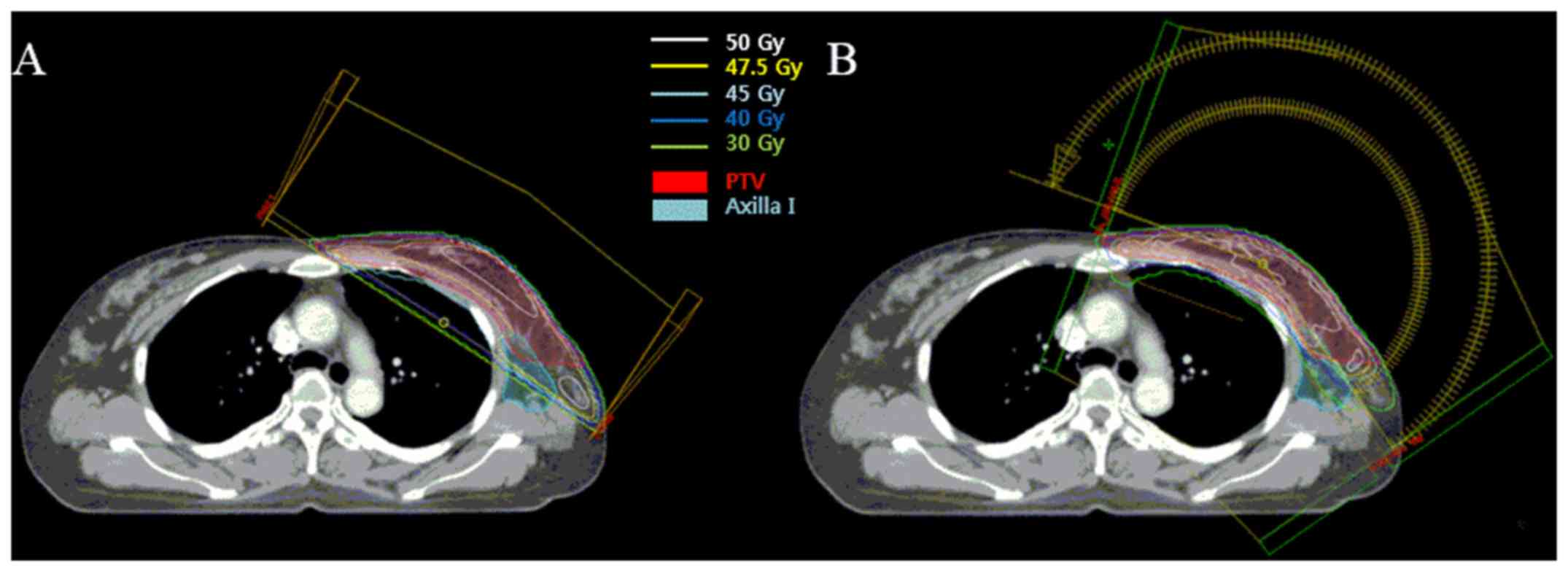
differ between the two plans. Fig. 1
shows a representative image of both plans with the PTV and isodose
lines.
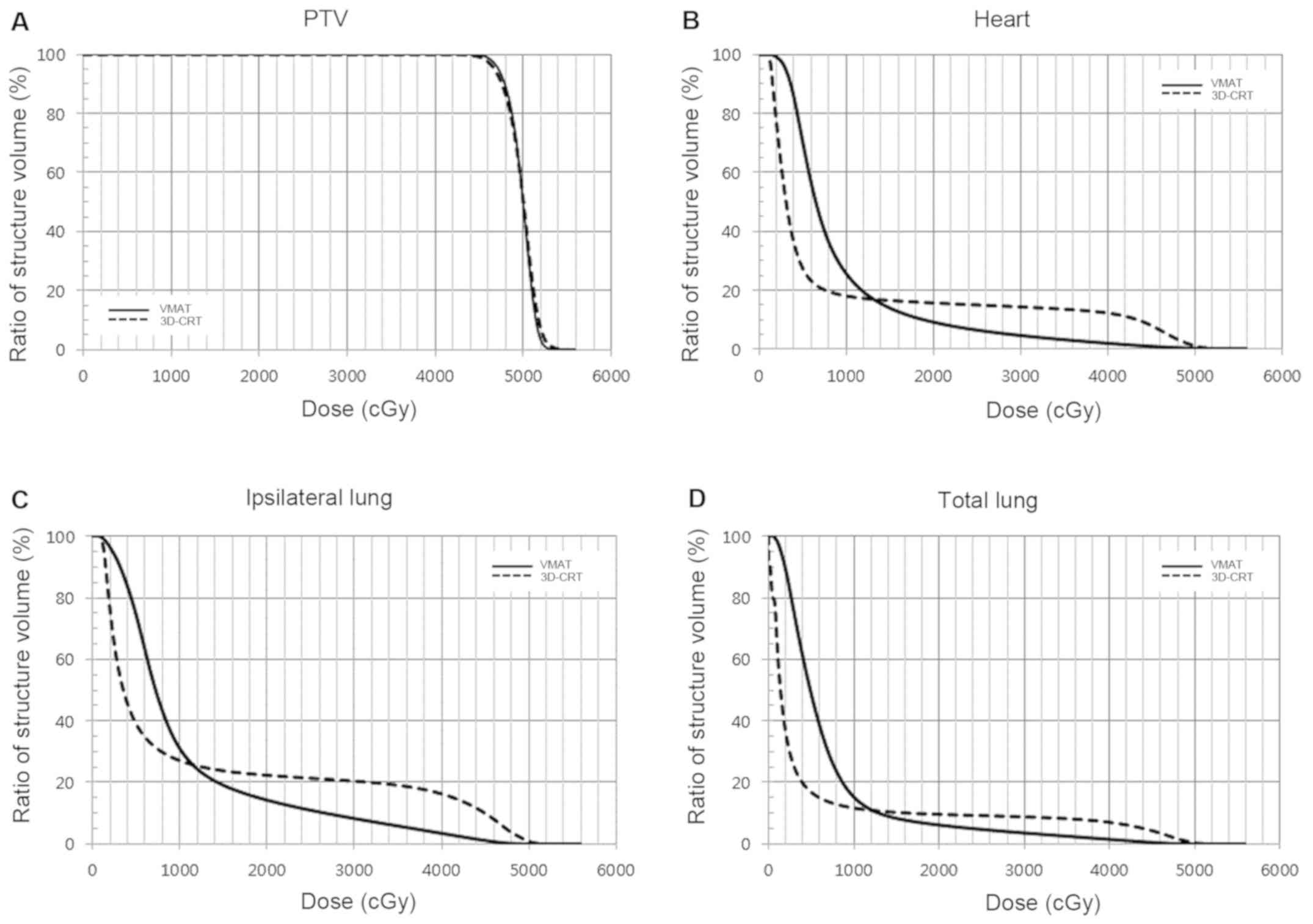
Table II lists the
OAR and ID data of the two plans and Fig. 2 shows the cumulative dose-volume
histograms. In brief, moderate-to-high doses (V25Gy and
V40Gy) of VMAT were lower than the 3D-CRT doses in all
OARs. For the heart, VMAT was significantly lower than 3D-CRT
(V25Gy, 6.3% vs. 14.8%; V40Gy, 1.8% vs.
12.1%, respectively, P<0.001). For the ipsilateral lung, the
moderate-to-high VMAT doses were lower than those for 3D-CRT
(V25Gy, 10.9% vs. 21.3%; V40Gy 3.4% vs.
16.2%, respectively, P<0.001). However, V5Gy was
higher for VMAT than 3D-CRT. For the heart, the V5Gy for
VMAT was higher than that for 3D-CRT (69.9% vs. 27.1%,
respectively, P<0.001). For the ipsilateral lung, the
V5Gy for VMAT was higher than that for 3D-CRT (74.5% vs.
39.1%, respectively, P<0.001). The mean dose to the ipsilateral
lung and spinal cord was higher for VMAT than 3D-CRT (P<0.001),
while for the heart there was no difference between the two plans
(9.5 Gy vs. 9.3 Gy, respectively). Lastly, the ID to the body was
higher for VMAT than 3D-CRT (82.7 Gy·L vs. 64.6 Gy·L, respectively,
P<0.001).
 | Table IIDosimetric comparison of radiation
dose delivered to PTV, organs at risk and integral dose between
3D-CRT and VMAT plans. |
Table II
Dosimetric comparison of radiation
dose delivered to PTV, organs at risk and integral dose between
3D-CRT and VMAT plans.
| Structure | Parameters | 3D-CRT | VMAT | P-value |
|---|
| PTV | DMean
(Gy) | 49.8±0.2 | 49.8±0.0 | 0.709 |
| | V45Gy
(%) | 99.2±0.9 | 99.7±0.2 | 0.002 |
| | V47.5Gy
(%) | 90.4±2.5 | 93.1±1.7 | 0.001 |
| Heart | DMean
(Gy) | 9.5±0.8 | 9.3±1.8 | 0.575 |
| | V5Gy
(%) | 27.1±8.9 | 69.9±14.4 | <0.001 |
| | V25Gy
(%) | 14.8±5.4 | 6.3±2.8 | <0.001 |
| | V40Gy
(%) | 12.1±5.0 | 1.8±1.4 | <0.001 |
| Ipsilateral
lung | DMean
(Gy) | 12.4±2.1 | 10.9±1.5 | 0.001 |
| | V5Gy
(%) | 39.1±5.3 | 74.5±10.7 | <0.001 |
| | V25Gy
(%) | 21.3±4.3 | 10.9±3.0 | <0.001 |
| | V40Gy
(%) | 16.2±4.5 | 3.4±1.8 | <0.001 |
| Total lung | DMean
(Gy) | 5.9±1.1 | 7.1±1.0 | <0.001 |
| | V5Gy
(%) | 16.9±2.9 | 48.8±9.4 | <0.001 |
| | V25Gy
(%) | 9.2±2.2 | 4.7±1.4 | <0.001 |
| | V40Gy
(%) | 7.0±2.2 | 1.5±0.8 | <0.001 |
| Spinal cord | DMean
(Gy) | 0.7±0.2 | 1.7±0.5 | <0.001 |
| | V5Gy
(%) | 0.0±0.0 | 0.1±0.1 | 0.001 |
| | V25Gy
(%) | 0.0±0.0 | 0.0±0.0 | - |
| | V40Gy
(%) | 0.0±0.0 | 0.0±0.0 | - |
| Body | ID (Gy·L) | 64.6±17.7 | 82.7±20.3 | <0.001 |
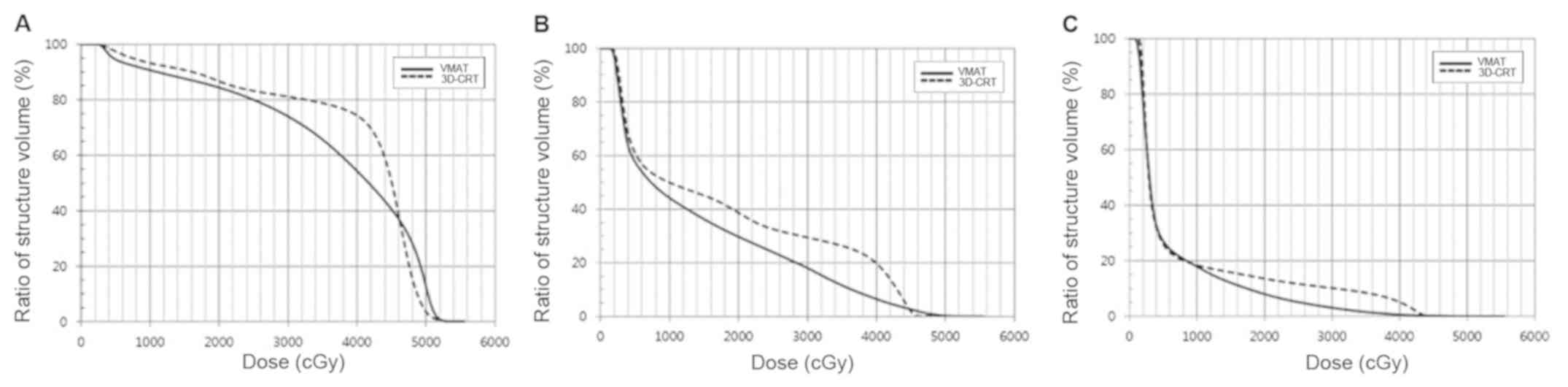
Comparison of axillary doses
The doses to axillary levels I-III were analyzed
separately. The mean, maximum, V25Gy and
V40Gy are shown in Table
III and Fig. 3. The mean doses
to each axillary level were significantly lower for VMAT vs.
3D-CRT. The V25Gy and V40Gy for VMAT were
significantly lower than those for 3D-CRT, except the
V25Gy of axillary level I. The max VMAT doses to
axillary levels II and III were lower than those for 3D-CRT, while
that to axillary level I was not.
 | Table IIIDosimetric comparison of radiation
dose delivered to axilla level I-III between 3D-CRT and VMAT. |
Table III
Dosimetric comparison of radiation
dose delivered to axilla level I-III between 3D-CRT and VMAT.
| Structure | Parameters | 3D-CRT | VMAT | P-value |
|---|
| Level I | DMean
(Gy) | 39.5±4.7 | 36.8±6.4 | 0.010 |
| | DMax
(Gy) | 49.3±7.2 | 52.4±0.8 | <0.001 |
| | V25Gy
(%) | 83.2±12.2 | 80.0±17.2 | 0.313 |
| | V40Gy
(%) | 74.3±15.1 | 54.3±16.1 | <0.001 |
| Level II | DMean
(Gy) | 18.0±12.4 | 14.2±11.3 | <0.001 |
| | DMax
(Gy) | 37.4±11.7 | 33.5±18.9 | 0.391 |
| | V25Gy
(%) | 32.6±32.1 | 24.0±30.3 | 0.001 |
| | V40Gy
(%) | 19.8±26.9 | 6.5±11.8 | 0.002 |
| Level III | DMean
(Gy) | 8.0±8.7 | 6.3±6.5 | 0.025 |
| | DMax
(Gy) | 24.0±18.2 | 19.1±17.1 | 0.003 |
| | V25Gy
(%) | 11.7±20.6 | 5.1±11.9 | 0.005 |
| | V40Gy
(%) | 5.2±11.2 | 0.6±2.2 | 0.018 |
Thus, target conformity was better for VMAT than the
3D-CRT; the incidental axillary dose with VMAT was lower than that
with 3D-CRT, despite non-imposition of an axillary dose-volume
constraint. The moderate-to-high doses delivered to the OARs were
lower with VMAT than 3D-CRT, while the low dose and ID to the body
were higher with VMAT than 3D-CRT.
Discussion
Radiation-related side effects in breast cancer
patients vary. One of the most common is ipsilateral arm edema
(3-7).
In the After Mapping of the Axilla: Radiotherapy Or Surgery
(AMAROS) study, 15% of patients showed clinical signs of lymphedema
1 year after axillary RT without ALND, 14% at 3 years, and 11% at 5
years (6). Interruption of the
axillary lymphatic system due to breast cancer treatment results in
BCRL, which has a negative effect on quality of life in breast
cancer patient (26-28).
We did not impose a dose-volume constraint on the
axilla to ensure similar conditions between VMAT and 3D-CRT.
Nevertheless, we found that VMAT significantly lowered the
incidental axillary radiation dose and, at the same time, increased
the target conformity compared with 3D-CRT. During the VMAT
optimization process, an option of normal tissue objective and the
ring structure would also have been helpful to reduce axillary
irradiation and improve target conformity (29,30). The
better conformity reduces unnecessary radiation doses to tissues
other than targets, thereby effectively reducing RT-related side
effects, including lymphedema (31,32).
A few studies have reported on reducing incidental
axillary irradiation in breast cancer patients using several RT
techniques. According to Kataria et al, who did not impose a
dose-volume constraint on the axilla, as in the present study, when
comparing IMRT (two tangential semi-opposed beams with gantry
angles of 130-145 and 305-320 for the medial and lateral fields,
respectively) with 3D-CRT (tangential beams with the same angles
and orientations as IMRT), the incidental axillary irradiation with
IMRT was lower than with 3D-CRT at levels I and II (19). Zhang et al also imposed no
constraint on the axilla and compared between conformal techniques
such as simplified-IMRT (s-IMRT; five to seven beams) with forward
IMRT (for-IMRT; two tangential opposite beams with the
field-in-field technique) (33). The
unintended dose to the axilla, especially level I, was lower with
s-IMRT than for-IMRT. The mean V40Gy, V45Gy,
and V47.5Gy of axillary levels II and III were very low
in both conformal techniques, and the results were similar to the
VMAT plans in the present study. Lee et al showed that IMRT
(seven fields with a skin-sparing technique) delivered
significantly less incidental axilla radiation than 3D-CRT
(parallel-opposite tangential fields with the field-in-field
technique), although they did not report whether axilla constraint
was considered during IMRT (34). To
our knowledge, some studies have compared incidental axillary
irradiation with IMRT, but none discussed incidental axillary
irradiation with VMAT. According to the aforementioned studies and
the present study, even if there is no constraint on the axillary
region, it is possible to reduce the incidentally irradiated dose
to the axilla by using advanced RT techniques, especially with
VMAT.
However, this incidental or unintended axillary
irradiation may not always be harmful. Although it comes short of a
therapeutic dose, incidental irradiation of the axilla from
tangential fields may exert some effects on controlling axillary
occult disease (35). Krasin et
al stated that treatment with tangential fields using
three-dimensional techniques still had a role in controlling
subclinical disease of axilla, despite a significant incidental
dose to the axilla (36). Fisher
et al reported that in early breast cancer patients, the
axillary recurrence rate following postoperative breast RT was 4.5%
compared with 7.2% in the surgery alone group (37). Further studies are necessary to
determine whether reducing incidental axillary irradiation with
advanced RT techniques, including VMAT, will have a negative impact
on regional axillary recurrence.
There is a concern that patients treated with the
VMAT technique are exposed to a higher ID to the body, unwanted
spinal cord irradiation and higher low-level doses to other OARs
such as lung and heart, compared with 3D-CRT. Because patients with
early-stage breast cancer have a relatively long life expectancy,
the higher ID to the body and low-dose bath of normal organs has
been criticized due to the potential risk of secondary cancer
(38,39). However, there is no evidence that
this has a role in carcinogenesis (40), and even if secondary cancers develop
from RT, the attributable mortality rate was estimated to be only
1~2% after 10 years (41). Although
malignant potential risks are suspected low, it should be noted
that other clinical side effects such as symptomatic lung injury or
cardiac events can be caused by low radiation doses (42,43).
Therefore, the risk should be balanced against the apparent
benefits of decreasing the side effects.
In conclusion, this study showed that VMAT, even
without dose-volume constraint on the axilla, significantly reduces
incidental axillary irradiation compared with 3D-CRT. Clinical
studies should be followed to prove that this dosimetric change
with advanced RT techniques can reduce BCRL while maintaining
efficacy in disease control.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Soonchunhyang
University Research Fund.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
IYJ analyzed and interpreted patient data, and wrote
the manuscript. ESK analyzed and interpreted data. WCK and CKM
acquired and analyzed data. SGY designed the study and critically
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by The Institutional Review
Board of Soonchunhyang University Cheonan Hospital (approval no.
SCHCA 2019-11-015-001) (Cheonan, Republic of Korea). Due to the
retrospective nature, the requirement to obtain informed consent of
the patients was waived by the board. All the procedures in this
study were in accordance with the Declaration of Helsinki. Patients
who participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dominici LS, Morrow M, Mittendorf E,
Bellon J and King TA: Trends and controversies in multidisciplinary
care of the patient with breast cancer. Curr Probl Surg.
53:559–595. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yeo SG, Kim J, Kwak GH, Kim JY, Park K,
Kim ES and Han S: Accelerated partial breast irradiation using
multicatheter brachytherapy for select early-stage breast cancer:
Local control and toxicity. Radiat Oncol. 5(56)2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Martel S, Maurer C, Lambertini M, Ponde N
and De Azambuja E: Breast cancer treatment-induced cardiotoxicity.
Expert Opin Drug Saf. 16:1021–1038. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Meattini I, Guenzi M, Fozza A, Vidali C,
Rovea P, Meacci F and Livi L: Overview on cardiac, pulmonary and
cutaneous toxicity in patients treated with adjuvant radiotherapy
for breast cancer. Breast Cancer. 24:52–62. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Petersen C and Würschmidt F: Late toxicity
of radiotherapy: A problem or a challenge for the radiation
oncologist? Breast Care (Basel). 6:369–374. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Donker M, van Tienhoven G, Straver ME,
Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH,
Klinkenbijl JH, Orzalesi L, et al: Radiotherapy or surgery of the
axilla after a positive sentinel node in breast cancer (EORTC
10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3
non-inferiority trial. Lancet Oncol. 15:1303–1310. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Warren LE, Miller CL, Horick N, Skolny MN,
Jammallo LS, Sadek BT, Shenouda MN, O'Toole JA, MacDonald SM,
Specht MC and Taghian AG: The impact of radiation therapy on the
risk of lymphedema after treatment for breast cancer: A prospective
cohort study. Int J Radiat Oncol Biol Phys. 88:565–571.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rockson SG: Lymphedema after breast cancer
treatment. N Engl J Med. 379:1937–1944. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kilbreath SL, Refshauge KM, Beith JM, Ward
LC, Ung OA, Dylke ES, French JR, Yee J, Koelmeyer L and Gaitatzis
K: Risk factors for lymphoedema in women with breast cancer: A
large prospective cohort. Breast. 28:29–36. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gross JP, Sachdev S, Helenowski IB, Lipps
D, Hayes JP, Donnelly ED and Strauss JB: Radiation therapy field
design and lymphedema risk after regional nodal irradiation for
breast cancer. Int J Radiat Oncol Biol Phys. 102:71–78.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gera R, Kasem A and Mokbel K: Can complete
axillary node dissection be safely omitted in patients with early
breast cancer when the sentinel node biopsy is positive for
malignancy? An update for clinical practice. In Vivo. 32:1301–1307.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kestin LL, Sharpe MB, Frazier RC, Vicini
FA, Yan D, Matter RC, Martinez AA and Wong JW: Intensity modulation
to improve dose uniformity with tangential breast radiotherapy:
Initial clinical experience. Int J Radiat Oncol Biol Phys.
48:1559–1568. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jo IY, Kim SW and Son SH: Dosimetric
evaluation of the skin-sparing effects of 3-dimensional conformal
radiotherapy and intensity-modulated radiotherapy for left breast
cancer. Oncotarget. 8:3059–3063. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mansouri S, Naim A, Glaria L and Marsiglia
H: Dosimetric evaluation of 3-D conformal and intensity-modulated
radiotherapy for breast cancer after conservative surgery. Asian
Pac J Cancer Prev. 15:4727–4732. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vugts G, Maaskant-Braat AJ, Voogd AC, van
Riet YE, Luiten EJ, Rutgers EJ, Rutten HJ, Roumen RM and
Nieuwenhuijzen GA: Repeat sentinel node biopsy should be considered
in patients with locally recurrent breast cancer. Breast Cancer Res
Treat. 153:549–556. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Giuliano AE, Ballman KV, McCall L, Beitsch
PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW,
Blumencranz PW, et al: Effect of axillary dissection vs no axillary
dissection on 10-year overall survival among women with invasive
breast cancer and sentinel node metastasis: The ACOSOG Z0011
(Alliance) randomized clinical trial. JAMA. 318:918–926.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Galimberti V, Cole BF, Viale G, Veronesi
P, Vicini E, Intra M, Mazzarol G, Massarut S, Zgajnar J, Taffurelli
M, et al: Axillary dissection versus no axillary dissection in
patients with breast cancer and sentinel-node micrometastases
(IBCSG 23-01): 10-year follow-up of a randomised, controlled phase
3 trial. Lancet Oncol. 19:1385–1393. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dogan N, Cuttino L, Lloyd R, Bump EA and
Arthur DW: Optimized dose coverage of regional lymph nodes in
breast cancer: The role of intensity-modulated radiotherapy. Int J
Radiat Oncol Biol Phys. 68:1238–1250. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kataria T, Bisht SS, Gupta D, Goyal S,
Jassal K, Abhishek A, Sharma K, Pareek P, Kumar V, Jain S, et al:
Incidental radiation to axilla in early breast cancer treated with
intensity modulated tangents and comparison with conventional and
3D conformal tangents. Breast. 22:1125–1129. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Henke G, Knauer M, Ribi K, Hayoz S, Gérard
MA, Ruhstaller T, Zwahlen DR, Muenst S, Ackerknecht M, Hawle H, et
al: Tailored axillary surgery with or without axillary lymph node
dissection followed by radiotherapy in patients with clinically
node-positive breast cancer (TAXIS): Study protocol for a
multicenter, randomized phase-III trial. Trials.
19(667)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rastogi K, Sharma S, Gupta S, Agarwal N,
Bhaskar S and Jain S: Dosimetric comparison of IMRT versus 3DCRT
for post-mastectomy chest wall irradiation. Radiat Oncol J.
36:71–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Balaji K, Subramanian B, Yadav P, Anu
Radha C and Ramasubramanian V: Radiation therapy for breast cancer.
Med Dosim. 41:253–257. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Teoh M, Clark CH, Wood K, Whitaker S and
Nisbet A: Volumetric modulated arc therapy: A review of current
literature and clinical use in practice. Br J Radiol. 84:967–996.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gentile MS, Usman AA, Neuschler EI,
Sathiaseelan V, Hayes JP and Small W Jr: Contouring guidelines for
the axillary lymph nodes for the delivery of radiation therapy in
breast cancer: Evaluation of the RTOG breast cancer atlas. Int J
Radiat Oncol Biol Phys. 93:257–265. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Badakhshi H, Kaul D, Nadobny J, Wille B,
Sehouli J and Budach V: Image-guided volumetric modulated arc
therapy for breast cancer: A feasibility study and plan comparison
with three-dimensional conformal and intensity-modulated
radiotherapy. Br J Radiol. 86(20130515)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Penha TR, Botter B, Heuts EM, Voogd AC,
von Meyenfeldt MF and van der Hulst RR: Quality of life in patients
with breast cancer-related lymphedema and reconstructive breast
surgery. J Reconstr Microsurg. 32:484–490. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ganz PA: The quality of life after breast
cancer-solving the problem of lymphedema. N Engl J Med.
340:383–385. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cornelissen AJM, Kool M, Keuter XHA, Heuts
EM, Piatkowski de Grzymala AA, van der Hulst RRWJ and Qiu SS:
Quality of life questionnaires in breast cancer-related lymphedema
patients: Review of the literature. Lymphat Res Biol. 16:134–139.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fadda G, Massazza G, Zucca S, Durzu S,
Meleddu G, Possanzini M and Farace P: Quasi-VMAT in high-grade
glioma radiation therapy. Strahlenther Onkol. 189:367–371.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jolly D, Alahakone D and Meyer J: A
rapidArc planning strategy for prostate with simultaneous
integrated boost. J Appl Clin Med Phys. 12(3320)2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rudat V, Alaradi AA, Mohamed A, Ai-Yahya K
and Altuwaijri S: Tangential beam IMRT versus tangential beam
3D-CRT of the chest wall in postmastectomy breast cancer patients:
A dosimetric comparison. Radiat Oncol. 6(26)2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xie X, Ouyang S, Wang H, Yang W, Jin H, Hu
B and Shen L: Dosimetric comparison of left-sided whole breast
irradiation with 3D-CRT, IP-IMRT and hybrid IMRT. Oncol Rep.
31:2195–2205. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang L, Yang ZZ, Chen XX, Tuan J, Ma JL,
Mei X, Yu XL, Zhou ZR, Shao ZM, Liu GY and Guo XM: Dose coverage of
axillary level I-III areas during whole breast irradiation with
simplified intensity modulated radiation therapy in early stage
breast cancer patients. Oncotarget. 6:18183–18191. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee J, Kim SW and Son SH: Dosimetric
evaluation of incidental irradiation to the axilla during whole
breast radiotherapy for patients with left-sided early breast
cancer in the IMRT era. Medicine (Baltimore).
95(e4036)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Reed DR, Lindsley SK, Mann GN,
Austin-Seymour M, Korssjoen T, Anderson BO and Moe R: Axillary
lymph node dose with tangential breast irradiation. Int J Radiat
Oncol Biol Phys. 61:358–364. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Krasin M, McCall A, King S, Olson M and
Emami B: Evaluation of a standard breast tangent technique: A
dose-volume analysis of tangential irradiation using
three-dimensional tools. Int J Radiat Oncol Biol Phys. 47:327–333.
2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fisher B, Redmond C, Poisson R, Margolese
R, Wolmark N, Wickerham L, Fisher E, Deutsch M, Caplan R, Pilch Y,
et al: Eight-year results of a randomized clinical trial comparing
total mastectomy and lumpectomy with or without irradiation in the
treatment of breast cancer. N Engl J Med. 320:822–828.
1989.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hall EJ and Wuu CS: Radiation-induced
second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol
Biol Phys. 56:83–88. 2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Han EY, Paudel N, Sung J, Yoon M, Chung WK
and Kim DW: Estimation of the risk of secondary malignancy arising
from whole-breast irradiation: Comparison of five radiotherapy
modalities, including TomoHDA. Oncotarget. 7:22960–22969.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Alongi F, Giaj-Levra N, Fiorentino A,
Mazzola R, Fersino S, Ricchetti F and Ruggieri R: Low-dose bath
with volumetric modulated arc therapy in breast cancer: ‘Much ado
about nothing?’. Tumori. 102:335–336. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Darby SC, McGale P, Taylor CW and Peto R:
Long-term mortality from heart disease and lung cancer after
radiotherapy for early breast cancer: Prospective cohort study of
about 300,000 women in US SEER cancer registries. Lancet Oncol.
6:557–565. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen J, Hong J, Zou X, Lv W, Guo F, Hong H
and Zhang W: Association between absolute volumes of lung spared
from low-dose irradiation and radiation-induced lung injury after
intensity-modulated radiotherapy in lung cancer: A retrospective
analysis. J Radiat Res. 56:883–888. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ni L, Koshy M, Connell P, Pitroda S,
Golden DW, Al-Hallaq H, Hubert G, Kauffman G, McCall A and Malik R:
Heart V5 predicts cardiac events in unresectable lung cancer
patients undergoing chemoradiation. J Thorac Dis. 11:2229–2239.
2019.PubMed/NCBI View Article : Google Scholar
|