Introduction
Over the world, ovary cancer is the 5th cause of
death by cancer in women and the first cause of death among
gynecological cancers. Despite a good response to platin-based
first line treatment, >70% of patients will progress within 2
years following diagnosis. PARP inhibitors are drugs that inhibit
DNA repair, with a maximum efficiency in homologous repair
deficient cells. The complete blockage of PARP enzymes in
homologous repair deficient cells triggers cell death by a
phenomenon called synthetic lethality. The development of PARP
inhibitors has revolutionized the management of BRCA mutated
ovary tumors. Indeed, the first available PARP inhibitor, Olaparib,
showed dramatic increase of progression free survival at metastatic
stage, and as a maintenance treatment for newly diagnosed patients
(1,2). In parallel, other PARP inhibitors,
Niraparib and Rucaparib, also showed efficiency on BRCA
mutated tumors and also on BRCA wild-type tumors for
Niraparib (3,4). As the efficiency of PARP inhibitors was
observed in tumors presenting a complete or partial response to
platin salt, its therapeutic use is limited to these platin
sensitive tumors. Cerebral progression of ovary cancer is a rare
event with a dark prognosis, death happening within a few weeks.
Here, we report the case of a BRCA mutated patient who has
lived >1 year with carcinomatous meningitis thanks to Olaparib
treatment.
Case report
A 54-year-old woman was diagnosed with ovarian
cancer in 2010. After an initial treatment with neoadjuvant
chemotherapy (Paclitaxel, Carboplatin), she underwent optimal
debulking surgery. In 2012, she presented a first peritoneal
relapse and received numerous lines of chemotherapy until August
2014. Oxaliplatin was the last platinum salt administered, since
the patient developed a Carboplatin allergy. In the absence of any
detectable disease, the treatment was stopped and clinical survey
was initiated.
Six months later, patient complained of headaches,
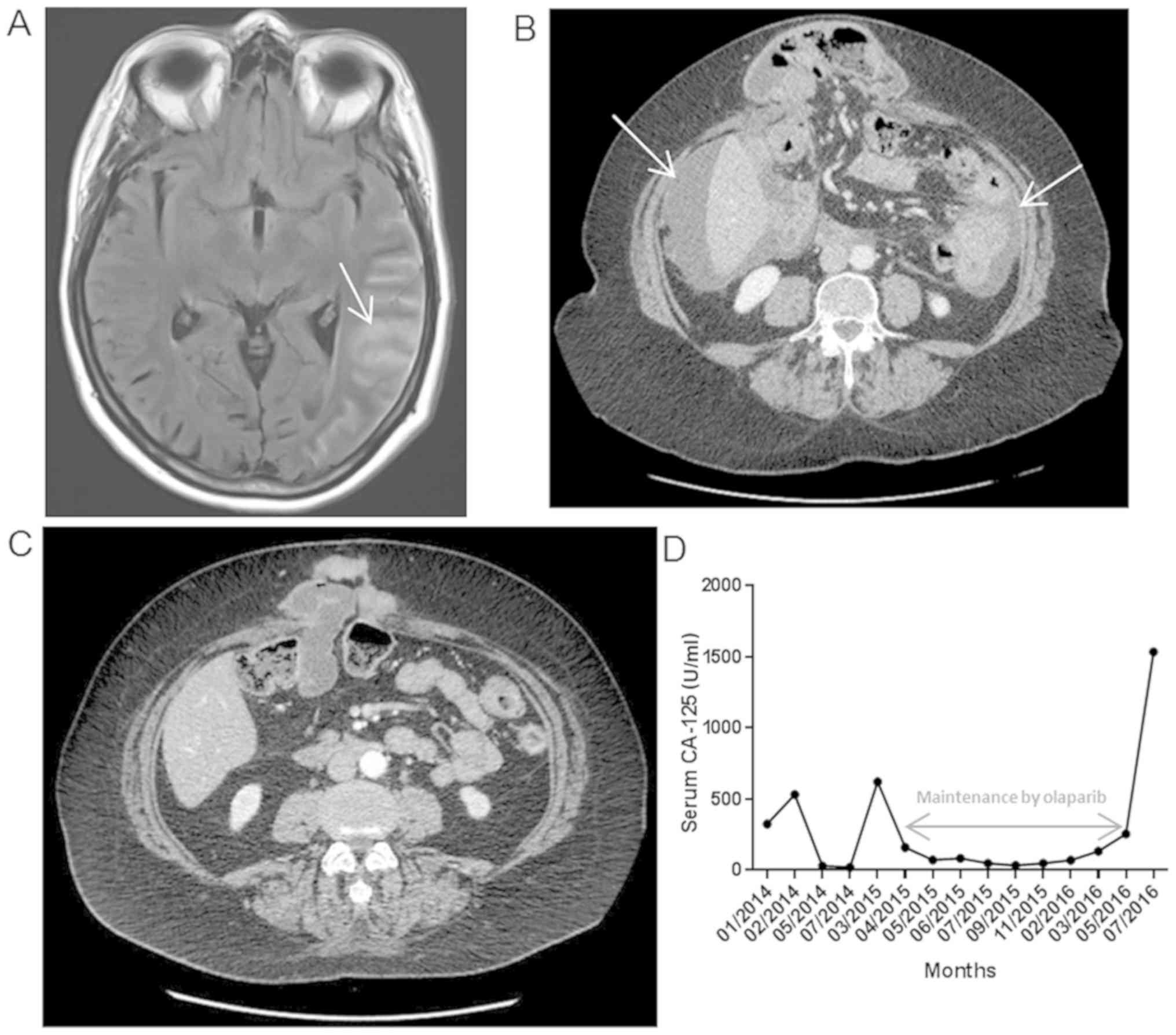
and magnetic resonance imaging (MRI) revealed nodular meningitis
(Fig. 1A). Lumbar puncture confirmed
carcinomatous cell presence. Additionally, computed tomography (CT)
scan showed peritoneal progression (Fig.
1B) without other lesions.
Due to her young age and to the pathogenic
BRCA2 mutation (c.7617+1G>T) identified in her family, a
germline genetic test was initiated and revealed that patient
carried the familial pathogenic BRCA2 mutation. Upon
confirmation of tumor cells presence by a pathologist, lumbar
puncture DNA was extracted using the Maxwell 16 FFPE Plus LEV DNA
purification kit (Promega Corporation) according to manufacturer's
protocol. Corresponding normal DNA was extracted from 500 µl EDTA
blood samples with the Maxwell 16 Blood DNA Purification system
(Promega Corp.) according to manufacturer's instructions. DNA
quality was assessed by spectrophotometry with absorbance at 230,
260 and 280 nm. DNA was quantified using a fluorimetric assay with
a Qubit device.
Genomic DNA from meningeal cells was fragmented with
a Covaris device to obtain fragments around 180-200 bp.
Subsequently, libraries were constructed and captured by using
SureSelect Human All Exon v5 kit (Agilent Technologies, Inc.)
following manufacturer's protocol. Paired-end (2x151 bases)
sequencing was performed on a NextSeq500 device (Illumina, Inc.).
Obtained sequences were aligned and annotated with the human Hg19
genome based on SureSelect Human All exon v5 manifest by using BWA
and GATK algorithms. Only sequences with a read depth of 10X and a
mutation allele frequency superior to 5% were analyzed. Exome
analysis on meningeal cells confirmed the presence of the
pathogenic BRCA2 mutation. Moreover, the tumor homozygous
pathogenic BRCA2 mutation status (mutated allele frequency
of 98%) suggested a loss of wild-type allele in tumor cells. Whole
brain radiotherapy was carried out and Cisplatin monotherapy
treatment was administrated from March to June 2015. CT scan
confirmed a positive response to chemotherapy (Fig. 1C) and Olaparib treatment was
proposed. This treatment allowed a 14 month disease control
(Fig. 1D) with a good quality of
life. Standard dose was administered without any modification due
to excellent tolerance.
On August 2016, meningeal and peritoneal progression
was diagnosed. Platinum based chemotherapy was tried without
improvement. Patient died on September 2016.
Discussion
The most frequent metastasis site of ovarian cancer
is the peritoneum. Meningeal and cerebral metastases seem to be a
rare event. Jernigan et al (5) have found an incidence of 2.58% of
central nervous system metastasis in their series. Cerebral
metastasis incidence seems to be correlated to BRCA
mutation. Sixty seven percent of patients with CNS metastasis had a
familial history of hereditary breast and ovarian cancer (5). Carcinomatous meningitis leads to poor
prognosis and treatments are limited. The FRENCH ANOCEF group has
published recently online guidelines on the treatment of
carcinomatous meningitis (6).
PARP inhibitors are described as a major therapeutic
advance in ovarian cancer (1,7). The
first drug of this pharmaceutical class is Olaparib. Olaparib is
only indicated in platinum sensitive recurrent ovarian cancer with
BRCA mutation. Nevertheless, several other agents such as
Rucaparib or Niraparib are in development and nearly available
(2,4). These molecules seem to be active in
tumors with homologous recombination deficiency, independently of
BRCA status (2,4). Studies showed good tolerance and the
most described side effect is hematological toxicity such as
anemia. Meningeal efficacy has not been described to date. This
case report shows long-term survival despite CNS metastases (20
months with 14 months under Olaparib treatment) with a good
clinical control. In the literature, the median survival without
treatment ranges between four to six weeks and 15.9 weeks with
intrathecal treatment (6), which was
not used in our case. Therefore it seems that Olaparib, a small
molecule, is able to cross the leptomeningeal barrier.
This is the first report on the efficacy of a PARP
inhibitor on meningeal disease of a BRCA mutated ovarian
cancer. This case report also illustrates that exome or Next
Generation Sequencing (NGS) analysis can be performed on a small
amount of cells with a good overlap with germinal BRCA
abnormalities. Moreover, this kind of analysis revealed its
complementarity with germline analysis, as the somatic second hit
could be observed. In this case report, the strong efficiency of
Olaparib could be explained by the homozygous status of the
pathogenic BRCA2 mutation in cancer cells.
In conclusion, carcinomatous meningitis is a rare
event with a poor prognosis in ovarian cancer. We illustrated a
good response with Olaparib, the first PARP inhibitor available in
clinical practice, suggesting efficient meningeal diffusion. NGS
could be performed in a small number of cells obtained from lumbar
puncture, with a good overlap with germ line mutations, allowing
complementary information on tumor status and pathogenic mutation
characterization. This first encouraging result should be confirmed
in clinical practice. Results should be confirmed using other PARP
inhibitors.
Acknowledgements
The authors would like to thank Dr Isabel Gregoire
(Georges-François Leclerc Anticancer Center, Dijon, France) for
manuscript editing.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are not publicly available due to presence of
identifying genetic information but are available from the
corresponding author on reasonable request.
Authors' contributions
LF, GT and LBL treated the patient. RB performed
genetic analysis. LF, RB, and LBL wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The paper was read and validated by the local Ethics
Committee of the Centre Georges-François Leclerc (Dijon,
France).
Patient consent for publication
The local Ethics Committee of the Centre
Georges-François Leclerc waives the necessity of consent for
publication by the patient because the patient has died, and the
content of the publication does not provide any identifying data on
the patient and the establishment does not wish to disturb the
patient's family in these unfortunate circumstances.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott C, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
platinum-sensitive relapsed ovarian cancer. N Engl J Med.
366:1382–1392. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mirza MR, Monk BJ, Herrstedt J, Oza AM,
Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I,
et al: Niraparib maintenance therapy in platinum-sensitive,
recurrent ovarian cancer. N Engl J Med. 375:2154–2164.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Coleman RL, Oza AM, Lorusso D, Aghajanian
C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G,
et al: Rucaparib maintenance treatment for recurrent ovarian
carcinoma after response to platinum therapy (ARIEL3): A
randomised, double-blind, placebo-controlled, phase 3 trial.
Lancet. 390:1949–1961. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jernigan AM, Mahdi H and Rose PG:
Epithelial ovarian cancer metastatic to the central nervous system
and a family history concerning for hereditary breast and ovarian
cancer-a potential relationship. Int J Gynecol Cancer.
25:1232–1238. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grossman SA, Finkelstein DM, Ruckdeschel
JC, Trump DL, Moynihan T and Ettinger DS: Randomized prospective
comparison of intraventricular methotrexate and thiotepa in
patients with previously untreated neoplastic meningitis. Eastern
cooperative oncology group. J Clin Oncol. 11:561–569.
1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pujade-Lauraine E, Ledermann JA, Selle F,
Gebski V, Penson RT, Oza AM, Korach J, Huzarski T, Poveda A,
Pignata S, et al: Olaparib tablets as maintenance therapy in
patients with platinum-sensitive, relapsed ovarian cancer and a
BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised,
placebo-controlled, phase 3 trial. Lancet Oncol. 18:1274–1284.
2017.PubMed/NCBI View Article : Google Scholar
|