Introduction
Gastric cancer (GC) is the fourth most common
malignancy and the second leading cause of cancer-related mortality
worldwide (1). Following surgery or
late during the clinical course, GC frequently spreads to the
regional lymph nodes, liver, peritoneum and lungs (2), but rarely disseminates to the bones.
Thus, the incidence of bone metastasis (BM) from GC is only
0.9-10.5% (3-7),
although the reported frequency of BM in GC patients is 13.4% in
autopsy series and increases up to 45.3% in BM screening studies
(8,9). Therefore, the incidence of
asymptomatic BM may be underestimated, and the rate of BM in
clinical cases may be markedly higher compared with the reported
incidence.
The bone is a frequent metastatic site of breast,
prostate and lung cancers, and the presence of BM typically
indicates a poor prognosis (10-12).
The incidence and prevalence of BM have increased proportionally
with the aging population. Skeletal-related events (SREs),
including pathological fractures, spinal cord compression and
hypercalcemia, are the result of BM, may cause reduced physical
function and quality of life (QoL), and require treatment with
radiotherapy or surgery (13). A
multidisciplinary approach using modalities such as radiotherapy,
surgery and various medical treatments, including chemotherapy,
hormone therapy and bone-modifying agents (BMA), is therefore
warranted for patients with BM (10).
The presence of BM in metastatic GC has been
reported as an independent poor prognostic factor, and GC patients
with BMs exhibited the poorest median survival time compared with
patients with metastases to other sites, including the chest,
liver, or abdomen (14). However,
to date, there have been only few studies examining the clinical
presentation and prognosis of BM from GC.
The aim of the present study was to retrospectively
investigate the clinicopathological characteristics, treatment
outcomes, prognostic factors and SREs in GC patients with BM who
underwent treatment at our institution.
Patients and methods
Study design and patients
The medical records of 60 GC patients with BM who
were treated at the Osaka International Cancer Institute between
January 2005 and December 2017 were anonymized and retrospectively
reviewed following BM diagnosis by physicians, radiologists and
orthopedic surgeons. The diagnosis of BM was based on clinical
symptoms and signs, as well as radiographic imaging studies, such
as X-ray, computed tomography (CT), magnetic resonance imaging
(MRI), bone scintigraphy, and/or fluorodeoxyglucose-positron
emission tomography (FDG-PET)/CT. Pathological fractures, spinal
cord compression and hypercalcemia were defined as SREs, as were
radiotherapy and orthopedic surgery that were performed for BM. The
protocol of the present study was approved by the Institutional
Review Board of the Osaka International Cancer Institute.
Data collection
The data collected for the present study were as
follows: Patient-related characteristics, including age, sex and
Eastern Cooperative Oncology Group performance status (ECOG PS)
score; tumor-related characteristics, including histology,
differentiation, stage, resection of primary site, visceral or
brain metastasis, and levels of serum carcinoembryonic antigen
(CEA), carbohydrate antigen 19-9 (CA19-9), C-reactive protein
(CRP), lactate dehydrogenase (LDH), albumin and alkaline
phosphatase (ALP); BM-related characteristics, including clinical
symptoms, the presence of BM at the initial diagnosis of GC,
number, location, type, treatment received (chemotherapy before and
after the BM diagnosis, and BMA) and SREs; follow-up period; and
outcome at last follow-up.
Follow-up and outcomes
The median follow-up period for all patients was 5
months (range, 0-43 months). Overall survival (OS) was defined as
the time from the date of diagnosis of BM to the date of death from
any cause or the date of the last follow-up visit. SRE-free
survival (SRS) was defined as the time from the date of BM
diagnosis to the date of the first SRE occurrence or the last
follow-up visit.
Statistical analysis
The Kaplan-Meier method was used to calculate OS and
SRS. The impact of prognostic factors on OS and SRS was first
assessed using the log-rank test in univariate analysis, and
multivariate analysis was then performed using the Cox proportional
hazard model. The significant variables in the univariate model
except for intervening variables were used to build the
multivariate model of survival. P<0.05 was considered to
indicate statistically significant differences. Statistical
analyses were performed using EZR software version 1.35 (Saitama
Medical Center, Jichi Medical University, Saitama, Japan), which is
a graphical user interface for R (The R Foundation for Statistical
Computing, Vienna, Austria).
Results
Patient, tumor and BM-related
characteristics
The patient, tumor and BM-related characteristics of
the 60 cases are summarized in Table
I. A total of 33 patients (55%) were male. The median age at
diagnosis of BM was 63.5 years (range, 26-83 years). The ECOG PS
score was 0-2 in 42 patients (70%) and 3-4 in 18 patients (30%).
Tumor histology of the primary lesion was adenocarcinoma in 56
patients (93.3%) and scirrhous carcinoma in 4 patients (6.7%). A
total of 14 patients (23.3%) had well- to moderately differentiated
tumors, and 46 (76.7%) had poorly differentiated tumors. A total of
4 patients (6.7%) had stage I, 6 (10%) had stage II, 11 (18.3%) had
stage III, 36 (60%) had stage IV, and 3 (5%) had unknown-stage
disease at initial diagnosis. In addition, 26 patients (43.3%)
underwent surgery for the primary tumor. Visceral or brain
metastasis coexisted with BM at the time of BM diagnosis in 37
patients (61.7%). The proportion of patients with elevated levels
of serum CEA (>5 ng/ml), CA19-9 (>37 U/ml), CRP (>0.3
mg/dl), LDH (>250 U/l) and ALP (>350 U/l) at the time of BM
diagnosis were 62.7, 60, 44.1, 38.3 and 63.3%, respectively.
Decreased serum albumin level (≤3.7 g/dl) was detected in 49.2% of
the patients.
 | Table IPatient-, tumor- and BM-related
characteristics and univariate analysis of prognostic factors for
OS (n=60). |
Table I
Patient-, tumor- and BM-related
characteristics and univariate analysis of prognostic factors for
OS (n=60).
| Factors | N (%) | Median OS
(months) | P-value |
|---|
| Age (years) | | | 0.426 |
|
≤60 | 26 (43.3) | 10 | |
|
>60 | 34 (56.7) | 8 | |
| ECOG PS score | | | <0.001 |
|
0-2 | 42(70) | 12 | |
|
3-4 | 18(30) | 3 | |
| Resection of primary
site | | | 0.251 |
|
Present | 26 (43.3) | 8 | |
|
Absent | 34 (56.7) | 11 | |
| Visceral or
brain | | | 0.009 |
| metastasis | | | |
|
Present | 37 (61.7) | 8 | |
|
Absent | 23 (38.3) | 14 | |
| CEA (ng/ml) | | | 0.341 |
|
≤5 | 22 (37.3) | 12 | |
|
>5 | 37 (62.7) | 9 | |
|
N/A | 1 | | |
| CA19-9 (U/ml) | | | 0.329 |
|
≤37 | 24(40) | 10 | |
|
>37 | 36(60) | 9 | |
| CRP (mg/dl) | | | 0.041 |
|
≤0.3 | 33 (55.9) | 12 | |
|
>0.3 | 26 (44.1) | 8 | |
|
N/A | 1 | | |
| LDH (U/l) | | | 0.005 |
|
≤250 | 37 (61.7) | 12 | |
|
>250 | 23 (38.3) | 5 | |
| Albumin (g/dl) | | | 0.009 |
|
≤3.7 | 29 (49.2) | 5 | |
|
>3.7 | 30 (50.8) | 16 | |
|
N/A | 1 | | |
| ALP (U/l) | | | 0.574 |
|
≤350 | 22 (36.7) | 9 | |
|
>350 | 38 (63.3) | 10 | |
| BM at GC
diagnosis | | | 0.380 |
|
Present | 19 (31.7) | 12 | |
|
Absent | 41 (68.3) | 8 | |
| Number of BM | | | 0.130 |
|
Solitary | 10 (16.7) | 9 | |
|
Multiple | 50 (83.3) | 10 | |
| Chemotherapy before
BM diagnosis | | | 0.011 |
|
Present | 32 (53.3) | 5 | |
|
Absent | 28 (46.7) | 14 | |
| Chemotherapy after
BM diagnosis | | | <0.001 |
|
Present | 46 (76.7) | 12 | |
|
Absent | 14 (23.3) | 2 | |
| Use of BMA | | | 0.152 |
|
Present | 34 (56.7) | 11 | |
|
Absent | 26 (43.3) | 5 | |
| SRE at BM
diagnosis | | | <0.001 |
|
Present | 23 (38.3) | 4 | |
|
Absent | 37 (61.7) | 12 | |
BM diagnosis was confirmed with CT, MRI, bone
scintigraphy and FDG-PET/CT in 32, 36, 20 and 25 patients,
respectively. Clinical symptoms, such as pain and paralysis, were
present in 34 patients (56.7%) at diagnosis of BM, whereas 26
patients (43.3%) were asymptomatic. A total of 19 patients (31.7%)
had already developed BM at GC diagnosis. Among the remaining 41
patients (68.3%) who developed BM after the diagnosis of GC, the
median interval from GC diagnosis to detection of BM was 15 months
(range, 1-126 months). A solitary BM was found in 10 patients
(16.7%) and multiple BMs were detected in 50 patients (83.3%). The
spine was the most common site for BM (49 patients, 81.7%),
followed by the pelvis (37 patients, 61.7%), ribs (24 patients,
40%) and sternum (15 patients, 25%). The lesions were osteoblastic
in 15 patients (25%), osteolytic in 24 (40%), mixed in 15 (25%),
and intertrabecular in 6 patients (10%). Chemotherapy was
administered before the diagnosis of BM in 32 patients (53.3%), and
46 patients (76.7%) received palliative chemotherapy after BM
diagnosis. BMA, such as zoledronic acid and denosumab, were
administered after diagnosis of BM to 34 patients (56.7%).
SREs
A total of 46 patients (76.7%) experienced SREs. The
most frequent SREs were radiotherapy (44 patients, 73.3%) followed
by spinal cord compression (17 patients, 28.3%), pathological
fractures (13 patients, 21.7%), orthopedic surgery (6 patients,
10%) and hypercalcemia (4 patients, 6.7%). A total of 23 patients
(38.3%) presented with SREs at the time of BM diagnosis, and the
remaining 23 patients (38.3%) developed SREs during follow-up.
Predictive factors of OS
Among the 60 GC patients with BMs, the OS rates
after the diagnosis of BM were 62.3% (6 months), 44.5% (1 year),
and 12.7% (2 years). The median OS duration after the diagnosis of
BM was 9 months (range, 0-43 months). A total of 46 patients who
experienced SREs had a median OS duration of 5 months (range, 0-36
months) after the diagnosis of SREs.
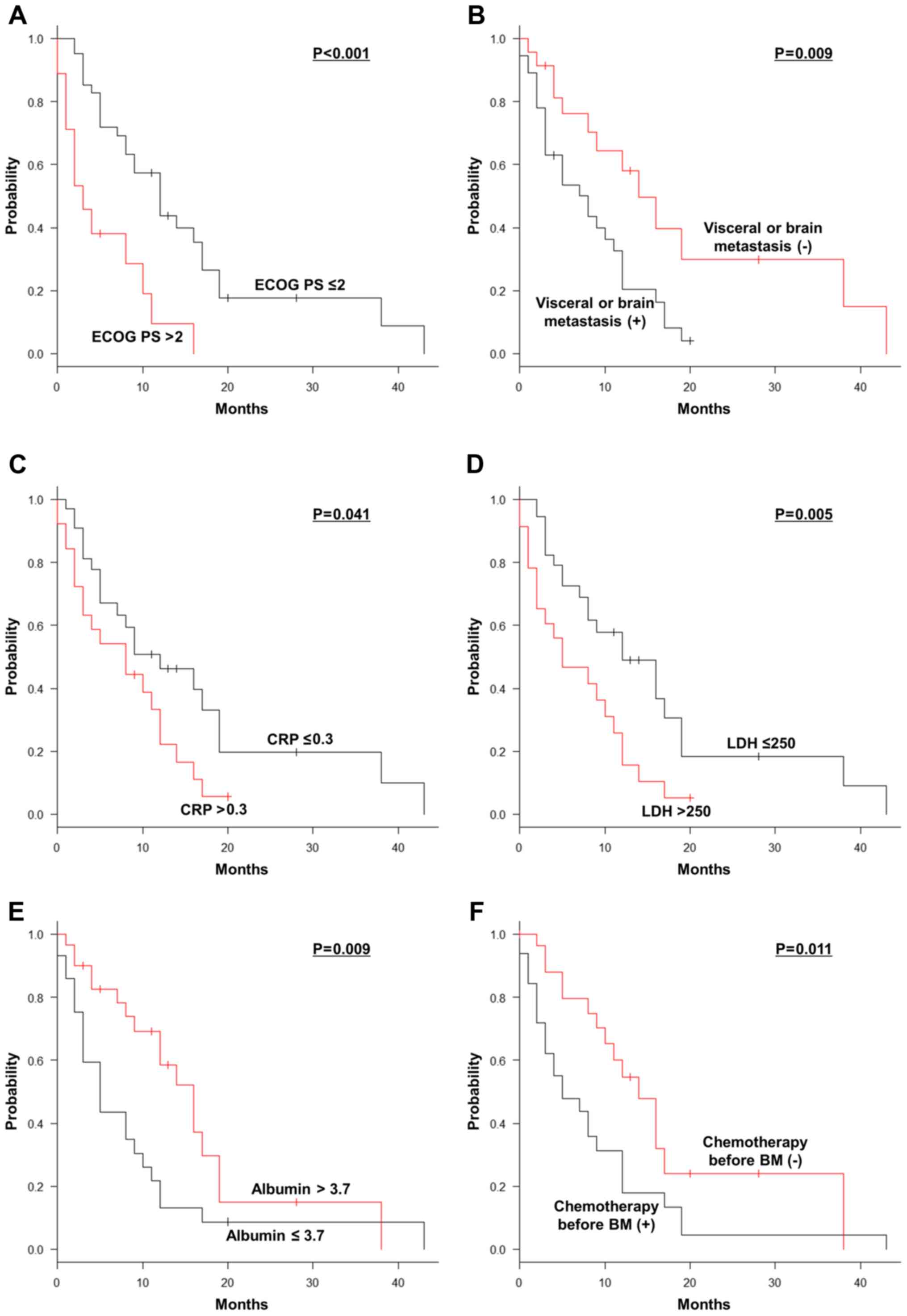
On univariate analyses, ECOG PS score
(P<0.001), visceral or brain metastasis (P=0.009), serum
levels of CRP (P=0.041), LDH (P=0.005) and albumin (P=0.009),
patients undergoing chemotherapy prior to (P=0.011) and after
(P<0.001) the diagnosis of BM and SRE at BM diagnosis
(P<0.001) were significant prognostic factors for OS after the
diagnosis of BM (Table I, Fig. 1A-H). Multivariate analyses revealed
that ECOG PS score >2 [hazard ratio (HR)=3.165; 95% confidence
interval (CI): 1.120-8.945; P=0.030], undergoing chemotherapy prior
to BM diagnosis (HR=3.802; 95% CI: 1.812-7.976; P<0.001), and
lack of chemotherapy after BM diagnosis (HR=5.897; 95% CI:
1.926-18.050; P=0.002) were independently correlated with a shorter
OS after the occurrence of BM (Table
II).
 | Table IIMultivariate analysis of prognostic
factors for OS. |
Table II
Multivariate analysis of prognostic
factors for OS.
| Factors | HR | 95% CI | P-value |
|---|
| ECOG PS score
>2 | 3.165 | 1.120-8.945 | 0.030 |
| Visceral or brain
metastasis | 1.965 | 0.950-4.065 | 0.069 |
| Chemotherapy before
BM diagnosis | 3.802 | 1.812-7.976 | <0.001 |
| No chemotherapy
after BM diagnosis | 5.897 | 1.926-18.050 | 0.002 |
| SRE at BM
diagnosis | 2.000 | 0.655-6.112 | 0.224 |
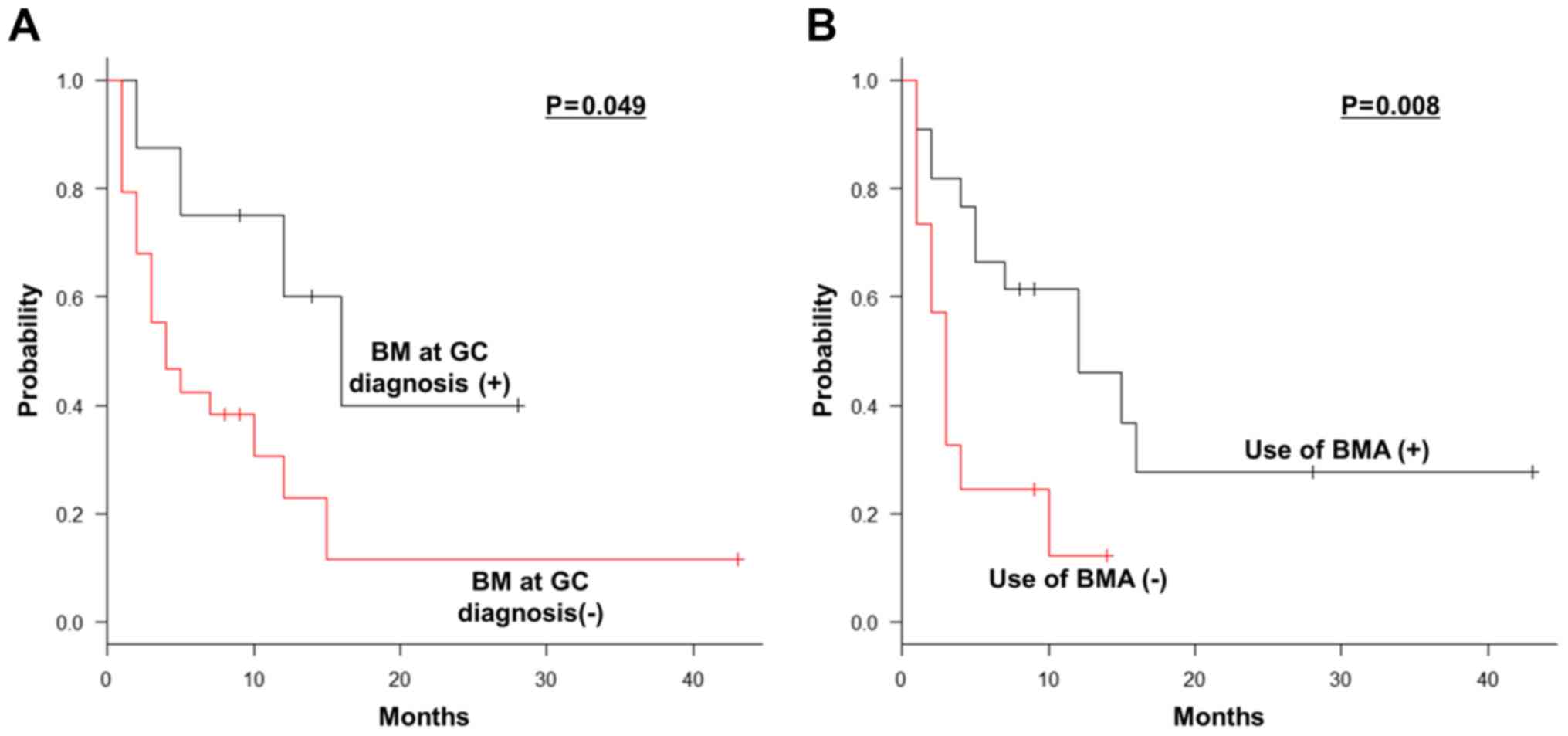
Predictive factors of SRS
Among the 37 patients without SREs at BM diagnosis,
the median SRS duration was 7 months (range, 0-43 months). On
univariate analyses, BM at GC diagnosis (P=0.049) and the use of
BMA (P=0.008) were significant prognostic factors for SRS (Table III, Fig. 2A and B). Multivariate analyses revealed that the
non-use of BMA (HR=2.868; 95% CI: 1.163-7.076; P=0.022) was the
only independent significant prognostic factor for unfavorable SRS
(Table IV).
 | Table IIIPatient-, tumor- and BM-related
characteristics and univariate analysis of prognostic factors for
SRS (n=37). |
Table III
Patient-, tumor- and BM-related
characteristics and univariate analysis of prognostic factors for
SRS (n=37).
| Factors | N (%) | Median SRS
(months) | P-value |
|---|
| Age (years) | | | 0.100 |
|
≤60 | 20 (54.1) | 4.5 | |
|
>60 | 17 (45.9) | 16 | |
| ECOG PS score | | | 0.510 |
|
0-2 | 34 (91.9) | 5 | |
|
3-4 | 3 (8.1) | 16 | |
| Resection of
primary site | | | 0.283 |
|
Present | 17 (45.9) | 7 | |
|
Absent | 20 (54.1) | 8.5 | |
| Visceral or
brain | | | 0.139 |
| metastasis | | | |
|
Present | 23 (62.2) | 5 | |
|
Absent | 14 (37.8) | 15 | |
| CEA (ng/ml) | | | 0.346 |
|
≤5 | 12 (33.3) | 12 | |
|
>5 | 24 (66.7) | 5 | |
|
N/A | 1 | | |
| CA19-9 (U/ml) | | | 0.107 |
|
≤37 | 15 (40.5) | 12 | |
|
>37 | 22 (59.5) | 4 | |
| CRP (mg/dl) | | | 0.582 |
|
≤0.3 | 26 (72.2) | 7 | |
|
>0.3 | 10 (27.8) | 5 | |
|
N/A | 1 | | |
| LDH (U/l) | | | 0.903 |
|
≤250 | 26 (70.3) | 5 | |
|
>250 | 11 (29.7) | 10 | |
| Albumin (g/dl) | | | 0.593 |
|
≤3.7 | 15 (41.7) | 4 | |
|
>3.7 | 21 (58.3) | 10 | |
|
N/A | 1 | | |
| ALP (U/l) | | | 0.486 |
|
≤350 | 18 (48.6) | 10 | |
|
>350 | 19 (51.4) | 4 | |
| BM at GC
diagnosis | | | 0.049 |
|
Present | 8 (21.6) | 16 | |
|
Absent | 29 (78.4) | 4 | |
| Number of BM | | | 0.800 |
|
Solitary | 8 (21.6) | 7.5 | |
|
Multiple | 29 (78.4) | 7 | |
| Chemotherapy
before | | | 0.103 |
| BM diagnosis | | | |
|
Present | 23 (62.2) | 4 | |
|
Absent | 14 (37.8) | 12 | |
| Chemotherapy
after | | | 0.970 |
| BM diagnosis | | | |
|
Present | 34 (91.9) | 7 | |
|
Absent | 3 (8.1) | 5 | |
| Use of BMA | | | 0.008 |
|
Present | 22 (59.5) | 12 | |
|
Absent | 15 (40.5) | 3 | |
 | Table IVMultivariate analysis of prognostic
factors for SRS. |
Table IV
Multivariate analysis of prognostic
factors for SRS.
| Factors | HR | 95% CI | P-value |
|---|
| No BM at GC
diagnosis | 2.699 | 0.863-8.439 | 0.088 |
| Non-use of BMA | 2.868 | 1.163-7.076 | 0.022 |
Discussion
BM results in disruption of the normal bone
homeostasis, which is a dynamic process involving
osteoclast-mediated osteolysis and osteoblast-mediated
osteogenesis. This frequently decreases bone integrity and causes
severe bone pain, an increased risk of fracture, and the release of
minerals from the bone matrix, resulting in hypercalcemia (13,15).
Complications associated with BM include SREs, such as radiotherapy
or surgery of the bone, pathological fractures, spinal cord
compression and hypercalcemia, which result in morbidity,
deterioration of the QoL and poor prognosis. Therefore, early
diagnosis and appropriate treatment of BM are crucial for
preventing the development of SREs and improving patient
survival.
Early detection of BM relies on its clinical
characteristics. There were clinical symptoms in 56.7% of the
patients at diagnosis of BM. CT scan is the most commonly used
imaging technique for the surveillance of GC patients. Although MRI
was not routinely used for patient follow-up monitoring, MRI was
the technique mostly used for diagnosing BM in the present study.
Our study demonstrated that skeletal metastatic lesions arising
from GC were frequently found at multiple locations (83.3%), were
metachronous (68.3%), coexisted with visceral or brain metastasis
(61.7%), were of the osteolytic type (40%), and occurred most
commonly in the spine (81.7%) and pelvis (61.7%). Moreover, the
serum ALP level, known to be the most predictive biological marker
for the presence of BM in GC (9,16), was
elevated in 63.3% of the patients in the present study. In general,
liver metastasis, in addition to BM, may be associated with
elevated levels of ALP. However, although γ-glutamyl transpeptidase
(γ-GTP) activity correlates with cholestatic liver disease, γ-GTP
activity is not usually increased in BM. In the present study,
γ-GTP levels were not increased in 18 of the 21 (85.7%) patients
with high serum levels of ALP who did not have liver metastasis
(data not shown). Therefore, it is suggested that, in patients
diagnosed with GC, if the ALP value is atypically elevated,
measurement of γ-GTP should be included in the evaluation of BM.
Even if GC patients are asymptomatic, elevated serum levels of ALP
and tumor markers, such as CEA and CA19-9, indicate BM and
evaluation of BM is required. In the present study, 43.3% of the
patients were asymptomatic at diagnosis of BM, and either bone
scintigraphy or FDG-PET/CT was performed in such patients. However,
as the serum ALP level is not always elevated when BM is present,
using appropriate modalities, such as CT, MRI, bone scintigraphy
and FDG-PET/CT, may also be necessary in routine practice, even in
asymptomatic patients, in order to detect BM at an early stage.
Patients with metastatic GC with good ECOG PS scores
and organ function should be offered systemic chemotherapy for
palliation in order to improve survival. The most frequently used
standard first-line chemotherapy regimen for metastatic GC is a
combination of a fluoropyrimidine with platinum, although triple
regimens including docetaxel may be useful in otherwise healthy
patients with a high tumor burden (17,18).
In patients with good ECOG PS score and organ function, second-line
treatment with agents not used in the first-line treatments, such
as taxanes and irinotecan, may confer a modest survival benefit
(19,20). By contrast, chemotherapy is rarely
administered to patients with poor ECOG PS. Previous studies have
reported poor prognosis of BM from GC, with a median OS of 2-7
months after BM diagnosis (3-7,14,21).
However, the median OS period after BM diagnosis in the present
study was 9 months, indicating that the prognosis of GC patients
with BM in our study population was better compared with that
previously reported in the literature. BM in GC is often associated
with a rapidly deteriorating clinical course and extremely poor
prognosis, due to combined bone marrow metastasis causing
hematological abnormalities, such as disseminated intravascular
coagulation (DIC). In the present study, 3.3% patients who had DIC
at diagnosis of BM succumbed to the disease within 2 months after
the diagnosis of BM and 21.7% of the patients developed DIC during
the clinical course (data not shown). Poor ECOG PS score was one of
the worst prognostic factors for OS on multivariate analyses.
Moreover, significantly poor OS was also found on multivariate
analyses in GC patients receiving chemotherapy prior to BM
diagnosis and without palliative chemotherapy after BM occurrence.
These data suggest that further treatment options may be available
for GC patients who have not received chemotherapy compared with
those with intensive chemotherapy prior to BM diagnosis;
furthermore, even if BM has been diagnosed, regardless of the
presence of visceral or brain metastasis, palliative chemotherapy
after BM diagnosis should be considered whenever possible.
Currently, palliative radiotherapy for BM associated
with pain symptoms is a well-established treatment. Previous
studies have reported that BM causes high rates of SREs in patients
with GC and radiotherapy is the most common SRE (3-6).
Concordantly, our data also revealed that SREs occurred in 76.7% of
our patients, and that radiotherapy for BM was performed in the
majority (73.3%) of those patients. In the present study, the
median survival after SRE occurrence was only 5 months, possibly
due to aggressive SREs affecting survival, or other complications
associated with SREs. Approximately 50% of patients who experienced
SREs were found to have had these SREs at BM diagnosis, which was a
significant prognostic factor for poor OS on univariate analysis.
The data of the present study demonstrated that, among patients who
did not present with SREs at BM diagnosis, 91.9% had ECOG PS scores
0-2 and were considered as candidates for palliative chemotherapy
after BM diagnosis. These data suggest that early detection of BM
before the occurrence of SRE improves patient survival.
It has been established that BMA, such as zoledronic
acid and denosumab, are beneficial for the treatment and prevention
of skeletal complications in patients with multiple myeloma and
breast and prostate cancers (22-24).
However, prospective data on the efficacy of BM in GC are lacking
in the literature. In the present study, although this treatment
modality did not significantly prolong OS, it was associated with a
significant extension of SRS. Therefore, our data support the
beneficial effects of BMA for BM that occurs from GC. At present,
preventive dental care to decrease the risk of developing
medication-related osteonecrosis of the jaw is usually offered
before administering BMAs in our institution, and BMAs are
generally well tolerated. Even if asymptomatic, the initiation of
BMA treatment upon diagnosis of BM is recommended to delay time to
SREs and reduce skeletal morbidity in GC patients with BM.
There were certain limitations to the present study.
This retrospective study was performed without randomization of
patient selection. The number of patients was insufficient to draw
a definitive conclusion. The majority of BM cases were not
confirmed pathologically. The standardized methods used for
detecting BMs were heterogenous, with each methodology having its
own limit of detection. We were unable to obtain tumor stage data
or laboratory test data in some of the cases. The present study did
not quantify and compare the therapeutic effects of chemotherapy
due to the wide range of chemotherapy regimens utilized. Finally,
the usage of BMAs depended on the discretion of the attending
physician.
In conclusion, poor ECOG PS score often prevents
patients from receiving further available treatment. Therefore,
early detection of BM and optimal treatment with BMA is imperative
for preventing or delaying SREs, leading to maintenance of a more
favorable ECOG PS score and continuation of chemotherapy in
patients with GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Japan
Orthopedics and Traumatology Research Foundation, Inc. (grant no.
372) and JSPS KAKENHI (grant no. JP19K18481).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YI and DT designed the study. DT, NS, AI, TW, HO,
TTan, HT, TY, NN and ST collected, interpreted and analyzed the
data. YI, DT, SO, TTab and ST performed statistical analyses. YI
wrote the manuscript. NS and TY treated the patients. DT, NS, AI,
TW, HO, TTan, HT, TY, NN, SO, TTab and ST revised the manuscript
critically for important intellectual content. All the authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of the Osaka International Cancer Institute (Osaka,
Japan).
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guadagni S, Catarci M, Kinoshita T,
Valenti M, De Bernardinis G and Carboni M: Causes of death and
recurrence after surgery for early gastric cancer. World J Surg.
21:434–439. 1997.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ahn JB, Ha TK and Kwon SJ: Bone metastasis
in gastric cancer patients. J Gastric Cancer. 11:38–45.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Park HS, Rha SY, Kim HS, Hyung WJ, Park
JS, Chung HC, Noh SH and Jeung HC: A prognostic model to predict
clinical outcome in gastric cancer patients with bone metastasis.
Oncology. 80:142–150. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Silvestris N, Pantano F, Ibrahim T,
Gamucci T, De Vita F, Di Palma T, Pedrazzoli P, Barni S, Bernardo
A, Febbraro A, et al: Natural history of malignant bone disease in
gastric cancer: Final results of a multicenter bone metastasis
survey. PLoS One. 8(e74402)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Turkoz FP, Solak M, Kilickap S, Ulas A,
Esbah O, Oksuzoglu B and Yalcin S: Bone metastasis from gastric
cancer: The incidence, clinicopathological features, and influence
on survival. J Gastric Cancer. 14:164–172. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim YJ, Kim SH, Kim JW, Lee JO, Kim JH,
Bang SM, Lee JS and Lee KW: Gastric cancer with initial bone
metastasis: A distinct group of diseases with poor prognosis. Eur J
Cancer. 50:2810–2821. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nishidoi H and Koga S: Clinicopathological
study of gastric cancer with bone metastasis. Gan To Kagaku Ryoho.
14:1717–1722. 1987.PubMed/NCBI(In Japanese).
|
|
9
|
Choi CW, Lee DS, Chung JK, Lee MC, Kim NK,
Choi KW and Koh CS: Evaluation of bone metastases by Tc-99m MDP
imaging in patients with stomach cancer. Clin Nucl Med. 20:310–314.
1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nielsen OS, Munro AJ and Tannock IF: Bone
metastases: Pathophysiology and management policy. J Clin Oncol.
9:509–524. 1991.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12 (Suppl)(6243S-6249S)2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Berenson JR, Rosen LS, Howell A, Porter L,
Coleman RE, Morley W, Dreicer R, Kuross SA, Lipton A and Seaman JJ:
Zoledronic acid reduces skeletal-related events in patients with
osteolytic metastases. Cancer. 91:1191–1200. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Riihimaki M, Hemminki A, Sundquist K,
Sundquist J and Hemminki K: Metastatic spread in patients with
gastric cancer. Oncotarget. 7:52307–52316. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lim SM, Kim YN, Park KH, Kang B, Chon HJ,
Kim C, Kim JH and Rha SY: Bone alkaline phosphatase as a surrogate
marker of bone metastasis in gastric cancer patients. BMC Cancer.
16(385)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, et al: Phase III study of docetaxel and cisplatin plus
fluorouracil compared with cisplatin and fluorouracil as first-line
therapy for advanced gastric cancer: A report of the V325 Study
Group. J Clin Oncol. 24:4991–4997. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman AR:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 358:36–46. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kang JH, Lee SI, Lim DH, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1518. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ford HE, Marshall A, Bridgewater JA,
Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S,
Middleton GW, et al: Docetaxel versus active symptom control for
refractory oesophagogastric adenocarcinoma (COUGAR-02): An
open-label, phase 3 randomised controlled trial. Lancet Oncol.
15:78–86. 2014. View Article : Google Scholar
|
|
21
|
Mikami J, Kimura Y, Makari Y, Fujita J,
Kishimoto T, Sawada G, Nakahira S, Nakata K, Tsujie M and Ohzato H:
Clinical outcomes and prognostic factors for gastric cancer
patients with bone metastasis. World J Surg Oncol. 15(8)2017.
View Article : Google Scholar
|
|
22
|
Rosen LS, Gordon D, Kaminski M, Howell A,
Belch A, Mackey J, Apffelstaedt J, Hussein MA, Coleman RE, Reitsma
DJ, et al: Long-term efficacy and safety of zoledronic acid
compared with pamidronate disodium in the treatment of skeletal
complications in patients with advanced multiple myeloma or breast
carcinoma: A randomized, double-blind, multicenter, comparative
trial. Cancer. 98:1735–1744. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fizazi K, Carducci M, Smith M, Damiao R,
Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al:
Denosumab versus zoledronic acid for treatment of bone metastases
in men with castration-resistant prostate cancer: A randomised,
double-blind study. Lancet. 377:813–822. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stopeck AT, Lipton A, Body JJ, Steger GG,
Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA,
Viniegra M, et al: Denosumab compared with zoledronic acid for the
treatment of bone metastases in patients with advanced breast
cancer: A randomized, double-blind study. J Clin Oncol.
28:5132–5139. 2010.PubMed/NCBI View Article : Google Scholar
|