Introduction
There are two predominant factors influencing the
carcinogenesis and survival of patients with squamous cell
carcinoma (SCC) of the head and neck (HNSCC): i) Infection with
human papillomavirus (HPV); and ii) the burden of tobacco and
alcohol consumption (1-3).
HPV accounts for a substantial proportion of SCCs, specifically of
the tonsils (oropharynx), with a positive impact on survival
(1,2), whereas the components found in tobacco
smoke and alcohol are associated with the genesis of laryngeal and
hypopharyngeal SCC (3). Although
there are some conflicting data (4-6),
it is widely accepted that smoking has a significant negative
effect on the survival of patients with HNSCC (1,2). There
have been various primarily retrospective studies into the course
of disease of smokers in comparison to former smokers and lifelong
non-smokers, with additional focus on pre- and post-treatment
smoking habit of patients in some of these studies (4-9).
The majority of studies analyzing patients' smoking habit prior to
diagnosis describe a clear survival advantage of non-smoking,
followed by ex-smoking and then active smokers (7-9).
Studies which focus on pre- and post-treatment smoking habit
describe an improved course of disease for patients who quit
smoking (8,9). In case of treatment with primary or
adjuvant radiochemotherapy (RCT), the latter is primarily
associated with peritumoral tissue hypoxia-related incomplete
response to RT (10), along with no
response to platinum-based induction CT (11). In case of treatment by surgery only,
the positive effect of smoking cessation at the time of diagnosis
is attributed to improved wound healing, fewer complications such
as pneumonia and a decrease in second primary malignancies
(12).
In a large population-based study, Sharp et
al (9) reported for the first
time that the positive effect of quitting can predominantly be
recognized in the group of patients that are treated by surgery
only, whereas survival data of patients who ceased or continued
smoking whilst receiving any form of RCT were more or less
indistinguishable. In Europe, and Germany in particular, the
proportion of smokers in the population with cancer is higher than
in countries with more successful anti-smoking campaigns (13), and surgery as single treatment
option, even in oropharyngeal SCC, plays a more pronounced role in
Europe when compared with the US.
Due to these findings, the effects of pre- and
post-treatment smoking habit were analyzed, with additional
stratification for treatment regimes. Moreover, it was investigated
as to whether the positive effects of alterations to smoking habit
at the time of first diagnosis can only be observed in the case of
complete smoking cessation, or whether smoking reduction may have
similar positive effects. The latter is encouraged, as a
substantial proportion of active smokers are more likely to
successfully achieve a reduction in rather than quit smoking.
Patients and methods
Study design
The files of 643 patients with HNSCC, treated
between 2013 and 2016 at the Department of Otorhinolaryngology,
Head and Neck Surgery of the Christian-Albrechts-University Kiel
(Kiel, Germany) were retrospectively analyzed.
The main dependent variables were overall survival
(OS) and disease-specific survival (DSS), with a follow-up range of
0.01-4.4 years and mean follow-up of 1.62 years. The independent
variable was smoking status, assessed either prior to or after
diagnosis. In addition, altered smoking after diagnosis was
investigated for separate treatment groups. Smoking habit prior to
diagnosis was classified as either ‘never smoked’, ‘active smoker’
or ‘former smoker’, when patients stopped smoking at least 2 years
prior to diagnosis. Smoking habit after diagnosis was classified as
‘no change’ (patients are still active smokers), ‘reduced’ (tobacco
consumption was reduced to <10 cigarettes per day) and quit
(patients stopped their tobacco consumption completely).
To increase numbers, patients treated by primary or
adjuvant RT or RCT were pooled and compared to patients treated by
surgery only. At the department of the study, the optimal target
dose for primary and adjuvant RCT is 300 mg cisplatin or
carboplatin per m2 body surface area. Usually, 100
mg/m2 were administered at week 1, 3, and 5. Target
doses for radiation are: 70 gy for primary RT and 60 gy for
adjuvant RT. Mortality was censored on 1st October, 2017.
Outcome variables
The main outcomes were OS and DSS. Survival was
defined as either the time in years from date of diagnosis until
date of death from any cause (OS), or as the time in years to
primary tumor-related death (DSS).
Statistical analysis
Kaplan-Meier plots and log-rank tests (SPSS 20.0;
IBM Corp.) were used to compare independent variables and
mortality. To analyze statistical differences between different
subgroups, pairwise log-rank comparisons with Bonferroni correction
were additionally performed. In addition, Cox univariate analysis
(forward stepwise; SPSS 20.0) was performed to analyze survival
advantages dependent on smoking habit alone or in combination with
patient treatment regimes. One-way ANOVA was performed to assess
age-related differences between active, never and former smokers,
whereas Student's t-test was performed to assess age-related
differences between patients that reduced or stopped tobacco
consumption after diagnosis (both SPSS 20.0). P≤0.05 was considered
to indicate a statistically significant difference.
Results
Patient demographics
Patient characteristics (n=643) are described in
Table I. The mean age was 64.5±10.2
years (range, 39.86-98.02 years). The majority of patients were
male [77.3% (497/643)]. Prior to diagnosis, 349 patients (54.4%)
reported a smoking habit (active smokers), 113 (17.6%) reported
having never smoked and 180 (28.0%) reported to have quit smoking
at least 2 years prior to diagnosis (former smokers). No
information regarding smoking status was available for 1 patient
treated by surgery only. Of those patients reporting to be active
smokers at time of diagnosis, 50 (14.3%) stopped smoking after
diagnosis and 38 (10.9%) reduced their tobacco consumption to
<10 cigarettes per day. While patients reporting to be active
smokers prior to diagnosis were significantly younger than never or
former smokers (never smoked, 68.6±10.9; former smokers, 68.4±9.7;
active smokers, 61.0±8.5; P<0.001), no age-related difference
was observed between those patients who continued smoking after
diagnosis and those who quit or reduced their tobacco consumption
(P>0.05; data not shown). Anatomical tumor sites were as
follows: Oral cavity, n=51 (7.9%); hypopharynx, n=94 (14.6%);
larynx, n=163 (25.3%); tonsil, n=95 (14.8%); oropharynx other than
tonsil, n=155 (24.1%); nasopharynx, n=49 (7.6%) and other sites,
n=36 (5.6%). Patients were treated as follows: i) The majority of
patients were treated by surgery only (315 patients; 49.0%); ii)
111 patients (17.3%) were treated by primary RCT; iii) 24 patients
(3.7%) received primary RT; iv) 121 patients (18.8%) were treated
by adjuvant RCT; and v) 72 patients (11.2%) were treated by
adjuvant RT. For further statistical analysis, all patients treated
by either primary RCT or RT, or by adjuvant RCT or RT were pooled,
resulting in a total of 328 patients treated by R(C)T; of these,
137 patients were treated with cisplatin, but data regarding the
cisplatin dosage were available for only 115 cases, rendering
further survival analysis in the present context impossible. Of all
328 R(C)T patients, 62 were never smokers, 89 were former smokers
and 177 were active smokers, of which 16 reduced and 21 quit
tobacco consumption after diagnosis. Similarly, of the 315 patients
treated by surgery, only 51 had never smoked, with 91 former
smokers and 172 active smokers, of which 22 reduced and 29 quit
their tobacco consumption after diagnosis. For 1 further patient,
no information regarding smoking habit was available.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variables | No | Percentage (%) |
|---|
| Sex |
|
Female | 146 | 22.7 |
|
Male | 497 | 77.3 |
| Smoking habit prior
to diagnosis |
|
Never
smoked | 113 | 17.6 |
|
Former
smokera | 180 | 28.0 |
|
Active
smoker | 349 | 54.4 |
| Altered smoking habit
after diagnosis |
|
Cessation | 50 | 14.3 |
|
Reductionb | 38 | 10.9 |
|
Continued
smoking | 261 | 74.8 |
| Status (as of 1st
October 2017) |
|
Alive | 501 | 77.9 |
|
Dead | 142 | 22.1 |
| Cause of death |
|
Primary
tumor | 87 | 61.3 |
|
Secondary
tumor | 6 | 4.2 |
|
Not tumor
related | 23 | 16.2 |
|
Unclear | 26 | 18.3 |
| Tumor site |
|
Oral
cavity | 51 | 7.9 |
|
Hypopharynx | 94 | 14.6 |
|
Larynx | 163 | 25.3 |
|
Tonsil | 95 | 14.8 |
|
Oropharynx
other than tonsil | 155 | 24.1 |
|
Nasopharynx | 49 | 7.6 |
|
Other | 36 | 5.6 |
| Therapy |
|
Surgery
only | 315 | 49.0 |
|
Primary
RCT | 111 | 17.3 |
|
Primary
RT | 24 | 3.7 |
|
Adjuvant
RCT | 121 | 18.8 |
|
Adjuvant
RT | 72 | 11.2 |
Kaplan-Meier analysis of OS and
DSS
The 3-year OS and DSS rates were 78.2% (111/142) and
80.6% (70/87), respectively. The detailed results of the
Kaplan-Meier analysis for OS and DSS, together with the 3-year
survival rates are shown in Table
II. Patients who stopped tobacco consumption at least 2 years
prior to diagnosis had no significant survival advantage when
compared with active smokers (P=0.165 and P=0.105 for OS and DSS,
respectively). In Fig. 1, the
survival curves of all patients separated by smoking habit are
shown. The results demonstrated a survival advantage for never
smoking (P=0.002 for OS and P=0.005 for DSS), with no significant
improvement in outcome for former smokers (P=0.157 for OS and
P=0.105 for DSS vs. active smokers). Further pairwise log-rank
tests demonstrated significantly improved OS for never smokers
compared with active (P<0.001) and former smokers (P=0.014), as
well as significantly improved DSS (P=0.002 and P=0.041,
respectively; Fig. 1A and B).
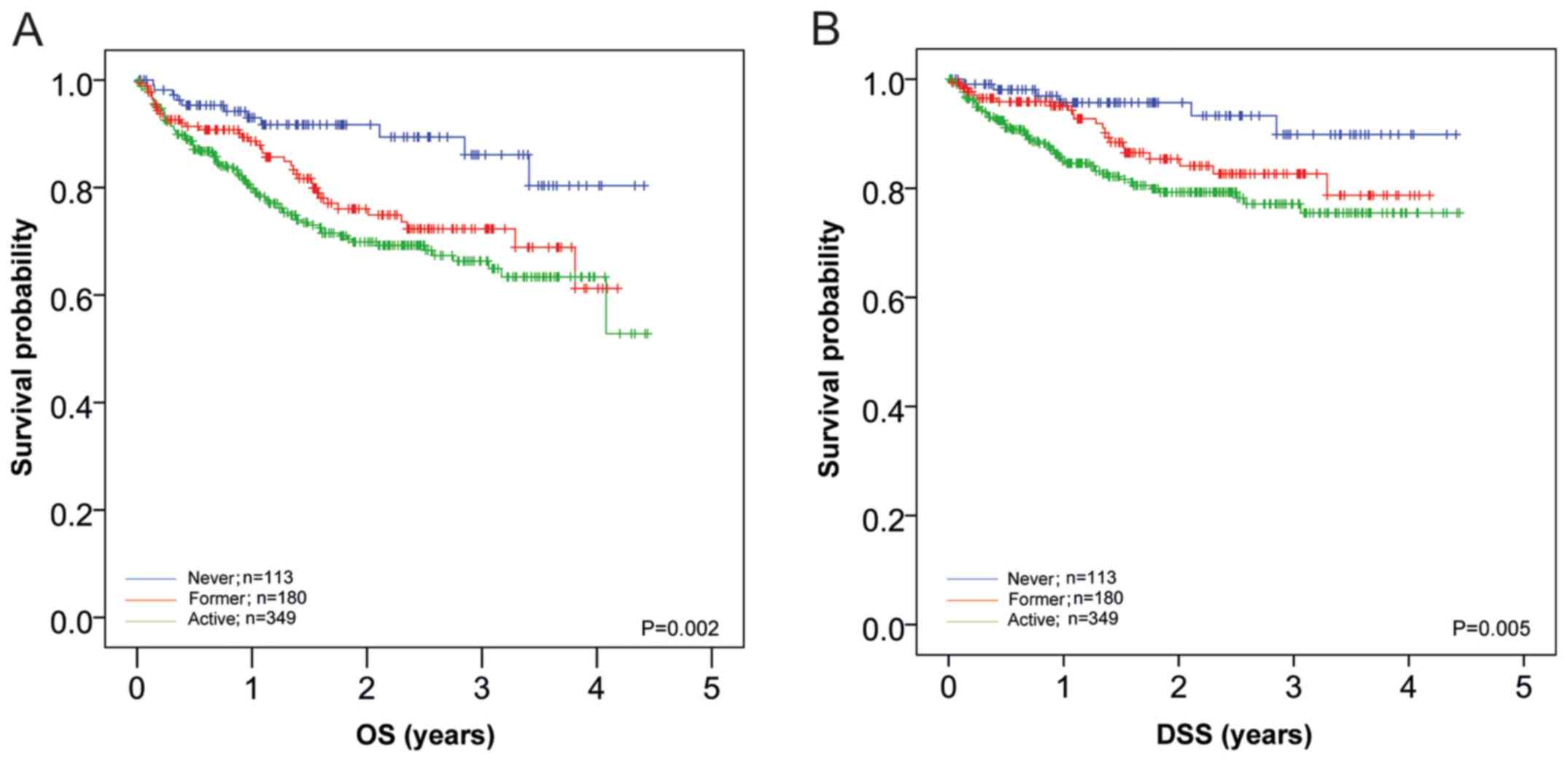 | Figure 1Effects of smoking habit prior to
diagnosis on OS and DSS of patients with HNSCC. (A) OS and (B) DSS
of patients with HNSCC in relation to smoking habit were analyzed.
OS in patients that never smoked (n=113; blue line), former smokers
(n=180; red line) and active smokers (n=349; green line) was 85.7,
72.3 and 66.3% after 3 years, respectively. The data indicated a
significant (P=0.002) effect of smoking on survival; however, no
significant difference was observed between former smokers and
active smokers (P=0.157). DSS in non-smokers, former smokers and
active smokers was 89.9, 82.7 and 77.1% after 3 years; a
significant (P=0.005) effect of smoking status on DSS was found.
However, no significant difference was observed between former and
active smokers (P=0.105). Further pairwise log-rank tests revealed
significant differences in OS between patients that never smoked
compared with active (P<0.001) and former smokers (P=0.014),
with similar differences in DSS (P=0.002 and P=0.041,
respectively). OS, overall survival; DSS, disease-specific
survival; HNSCC, squamous cell carcinoma of the head and neck. |
 | Table IIKaplan-Meier analysis showing OS and
DSS dependent upon smoking habit prior to and following tumor
diagnosis. |
Table II
Kaplan-Meier analysis showing OS and
DSS dependent upon smoking habit prior to and following tumor
diagnosis.
| Variable | N | Mean, years | SD, years | Minimum, years | Maximum, years | Median, years | 3-year OS, % | P-value | 3-year DSS, % | P-value |
|---|
| Smoking habit prior
to diagnosis | | | | | | | | | | |
|
Never
smoked | 113 | 1.72 | 1.18 | 0.02 | 4.41 | 1.47 | 85.7 | 0.002 | 89.9 | 0.005 |
|
Active
smoker | 349 | 1.55 | 1.14 | 0.01 | 4.44 | 1.26 | 66.3 | | 77.1 | |
|
Former
smokera | 180 | 1.69 | 1.14 | 0.02 | 4.18 | 1.54 | 72.3 | | 82.7 | |
| Smoking habit prior
to diagnosis, excluding non-smokers | | | | | | | | | | |
|
Former
smokera | 180 | 1.69 | 1.14 | 0.02 | 4.18 | 1.54 | 72.3 | 0.165 | 82.7 | 0.105 |
|
Active
smoker | 349 | 1.55 | 1.14 | 0.01 | 4.44 | 1.26 | 66.3 | | 76.9 | |
| Altered smoking
habit after diagnosis, all patients | | | | | | | | | | |
|
Never
smoked | 113 | 1.72 | 1.18 | 0.02 | 4.41 | 1.47 | 86.1 | 0.002 | 89.8 | 0.001 |
|
Reducedb | 38 | 1.79 | 1.11 | 0.04 | 3.97 | 1.61 | 75.2 | | 89.7 | |
|
Quit | 50 | 2.11 | 1.30 | 0.07 | 4.44 | 2.14 | 77.7 | | 85.9 | |
|
No
change | 261 | 1.40 | 1.08 | 0.01 | 4.42 | 1.12 | 61.7 | | 72.8 | |
| Altered smoking
habit after diagnosis, excluding non-smokers | | | | | | | | | | |
|
Not
changed | 261 | 1.40 | 1.08 | 0.01 | 4.42 | 1.12 | 61.6 | 0.05 | 72.8 | 0.045 |
|
Reducedb | 38 | 1.79 | 1.11 | 0.04 | 3.97 | 1.61 | 75.0 | | 89.7 | |
|
Quit | 50 | 2.11 | 1.31 | 0.07 | 4.44 | 2.14 | 77.0 | | 85.9 | |
| Altered smoking
habit after diagnosis, R(C)T patients | | | | | | | | | | |
|
Never
smoked | 62 | 1.76 | 1.11 | 0.02 | 4.33 | 1.47 | 84.6 | 0.110 | 87.5 | 0.195 |
|
Reducedb | 16 | 1.67 | 1.07 | 0.49 | 3.94 | 1,43 | 65.1 | | 90.9 | |
|
Quit | 21 | 2.36 | 1.16 | 0.24 | 4.33 | 2.30 | 75.2 | | 80.2 | |
|
No
change | 140 | 1.61 | 1.04 | 0.01 | 4.30 | 1.39 | 61.4 | | 75.3 | |
| Altered smoking
habit after diagnosis, R(C)T patients excluding non-smokers | | | | | | | | | | |
|
No
change | 141 | 1.60 | 1.03 | 0.01 | 4.30 | 1.39 | 61.5 | 0.664 | 75.3 | 0.588 |
|
Reducedb | 16 | 1.66 | 1.06 | 0.49 | 3.94 | 1.42 | 65.1 | | 90.9 | |
|
Quit | 20 | 2.40 | 1.16 | 0.24 | 4.33 | 2.47 | 79.2 | | 80.2 | |
| Altered smoking
habit after diagnosis, surgery-only patients | | | | | | | | | | |
|
Never
smoked | 51 | 1.67 | 1.28 | 0.03 | 3.31 | 1.41 | 86.9 | 0.017 | 92.4 | 0.005 |
|
Reducedb | 22 | 1.89 | 1.16 | 0.04 | 3.97 | 1.64 | 80.6 | | 88.9 | |
|
Quit | 29 | 1.94 | 1.39 | 0.07 | 4.44 | 1.71 | 81.5 | | 92.5 | |
|
No
change | 121 | 1.16 | 1.07 | 0.01 | 4.42 | 0.73 | 64.4 | | 71.2 | |
| Altered smoking
habit after diagnosis, surgery-only patients excluding
non-smokers | | | | | | | | | | |
|
No
change | 120 | 1.16 | 1.07 | 0.01 | 4.42 | 0.74 | 64.2 | 0.228 | 71.2 | 0.048 |
|
Reducedb | 22 | 1.89 | 1.16 | 0.04 | 3.97 | 1.64 | 80.6 | | 88.9 | |
|
Quit | 30 | 1.92 | 1.37 | 0.07 | 4.44 | 1.67 | 77.3 | | 92.5 | |
| Diagnosis | | | | | | | | | | |
|
Nasopharynx | 49 | 1.67 | 1.27 | 0.02 | 4.41 | 1.39 | 97.4 | 0.01 | 97.5 | 0.004 |
|
Larynx | 163 | 1.73 | 1.16 | 0.04 | 4.08 | 1.56 | 79.6 | | 91.9 | |
|
Other | 36 | 1.26 | 1.11 | 0.02 | 4.20 | 0.95 | 75.4 | | 87.4 | |
|
Tonsil | 95 | 1.74 | 1.16 | 0.08 | 4.30 | 1.59 | 74.9 | | 80.3 | |
|
Oral
cavity | 51 | 1.44 | 1.19 | 0.03 | 4.02 | 1.07 | 62.0 | | 67.3 | |
|
Oropharynx
other than tonsil | 155 | 1.62 | 1.09 | 0.03 | 4.44 | 1.48 | 61.5 | | 74.1 | |
|
Hypopharynx | 94 | 1.52 | 1.15 | 0.01 | 4.33 | 1.33 | 58.4 | | 70.0 | |
| Therapy | | | | | | | | | | |
|
Surgery
only | 315 | 1.47 | 1.21 | 0.01 | 4.44 | 1.14 | 73.1 | 0.001 | 82.0 | 0.070 |
|
Primary
RCT | 111 | 1.60 | 1.03 | 0.02 | 4.20 | 1.49 | 58.2 | | 73.7 | |
|
Primary
RT | 24 | 0.84 | 0.60 | 0.01 | 2.27 | 0.78 | 40.3 | | 64.6 | |
|
Adjuvant
RCT | 121 | 2.09 | 1.11 | 0.13 | 4.33 | 1.98 | 78.9 | | 83.7 | |
|
Adjuvant
RT | 72 | 1.75 | 0.97 | 0.22 | 4.33 | 1.49 | 80.8 | | 89.6 | |
Patients who either reduced tobacco consumption
<10 cigarettes per day [(38/349), 10.9%] or quit [(50/349),
14.3%] tobacco consumption after first tumor diagnosis had
significantly improved OS and DSS compared with patients who
continued smoking (P=0.05 and P=0.045 for OS and DSS, respectively;
Table II): The 3-year OS rates
were 75.0 and 77.0% for patients who reduced or quit smoking,
respectively, compared with 61.6% in patients who continued
smoking; 3 years DSS rates were 89.7 and 85.9% for patients who
reduced or quit smoking, respectively, compared with 72.8% in
patients who continued smoking (Table
II). In Fig. 2A and B, these results are shown as Kaplan-Meier
curves, including the survival curves of the patients who never
smoked, demonstrating a clear survival benefit for the never
smokers (86.1% OS and 89.8% DSS), with an overall P=0.002 for OS
and P=0.001 for DSS. Pairwise log-rank comparisons (stated in
detail in the Fig. 2 legend)
revealed significant differences in OS for never smokers compared
with continued smokers (P<0.001) and those that reduced their
smoking (P=0.047), but not those that quite smoking (P=0.111).
There were no significant differences in OS for patients that
reduced tobacco consumption compared with active smokers (P=0.347)
or those that quit smoking (P=0.347); however, patients who quit
smoking exhibited significantly different OS compared with active
smokers (P=0.049). In DSS, significant differences were only
observed between never smokers and active smokers (P<0.001), and
active smokers and those that quit smoking (P=0.047).
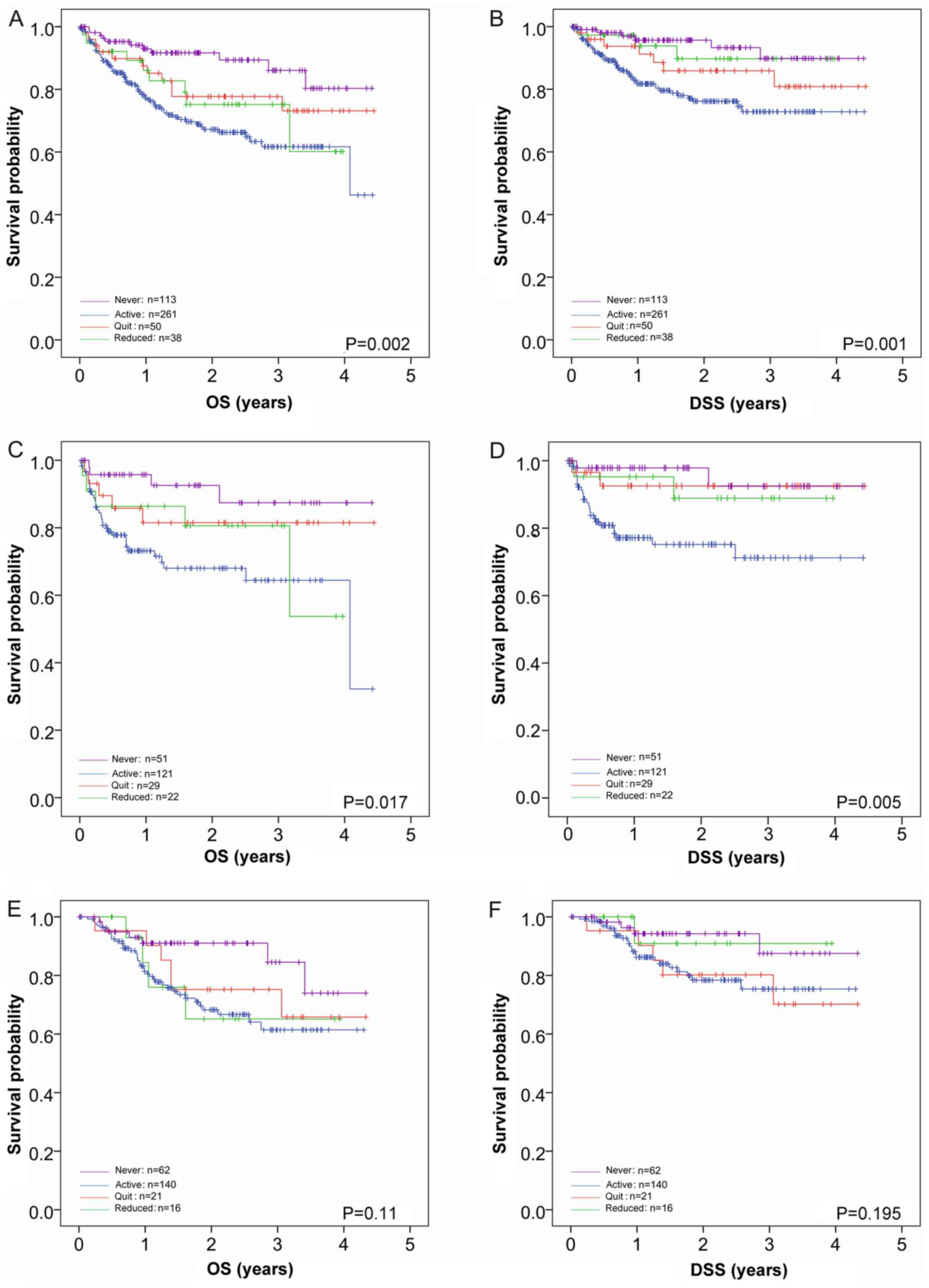 | Figure 2Effects of altered smoking habit at
time of diagnosis on OS and DSS in patients with HNSCC. (A) OS and
(B) DSS of patients with HNSCC irrespective of treatment is shown
(n=462). Patients who continued smoking (blue line; n=261)
exhibited the worst 3-year OS (61.7%) and DSS (72.8%), whereas
never smokers (n=113; magenta line) exhibit the highest survival
(86.1 and 89.8%, respectively). Those that reduced (n=38; green
line) and quit smoking (n=50; red line) exhibited intermediate
survival rates. The effects of smoking habit post-diagnosis on OS
(P=0.002) and DSS (P=0.001) were significant. Pairwise comparisons
revealed significant differences in OS for never smokers compared
with continued smokers (P<0.001) and those that reduced their
smoking (P=0.047), but not those that quite smoking (P=0.111).
There were no significant differences in OS for patients that
reduced tobacco consumption compared with active smokers (P=0.347)
or those that quit smoking (P=0.347); however, patients who quit
smoking exhibited significantly different OS compared with active
smokers (P=0.049). Pairwise comparisons revealed significant
differences in DSS only between never smokers and active smokers
(P<0.001), and active smokers and those that quit smoking
(P=0.047). (C) OS and (D) DSS of patients treated with surgery only
(n=223). Smoking habit significantly influenced OS (P=0.017) and
DSS (P=0.005) in patients treated with surgery only. Pairwise
comparisons revealed significant differences in OS between never
smokers and active smokers (P=0.003), and active smokers and
patients that quit smoking (P=0.049). Pairwise comparisons revealed
significant differences in DSS when active smokers were compared
with never smokers (P=0.004), patients that quit smoking (P=0.035)
and patients that reduced their smoking (P=0.045). (E) OS and (F)
DSS of patients treated by R(C)T (n=239). There were no significant
effects of smoking habit observed on OS (P=0.11) or DSS (P=0.195)
in patients treated with R(C)T. The 3-year OS of the never smokers,
active smokers, reduced smokers and those that quit were 84.6,
61.4, 65.1 and 75.2%, respectively. The 3-year DSS of the never
smokers, active smokers, reduced smokers and those that quit were
87.5, 75.3, 90.9 and 80.2%, respectively. In all plots, former
smokers (those that quit smoking >2 years prior to diagnosis)
were excluded. OS, overall survival; DSS, disease-specific
survival; HNSCC, squamous cell carcinoma of the head and neck;
R(C)T, radio(chemo)therapy. |
When stratifying the effect of continued tobacco
consumption, quitting, and/or reduction in relation to patient
treatment, log-rank tests for patients treated by surgery only
analyzing DSS (P=0.045) and OS (P=0.05) demonstrated a significant
survival advantage for patients who reduced or quit tobacco
consumption after diagnosis (Table
II). Including the survival data of the never-smoking patients
treated by surgery only into the equation demonstrated a survival
advantage for these patients (overall P=0.017 for OS; P=0.005 for
DSS; Fig. 2C and D). Pairwise comparisons of the survival
data of patients treated by surgery only again revealed significant
differences in OS between never smokers and active smokers
(P=0.003), and active smokers and patients that quit smoking
(P=0.049). For DSS, pairwise comparisons revealed significant
differences when active smokers were compared with never smokers
(P=0.004), patients that quit smoking (P=0.035) and patients that
reduced their smoking (P=0.045). In patients treated by RCT,
altered smoking habit at time of diagnosis had no significant
effect on OS (P=0.664) or DSS (P=0.588). Including the survival
data of the patients that never smoked into the equation did also
not reveal a significant effect of smoking habit on survival
(P=0.11 for OS; P=0.195 for DSS; Fig.
2E and F).
Cox univariate analysis of OS and DSS
in relation to smoking habit
To further analyze the effects of smoking habit
before or after cancer diagnosis, Cox univariate analysis was
performed. The results are shown in Table III, corroborating the results
obtained by Kaplan-Meier analysis. The data demonstrated that OS
and DSS was, when compared with never smokers, significantly worse
in active (P=0.001 for OS; P=0.021 for DSS) and former smokers
(P=0.017 for OS; P=0.041 for DSS), with no significant difference
between active and former smokers (P=0.253 for OS; P=0.159 for DSS;
Table III). Patients who either
reduced or quit their tobacco consumption exhibited worse OS when
compared with never smokers (P=0.016 and P=0.04 for smoking
reduction and smoking cessation, respectively, vs. never smokers),
whereas active smokers exhibited the worst OS (P<0.001 vs. never
smoked; Table III and Fig. 2A). Similar results were obtained in
patients treated by surgery only (P=0.035 and P=0.045 for smoking
reduction and smoking cessation, respectively, vs. never smoked).
Furthermore, when comparing active smokers with patients that never
smoked, OS was significantly worse (P<0.001; Table III and Fig. 2C). In patients treated by R(C)T, OS
was not affected by the smoking habit of the patients; none of the
former or active smokers had significantly different survival rates
compared with the never smokers (P=0.055 for smoking reduction,
P=0.079 for quitting, P=0.051 for patients who continued smoking;
Table III and Fig. 2E).
 | Table IIIUnivariate Cox analysis for OS and
DSS. |
Table III
Univariate Cox analysis for OS and
DSS.
| | OS | DSS |
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Smoking habit prior
to diagnosis vs. never smoked |
|
Active
smoker | 1.669 | 0.882-3.159 | 0.001 | 1.805 | 1.073-3.185 | 0.021 |
|
Former
smokera | 1.192 | 0.493-2.883 | 0.017 | 1.431 | 1.165-3.209 | 0.041 |
| Smoking habit prior
to diagnosis vs. former smoker |
|
Active
smoker | 1.495 | 0.916-2.440 | 0.253 | 1.309 | 0.900-1.904 | 0.159 |
| Altered smoking
habit after diagnosis vs. never smoked, all patients |
|
Reducedb | 2.990 | 1.224-7.307 | 0.016 | 1.063 | 0.151-4.364 | 0.955 |
|
Quit | 2.435 | 1.041-5.694 | 0.040 | 1.328 | 0.842-6.697 | 0.155 |
|
No
change | 4.522 | 2.343-8.728 | <0.001 | 4.766 | 1.843-12.178 | 0.001 |
| Altered smoking
habit after diagnosis vs. never smoked, patients treated by surgery
only |
|
Reducedb | 2.705 | 0.725-10.087 | 0.035 | 1.011 | 0.243-14.983 | 0.134 |
|
Quit | 1.967 | 0.527-7.330 | 0.045 | 1.521 | 0.245-11.030 | 0.146 |
|
No
change | 4.141 | 0.470-11.721 | 0.001 | 6.279 | 0.431-25.954 | 0.001 |
| Altered smoking
habit after diagnosis vs. never smoked, patients treated by
R(C)T |
|
Reducedb | 2.299 | 0.572-7.857 | 0.055 | 1.276 | 0.323-9.265 | 0.751 |
|
Quit | 1.942 | 0.651-5.709 | 0.079 | 2.217 | 0.836-7.615 | 0.321 |
|
No
change | 2.585 | 1.154-5.792 | 0.051 | 2.698 | 0.539-10.165 | 0.061 |
Analysis of DSS in all patients showed that
reduction or cessation of tobacco consumption shifted the survival
rates towards the survival rates of the never smokers (P=0.995 for
reduction vs. never smoked; P=0.155 for quitting); patients who did
not change their smoking habit had the worst DSS (P=0.001; Table III and Fig. 2B). Similar results were obtained in
patients treated by surgery only. Again, no significant differences
were seen between patients who never smoked compared with those who
reduced their tobacco consumption (P=0.134) or quit smoking
completely (P=0.146). Patients who continued smoking again had the
worst outcome (P=0.001; Table III
and Fig. 2D). In R(C)T patients,
however, no such differences were found (P=0.751, P=0.321 and
P=0.061 for reduction, quitting and continued smoking,
respectively, vs. never smoked).
Hence, the positive effects of reducing or quitting
smoking for OS and DSS observed in the entire cohort were based on
the favorable outcomes of patients treated with surgery only.
Discussion
In light of the present literature, there were three
results of specific interest observed by analyzing 643 patients
with HNSCC for the impact of smoking habit on survival in a single
center setting: i) Patients who quit smoking at least 2 years
before cancer diagnosis/treatment did not exhibit survival rates
that differed from those of active smokers; ii) reduction of
smoking at the time of cancer diagnosis led to comparable, albeit
slightly inferior, positive effects on survival rates when compared
with quitting smoking; and iii) this was specifically observed in
the group of patients treated with surgery only. These data were in
accordance with the findings reported by Sharp et al
(9). Rather expected was the
finding that smoking (active or past) was detrimental to OS and DSS
for all patient groups regardless of treatment. These data were
consistent with various other studies into the issue (1,2,14).
The majority of studies into smoking habit and
cancer describe the survival rates of former smokers to be
intermediate compared with active smokers (lower survival rate) and
those that never smoked (improved survival) (8,9,15,16).
In the present study, however, there were no significant
differences in survival between former and active smokers for DSS
or OS. Only one study could be identified showing comparable data
(17). One hypothesis explaining
these data would be that a person who quits smoking at least 2
years prior to a cancer diagnosis has a decreasing risk of
developing cancer over time; however, once this person does suffer
from HNSCC, there is no survival benefit when compared to a person
that continued to smoke. These results appear to somewhat
contradict the other findings from the present study that indicated
positive survival effects for patients who quit or reduced smoking
following first diagnosis and before treatment. However, the two
described effects of smoking history could be based on different
mechanisms: While quitting smoking following first time diagnosis
may have direct, most likely hypoxia-related positive effects on
post-operative wound healing and peri-operative complication rates
(12), both of which will improve
survival outcome, the negative long-term effects of extended
tobacco smoke exposure may be linked to genetic aberrations
(18) and smoking-associated
co-morbidity (16,17), leading to impaired survival
outcomes. In fact, in the present study, smokers who quit or
reduced smoking at first time diagnosis exhibited improved survival
rates compared with ex-smokers, with 3-year OS rates of 76.7 and
72.3% (DSS, 87.5 and 82.6%), respectively; however, the reason for
this remains unclear.
The improved survival of patients who reduced or
quit smoking at time of first cancer diagnosis, which was observed
across the entire study cohort, was specifically observed in the
group of patients that were treated with surgery only. Thus, the
present analysis validated the findings reported by Sharp et
al (9). This is noteworthy, as
the evidence in the literature is not unanimous, with various
studies reporting effects of smoking habit on survival outcomes
only when: i) Treatment regimens included radiotherapy; ii) the
tumors were HPV-negative; and iii) p16INK4A status in
immunohistochemistry was additionally stratified for (14,19,20),
whereas others did not see an association between smoking habit and
survival under any circumstances (4,5). In
the present study, neither HPV nor p16INK4A status were
included in the analysis, as all patients were treated prior to the
routine inclusion of these parameters into patient check-ups. A
review and meta-analysis by Grønkjær et al (12) summarized that pre-operative smoking
increased the risk of wound complications, general infections,
pulmonary infections, neurologic complications and admission to
intensive care units. The data from the present study suggested
that treatment-related and smoking-dependent co-morbidities might
be responsible for the impact of smoking on the OS and DSS of
patients with HNSCC. Moreover, the data reported in the present
study and by Sharp et al (9)
contradict the frequently stated assumption that smoking
compromises outcomes predominantly among those treated with
radiation therapy by decreasing oxygenation and/or reducing the
effects of radiation-induced tumor killing (7). The lack of consensus in this matter
becomes more evident when considering that Browman et al
(10) published a landmark article
suggesting that active smoking during radiation therapy was
detrimental with respect to OS, progression-free survival and
complication-free survival; a subsequent follow-up study the same
authors conducted, however, did not corroborate these findings
(21).
As previously described, positive effects on
survival have been found for patients quitting smoking before or at
the time of cancer diagnosis (9).
To the best of our knowledge, the present study is the first
showing that a reduction in smoking (<10 cigarettes per day)
leads to a beneficial effect on patient survival. This may be an
encouraging finding, as it is more likely for patients to achieve a
reduction in smoking than to quit smoking altogether, particularly
at times of mental distress due to a cancer diagnosis (8). In Germany and other European
Countries, it is well established that the proportion of smokers
among the general population is substantially higher than in the
USA, which has more successful anti-smoking campaigns (10). Our recent study reported that the
proportion of smokers among patients with HPV-negative and
HPV-positive tonsillar SCC is 70 and 50%, respectively (2). Thus, clinicians could be encouraged to
recommend at least a reduction of tobacco consumption at first
visit. According to the present data, to reduce or quit smoking
should be recommended to all patients that will receive treatment
for HNSCC, and this holds true whether or not the treatment
regimens include R(C)T. Of note, only 56% of physicians recommend
to their cancer patients who smoke that they stop smoking, and most
oncology providers do not provide smoking interventions beyond
advice to quit (8). In this
context, it is noteworthy that requirements for head and neck
oncology certificates formulated by the German Cancer Society, for
example, include, among other points, psycho-oncologic and
nutrition care, as well as other concomitant support, but no offers
for cessation interventions. Evidence in the literature strongly
advocates changing this and implementing such interventions in the
care of patients with cancer.
Limitations of this analysis are primarily based on
the retrospective nature of the study. This means that certain
specific questions remain unanswered due to a lack of precise
documentation in the patient´s files. One of these questions is
whether or not the patients that reduced or quit smoking at the
time of cancer diagnosis actually maintained the alterations in
smoking habit, as in follow-up examinations, smoking status is not
routinely questioned. Similarly, it cannot be fully excluded that
patients considered as former smokers did not relapse after cancer
diagnosis due to the intense mental distress. It has been estimated
that ~50% of patients with cancer who smoked prior to diagnosis
fail to stop smoking or relapse after diagnosis (8). In the case that a substantial
proportion of smokers not only failed to quit or reduce smoking but
relapsed to former smoking habit from prior to the cancer
diagnosis, the survival benefits of quitting or reducing smoking
may be underestimated in the present study.
In conclusion, the findings of the present study
support encouraging patients to alter their smoking habit, even if
this only entails reducing tobacco consumption, regardless of which
treatment regimen is planned. Smoking cessation interventions must
be integrated into the holistic care of patients with cancer.
Future efforts are required to clarify which factors exactly are
responsible for the survival advantages observed in the subgroup of
patients treated with surgery only who reduced or stopped their
tobacco consumption following their cancer diagnosis. Clinicians
should be motivated to support smoking cessation among their
patients.
Acknowledgements
The authors thank Dr Crystal Moore (Environmental
Agency, United Kingdom) for careful and critical reading of the
manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study was conceived and designed by MH, ESQ and
KH. Data were acquired by TS, KK, ALF, AH and MGD, statistically
analyzed by ESQ and MH, and interpreted by ASF, ML and MH. The
manuscript was prepared by AsF, ESQ and MH, and edited by AlF, KH
and AH. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Patients provided written informed consent to
participate in the study. The study was approved by the Ethics
Committee of the Medical Faculty of the
Christian-Albrechts-University of Kiel (Kiel, Germany) (permit no.
D 507/17).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hoffmann M, Quabius ES, Tribius S,
Gebhardt S, Görögh T, Hedderich J, Huber K, Dunst J and Ambrosch P:
Influence of HPV-status on survival of patients with tonsillar
carcinomas (TSCC) treated by CO2-laser surgery plus risk
adapted therapy-a 10 year retrospective single centre study. Cancer
Lett. 413:59–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Marur S and Forastier AA: Head and neck
squamous cell carcinoma: Update on epidemiology, diagnosis, and
treatment. Mayo Clin Proc. 91:386–936. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Farshadpour F, Kranenborg H, Calkoen EV,
Hordijk GJ, Koole R, Slootweg PJ and Terhaard CH: Survival analysis
of head and neck squamous cell carcinoma: Influence of smoking and
drinking. Head Neck. 33:817–823. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stucken CL, de Almeida JR, Sikora AG, Tong
CC and Genden EM: Impact of human papillomavirus and smoking on
survival outcomes after transoral robotic surgery. Head Neck.
38:380–386. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lassig AA, Yueh B and Joseph AM: The
effect of smoking on perioperative complications in head and neck
oncologic surgery. Laryngoscope. 122:1800–1808. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen AM, Chen LM, Vaughan A, Farwell DG,
Luu Q, Purdy JA and Vijayakumar S: Head and neck cancer among
lifelong never-smokers and ever-smokers: Matched-pair analysis of
outcomes after radiation therapy. Am J Clin Oncol. 34:270–275.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choi SH, Terrell JE, Bradford CR, Ghanem
T, Spector ME, Wolf GT, Lipkus IM and Duffy SA: Does quitting
smoking make a difference among newly diagnosed head and neck
cancer patients? Nicotine Tob Res. 18:2216–2224. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sharp L, McDevitt J, Carsin AE, Brown C
and Comber H: Smoking at diagnosis is an independent prognostic
factor for cancer-specific survival in head and neck cancer:
Findings from a large, population-based study. Cancer Epidemiol
Biomarkers Prev. 23:2579–2590. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Browman GP, Wong G, Hodson I, Sathya J,
Russell R, McAlpine L, Skingley P and Levine MN: Influence of
cigarette smoking on the efficacy of radiation therapy in head and
neck cancer. N Engl J Med. 328:159–163. 1993.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fountzilas G, Kosmidis P, Avramidis V,
Nikolaou A, Kalogera-Fountzila A, Makrantonakis P, Bacoyiannis C,
Samantas E, Skarlos D and Daniilidis J: Long-term survival data and
prognostic factors of a complete response to chemotherapy in
patients with head and neck cancer treated with platinum-based
induction chemotherapy: A Hellenic Co-operative oncology group
study. Med Pediatr Oncol. 28:401–410. 1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Grønkjær M, Eliasen M, Skov-Ettrup LS,
Tolstrup JS, Christiansen AH, Mikkelsen SS, Becker U and
Flensborg-Madsen T: Preoperative smoking status and postoperative
complications: A systematic review and meta-analysis. Ann Surg.
259:52–71. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wandt H, Schäfer-Eckart K and Greinacher
A: Platelet transfusion in hematology, oncology and surgery. Dtsch
Arztebl Int. 111:809–815. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gillison ML, Zhang Q, Jordan R, Xiao W,
Westra WH, Trotti A, Spencer S, Harris J, Chung CH and Ang KK:
Tobacco smoking and increased risk of death and progression for
patients with p16-positive and p16-negative oropharyngeal cancer. J
Clin Oncol. 30:2102–2111. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hong AM, Martin A, Chatfield M, Jones D,
Zhang M, Armstrong B, Lee CS, Harnett G, Milross C, Clark J, et al:
Human papillomavirus, smoking status and outcomes in tonsillar
squamous cell carcinoma. Int J Cancer. 132:2748–2754.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Beynon RA, Lang S, Schimansky S, Penfold
CM, Waylen A, Thomas SJ, Pawlita M, Waterboer T, Martin RM, May M
and Ness AR: Tobacco smoking and alcohol drinking at diagnosis of
head and neck cancer and all-cause mortality: Results from head and
neck 5000, a prospective observational cohort of people with head
and neck cancer. Int J Cancer. 143:1114–1127. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Peterson LA, Bellile EL, Wolf GT, Virani
S, Shuman AG, Taylor JM and Rozek LS: University of Michigan Head
and Neck Specialized Program of Research Excellence Program.
Cigarette use, comorbidities, and prognosis in a prospective head
and neck squamous cell carcinoma population. Head Neck.
38:1810–1820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dumanski JP, Rasi C, Lönn M, Davies H,
Ingelsson M, Giedraitis V, Lannfelt L, Magnusson PK, Lindgren CM,
Morris AP, et al: Mutagenesis Smoking is associated with mosaic
loss of chromosome Y. Sience. 347:81–83. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kawakita D, Hosono S, Ito H, Oze I,
Watanabe M, Hanai N, Haseqawa Y, Tajima H, Murakami S, Tanaka H and
Matsuo K: Impact of smoking status on clinical outcome in oral
cavity cancer patients. Oral Oncol. 48:186–191. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sethi S, Ali-Fehmi R, Franceschi S,
Struijk L, van Doorn LJ, Quint W, Albashiti B, Ibrahim M and Kato
I: Characteristics and survival of head and neck cancer by HPV
status: A cancer registry-based study. Int J Cancer. 131:1179–1186.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Browman GP, Mohide EA, Willan A, Hodson I,
Wong G, Grimard L, MacKenzie RG, El-Sayed S, Dunn E and Farrell S:
Association between smoking during radiotherapy and prognosis in
head and neck cancer: A follow-up study. Head Neck. 24:1031–1037.
2002.PubMed/NCBI View Article : Google Scholar
|
















