Introduction
Patients with a new diagnosis of early breast cancer
require axillary node staging to quantify regional metastatic
involvement, and this provides key prognostic information used for
determination of individual treatment plans. Staging of the axilla
prior to surgery using ultrasound ± axillary ultrasound
(AUS)-guided biopsy (AUSB) and routine fixation with haematoxylin
and eosin (H&E) staining, is frequently performed to obtain
detailed information in patients newly diagnosed with breast cancer
(1,2). The removal of all axillary nodes with
an axillary lymph node dissection (ALND) is recommended in patients
with a positive biopsy. When the biopsy is negative, operative
assessment is required with a sentinel lymph node (SLN) biopsy
(SLNB) (1). When the SLNB is found
to be free from metastatic disease, no further axillary treatment
is recommended.
Patients with early stage (T1-2) disease, ≤2
macro-metastatic SLNs who are treated with SLNB alone, breast
conservation surgery, whole-breast radiotherapy and adjuvant
systemic therapy exhibit similar disease-free and overall survival
rates compared with those who undergo ALND (3,4). As
such, ALND may be considered unnecessary or in excess for treatment
of patients with early breast cancer and a low burden of axillary
node involvement. A routine decision to proceed with ALND following
the return of a single positive biopsy from AUSB may thus be out of
step with emerging and contemporary practice.
A reliable intraoperative technique that identifies,
quantifies and predicts axillary lymph node involvement may offer
selective and more conservative surgical treatment of the axilla in
a single procedure. ALND, and its associated increased risk of
morbidity, may be avoided whilst also providing benefits to these
patients, conserving resources and remaining in compliance with
emerging clinical practice guidelines (3,5). SLNB
combined with an intraoperative molecular-based assessment,
one-step nucleic acid cytokeratin-19 (CK-19) amplification assay
(OSNA), provides an objective whole-node assessment of SLN disease
burden that is independent of the size or number of lymph nodes
tested (6). For these reasons, OSNA
possesses greater potential for predicting residual axillary nodal
involvement compared with routine histopathological assessment, as
histopathological assessment does not offer timely intraoperative
SLN evaluation, is subjective, categorical and is at increased risk
of sampling errors with sub-total node assessment (7,8). OSNA
amplifies CK19 mRNA in SLN samples, typically providing a
quantitative measurement of metastatic disease burden within 35
min. OSNA can be used to inform prognosis and stratify nodal
disease burden into negative, micro-metastatic, ≤2 node
macro-metastatic, and >2 and >4 node macro-metastatic levels
(7-11).
The total CK19 mRNA copy number of a SLNB [(total tumour load
(TTL)] may predict non-SLN (NSLN) involvement and axillary node
disease burden, facilitating the decision to proceed with, or avoid
complete ALND.
OSNA is currently used in >300 centres across
Europe and in Japan (Sysmex Europe GmbH). It has been fully
validated and was approved for routine clinical use in the UK by
the National Institute of Clinical Excellence (NICE) in
2013(12). In routine operating
practices, SLNB is performed first and the node(s) are sent to the
pathology department for OSNA. Whilst awaiting the results, the
surgeon will continue with the primary breast procedure and, when
appropriate, closure of the primary breast wound. However, ~70% of
OSNA procedures are negative. The latest analyser can return
results within 35 min so there is essentially no delay introduced
by the procedure. The cost-effective savings and improvements in
the patient care are provided by proceeding to perform a single
procedure for the ~30% of patients who actually require ALND. There
is no need for the patient to return to hospital for ALND. There is
no duplication of theatre time, anaesthetic or surgical resources
(including time, reusables, disposables and surgical instrument
re-sterilization). There is no inconvenience to the patient with a
return to hospital, and operating theatre availability is increased
downstream for other patients. NICE concluded that the RD-100i OSNA
system was likely to be a cost-effective use of resources, equally
or more cost effective than postoperative histopathology (12). Fiscal savings in terms of reduced
secondary surgeries and bed occupancy (in 2015) were potentially
worth €150 per patient (12-14).
The aim of the present study was to compare the
accuracy and utility of SLNB with OSNA to AUSB with routine
histochemistry in staging the axilla of patients with a new
diagnosis of early breast cancer. Using a retrospective cohort
study of consecutive patients over a 3-year period, an extended
role for OSNA to define a group of patients for whom pre-operative
assessment of nodal staging with AUSB is redundant and may
represent an unnecessary physical intervention with duplication of
pathology resources was identified.
Patients and methods
Patients
The present study was a retrospective, single-centre
cohort study of consecutive patients, treated between December 2012
and August 2015, with primary invasive cT1-3 breast carcinoma. All
patients received AUS ± AUSB, and the patients were divided into
two main groups: i) Patients with a single positive AUSB (AUSB+)
who proceeded to ALND (median age, 61.2 years; age range 23-93; 191
female, 0 male) and ii) patients with a normal AUS or negative
biopsy (AUSB-) followed by SLNB with OSNA ± ALND (median age, 54.2
years; age range, 23-92; 687 female, 4 male).
Patient data were anonymized and collected
retrospectively, without influence on patient therapy. The present
study was approved by the Clinical Effectiveness Unit of Sheffield
Teaching Hospitals NHS Trust. As part of a service evaluation of
two standardised diagnostic interventions, there was no requirement
to obtain informed consent from the patients. No patient was
interviewed or surveyed, and no identifiable patient data were used
in the study. Therefore, consent for participation from patients
was waived.
Patients who received neo-adjuvant chemotherapy
prior to AUS ± AUSB, underwent ipsilateral axillary surgery, or had
recurrent disease or extensive ductal carcinoma in situ
(DCIS) without invasion were excluded. Bilateral breast cancer
cases were treated as separate entities. A threshold of 1-2
metastatic SLNs was used as a surrogate measure of low-burden
axillary node disease. This threshold corresponds to the level
reported in the American College of Surgeons Oncology Group
(Alliance) ACOSOG Z0011 trial (3,4).
Analysis was performed on the whole patient group and a subset of
patients, separately identified from the whole group, who met the
full criteria of the ACOSOG Z0011 trial; that is, no pre-operative
chemotherapy, T1 or T2 tumour, 1 or 2 positive SLN's,
breast-conserving surgery and planned whole breast radiotherapy.
ACOSOG trial selection criteria compliant patient characteristics
are not detailed since patient outcomes (such as local recurrence,
disease-free or overall survival) are not measured or relevant, and
trial outcomes comparison unintended. In contrast with the ACOSOG
Z0011 study, all patients in the present study underwent AUS prior
to breast surgery. Those with an abnormal lymph node morphology
underwent immediate ultrasound guided biopsy, and those with
confirmed metastatic disease were recommended to undergo ALND
without SLNB ± OSNA, the protocol used at the time of this
study.
Pre-operative assessment of the
axilla
B mode ultrasound examination of the axilla was
performed by experienced breast radiologists and advanced breast
practitioners, following a standardised protocol (15). With the patient placed in a supine
position and the ipsilateral hand resting behind the head, the
axilla was scanned in a longitudinal (L) and transverse (T)
direction using a Siemens Acuson S2000 18L6 high-density high
frequency linear-array transducer (5.5-18.0 MHz; Siemens AG). Lymph
nodes with an abnormal appearance were identified using qualitative
criteria including size, oval or round shape, absent fatty hilum,
abnormal peripheral blood flow, sharpness of the margin and focal
thickening of the cortex (16-18).
These nodes were targeted and the L/T axis ratio (Solbiati index),
hilum and cortical thickness were measured. If there was >1
abnormal node in the axilla, the most morphologically abnormal node
was sampled. Lymph nodes with an absent fatty hilum, L/T index
<2 and/or cortical thickness >2.9 mm were biopsied, under
direct ultrasound guidance, using local anaesthetic and a 90 mm 18
g core-cut needle with automated throw (Achieve programmable
automatic biopsy system; CareFusion).
SLN identification and OSNA
SLNB was performed using a standard protocol using a
combination of radiopharmaceutical and blue dye (19). 99mTc-labelled albumin
nanocolloid (Nanocoll®; GE Healthcare) was injected
intradermally (0.1-0.5 ml) at a single periareolar site
corresponding to the tumour quadrant; 40 MBq the day before surgery
or 20 MBq on the day of surgery. Patent Blue V Dye (Laboratoire
Guebert; 2 ml undiluted) was injected subdermally at a single
periareolar site corresponding to the tumour quadrant immediately
prior to surgery. Under general anaesthetic, SLNs were identified
and removed prior to breast tumour excision, and sent on ice to the
Pathology Department; no more than 2 nodes were sent for assessment
by OSNA. Any additional SLNs were sent for routine fixation,
H&E staining and delayed reporting (20). Therapeutic local excision,
therapeutic mammoplasty or mastectomy was performed as part of the
planned breast cancer treatment. Each SLN, trimmed of fat, was
weighed and recorded. SLNs weighing <50 mg were too small to be
processed by OSNA, and therefore used for routine histological
assessment. SLNs weighing >600 mg were divided into two or more
pieces and processed separately, and the results combined. The OSNA
assay was performed according to the manufacturer's protocol
(Sysmex Europe GmbH) (6). Each SLN
was homogenized in 4 ml homogenizing buffer on ice. The lysate was
centrifuged to remove fat, cellular debris and other contaminants,
and the mRNA containing supernatant was extracted and diluted. A 2
µl aliquot of the buffered lymph node lysate was used for automated
quantitative amplification of CK19 mRNA via reverse transcription
loop-mediated isothermal amplification (RT-LAMP) using a ready-to
use reagent kit on a RD-100i system (Sysmex Europe GmbH). The rate
of amplification was measured spectrophotometrically and the CK19
copy number calculated by comparison to a standard curve. Based on
the number of CK19 mRNA copies/µl, the result was assessed in
accordance with the cut-off levels determined by Tsujimoto et
al (6) with non-metastatic as
<250 copies/µl, micro-metastasis as 250-5,000 copies/µl and
macro-metastasis defined as >5,000 copies/µl of CK19 mRNA. The
OSNA results were communicated by telephone to the surgeon within
45 min of sample receipt. Patients with at least one
macro-metastasis on intraoperative OSNA analysis underwent levels
I, II and III ALND. Between December 2012 and June 2013, a positive
OSNA result for one or two nodes with micro-metastases meant the
patient immediately underwent ALND. In June 2013, the departmental
protocol was amended to recommend the removal of two further nodes
for routine histological processing with a delayed ALND if these
returned macro-metastatic involvement. All remaining lymph nodes
not used for OSNA were processed according to the UK Breast Cancer
pathology protocol (20). Lymph
nodes <5 mm were bisected whereas larger nodes were sectioned
into 3 mm single sections and assessed using H&E staining.
Statistical analysis
For statistical analysis, when ≥2 SLNs were
involved, the combined value of CK19 mRNA copies was calculated.
The TTL was defined as total CK19 mRNA copy number in all the
positive SLNs (copies/µl). The TTL of the macro-metastatic SLNB
sample was compared with the total lymph node status and NSLN
status of ALND, following routine histological assessment with
H&E staining. For subset data interpretation, the TTL was set
at 5,000 copies/µl for recommendation of ALND and 15,000 copies/µl
as a threshold of 2 positive nodes (10). Tests for association were determined
using a χ2 test or Fisher's Exact test between the AUSB
group and the SLNB/ALND group. All tests were two-tailed and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Between December 2012 and August 2015, there were
1,315 new diagnoses of cT1-3 carcinoma in 1,306 consecutive
patients. Of these, 266 (20.4%) patients with cT1-3 cN0 staging
received 271 AUSBs as part of their assessment; 205 AUSB+ and 66
AUSB-. Based on the results, 23 cases (14 AUSB+ and 9 AUSB-) were
excluded from the 271 AUSB analyses; 9 patients were treated with
primary endocrine therapy only, 8 patients had DCIS only on
subsequent pathological reporting, 3 patients had metastatic
disease on presentation, 2 patients had recurrent disease and 1
patient had a contralateral lymph node biopsy diagnosing
intercurrent chronic lymphocytic leukaemia. Complete AUSB
assessment was performed on 191 AUSB+ and 57 AUSB-biopsies.
A total of 700 SLNB with OSNA procedures were
performed in 691 patients with an AUS-/AUSB-assessment. A total of
16 cases were excluded from this group; 15 patients had extensive
DCIS only on the final histological analysis, and 1 patient had
received incomplete neo-adjuvant therapy (Fig. 1). The remaining 349/1,306 patients
had normal AUS assessments and did not proceed to SLNB with OSNA
for multiple reasons including advanced age and/or concurrent
illness (unfit for surgery), metastatic disease at presentation,
previous ipsilateral axillary surgery or a decision to provide
neo-adjuvant chemotherapy or primary endocrine therapy.
Macro-metastatic lymph node involvement was finally diagnosed in
348/971 of the axillae studied (35.8%); 279 of these 348 axillae
were further characterised as >2 or ≤2 macro-metastatic disease
burden by ALND. The remaining 69/348 were lost to treatment naïve
ALND analysis by above and below mentioned exclusions, including
standard pre-ALND treatment means.
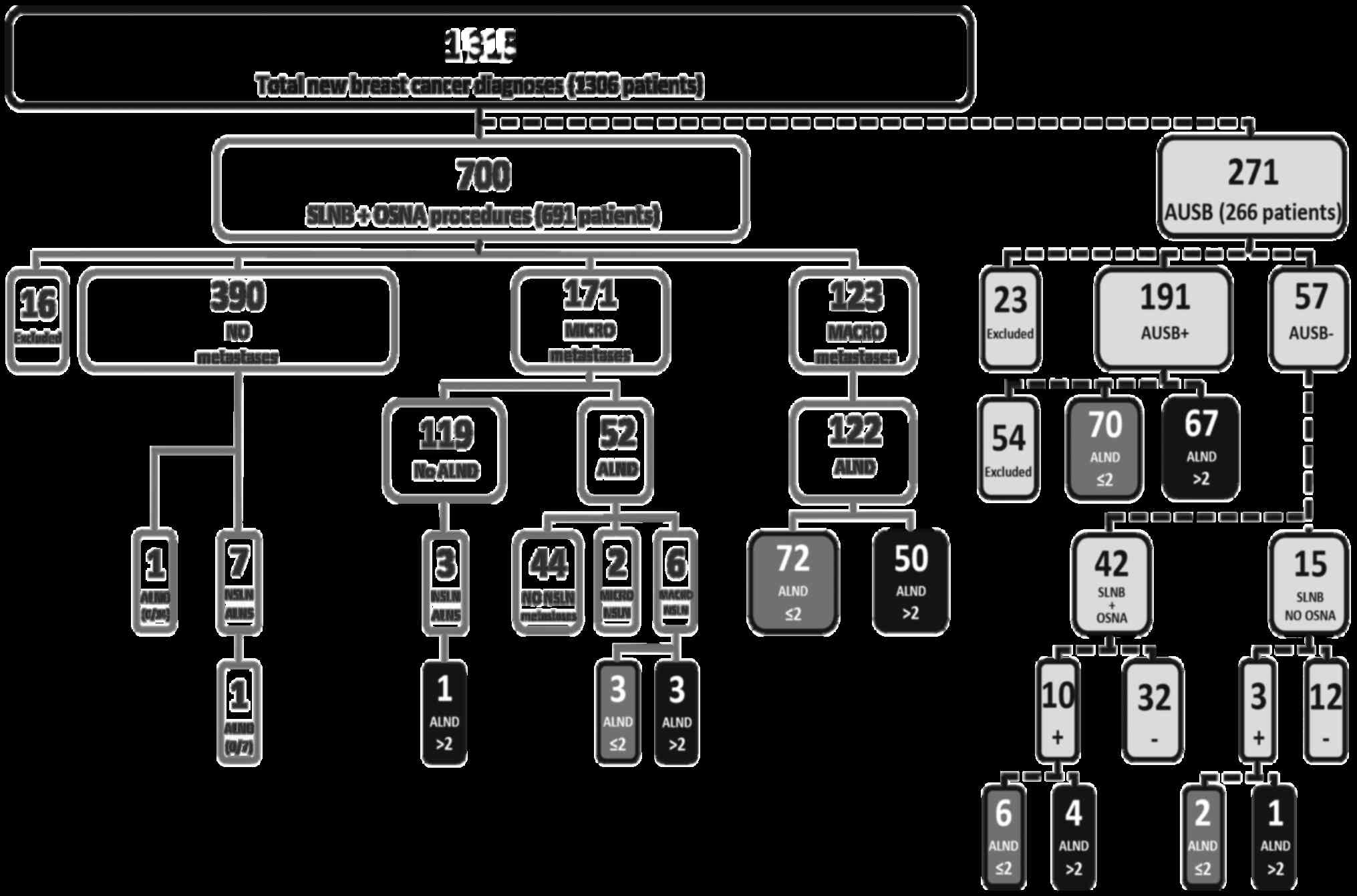 | Figure 1Cohort distribution flow chart. SLNB,
sentinel lymph node biopsy; OSNA, One-step Nucleic Acid
Amplification; ALND, axillary lymph node dissection; NSLN,
non-sentinel lymph node; AUSB, axillary ultrasound biopsy; +,
positive node (metastatic disease present); -, negative node
(metastatic disease absent); MICRO, micro-metastases; MACRO,
macro-metastases; ALNS, axillary lymph node sample. |
Of the 191 AUSB+ assessments, 137 proceeded directly
to ALND; 54 were censored after treatments that precluded further
analysis of nodal involvement (31 received chemotherapy, 19
received primary endocrine therapy only, 3 had breast only surgery
for local control and 1 received radiotherapy only). A total of
70/137 (51%) of the AUSB+ patients who subsequently underwent ALND
had ≤2 positive nodes. For an AUSB+, estimating a nodal burden of
>2 (positive) axillary lymph node metastases was expressed as
the sensitivity and negative predictive value (NPV) of AUS of 0.53
[(0.44-0.62), 95% confidence interval (CI)] and 0.58 (0.53-0.64,
95% CI), respectively (Table I).
The false negative rate (FNR) of AUSB correctly predicting an
axillary node burden of >2 macro-metastatic nodes was 47%
(accuracy, 53.8%).
 | Table ISensitivity, specificity and
predictive values of AUSB based on a threshold of 2 positive nodes
for selective axillary preservation for all axillary LN
dissections. |
Table I
Sensitivity, specificity and
predictive values of AUSB based on a threshold of 2 positive nodes
for selective axillary preservation for all axillary LN
dissections.
| AUSB | LN>2 | LN≤2 | Total |
|---|
| + | 67 | 70 | 137 |
| - | 59 | 83 | 142 |
| Total | 126 | 153 | 279 |
The sensitivity and NPV for SLNB + OSNA, using a TTL
cut-off of 15,000 copies/µl for predicting >2 macro-metastases,
were 0.82 (0.71-0.92, 95% CI) and 0.98 (0.97-0.99, 95% CI;
P<0.0001, OSNA vs. AUS) for all patients (Table II) and 0.87 (0.73-1.0) and 0.99
(0.99-1.0; P<0.0001, OSNA vs. AUS) for patients matched to the
ACOSOG Z0011 trial inclusion criteria, identified and analysed
separately (Table III). The FNR
of SLNB + OSNA correctly predicting an axillary node burden of
>2 macro-metastatic nodes was 18% (accuracy, 87.6%) in the whole
group and 13% (accuracy, 90.8%) for the Z0011 matched group. The
NPV for all axillae not associated with breast conserving surgery
was 0.949 (95% CI, 0.906-0.973).
 | Table IISensitivity, specificity and
predictive values of OSNA based on a threshold of 2 positive nodes
for selective axillary preservation for all axillae. |
Table II
Sensitivity, specificity and
predictive values of OSNA based on a threshold of 2 positive nodes
for selective axillary preservation for all axillae.
| OSNA |
LN>2a | LN≤2a | Total |
|---|
| + | 44 | 58 | 102 |
| - | 10 | 572 | 582 |
| Total | 54 | 630 | 684 |
 | Table IIISensitivity, specificity and
predictive values of OSNA based on a threshold of 2 positive nodes
for selective axillary preservation for all ACOSOG Z0011
criteria-matched axillaea. |
Table III
Sensitivity, specificity and
predictive values of OSNA based on a threshold of 2 positive nodes
for selective axillary preservation for all ACOSOG Z0011
criteria-matched axillaea.
| OSNA |
LN>2b | LN≤2b | Total |
|---|
| + | 20 | 35 | 55 |
| - | 3 | 441 | 444 |
| Total | 23 | 476 | 499 |
Discussion
The clinical utility of AUS ± AUSB to stage
clinically node-negative early breast cancer is contested. The
ACOSOG Z0011 trial, and several other studies have reported that
patients with low burden axillary node involvement do not require
ALND, demonstrating no adverse impact on locoregional control or
overall survival (3,4,21-23).
These findings are resulting in surgical practice moving towards
selective conservation of the node involved axilla (4,5,24,25).
In the present study, 51% of patients with AUSB+ had ≤2 involved
nodes following ALND. This observation is consistent with the
findings of other studies, which reported between 41-52% of cases
(26-30)
and supports the notion of excess treatment in a substantial
proportion of patients. As an instrument for deciding between ALND
and axillary conservation, AUSB+ alone does not provide sufficient
discrimination, particularly in Z0011 criteria compliant patients.
Meeting the demands of emerging clinical practices to confidently
deliver conservative management of the low-burden node-positive
axilla in early breast cancer requires scales of quantification and
stratification of axillary nodal disease burden greater than that
provided by AUS ± AUSB (31).
The sensitivity of AUS assessment is highly
dependent on the prevalence and extent of axillary tumour burden
(26,32-35).
Stachs et al (15) reported
AUS sensitivity of 45.2%, similar to the present study. With
regards to avoiding unnecessary ALND surgery and its associated
complications, the sensitivity and specificity of AUSB calculated
in the present study questions the logic of performing the
procedure when only one node, or possibly two or have appearances
considered indeterminate on imaging in this group of patients.
These findings suggest that AUSB should not be performed routinely
in patients with a new presentation of cT1-2 cN0 breast cancer, but
used selectively when the ultrasound analysis identifies multiple
(>2) node involved, in disagreement with the clinical
findings.
Nodal macro-metastases were present in 35.8%
(348/971) of the present cohorts' axillae. Just over half of these
(59%; 205/348) were detected, but not quantified, by AUS
assessment. On the other hand, a negative AUS assessment did not
exclude the possibility of lymph node metastases. Of the AUSBs,
66/271 (24.3%) were negative, of which 13/57 (22.8%) AUSB-axillae
and 130/700 (18.6%) AUS-axillae had macro-metastatic nodal disease
following ALND. The SLNB FNR is inversely related to the number of
nodes removed (36), supporting the
observation of a high FNR for AUSB with sub-total, core-cut biopsy
of a single node. In the present study, the observed mean yield of
1.94 SLNs for OSNA TTL per patient, is in agreement with a median
of 2 SLN observed in >11,000 patients in the ACOSOG Z0011 trial,
the New Start programme and the AMAROS trial, collectively
(4,37,38).
The standard technique of SLNB has an FNR of <9% for two nodes
(36). However, axillary recurrence
risk remains low, even with a range of reported SLNB FNR varying
between 6.7-14.8% (19,36,39,40).
OSNA, with an FNR of 1.4%, adds very little to the overall FNR
(41). The NPV for all axillae not
associated with breast conserving surgery is 0.949 (95% CI,
0.906-0.973). This finding suggests that whenever the OSNA TTL is
below the cut-off threshold of 15,000 copies/µl, it is likely to be
correct in predicting an axillary lymph node burden of ≤2 positive
nodes 94.9% of the time. This patient cohort (that is, those not
undergoing breast conserving surgery) includes patients with T3
tumours who are outside the criteria of ACOSOG but within the whole
cohort analysis of this study. The FNR of 5% in this group is also
well below the FNR of SLNB for one and two nodes.
Moorman et al (34) reported that only 9.6% of 1,060
patients (with T1-2 cN0 disease and a maximum of 2 SLNs with
macro-metastases) had >2 positive axillary nodes on ALND,
estimating the risk of having >2 positive nodes at 2.2% for
pT1-2. The present study reported a similar low risk of involvement
of >2 positive nodes of 7.9% (54/684 for cT1-3) and 4.6% (23/499
for cT1-2 cN0) for SLNB + OSNA, using a TTL cut-off of 15,000
copies/µl for predicting >2 macro-metastases. The reported rates
of isolated axillary recurrence in SLN-disease is ~0.6% after 3
years and 1.1% after 5 years (42),
similar to the 0.9% (5 years) and 1.5% (10 years) for SLN+ disease
without ALND in the Z0011 trial (3,4). Thus,
with such low estimates of risk, it seems illogical to remain
preoccupied with the need to perform AUSB, let alone ALND, on
patients with cT1-T2 disease and a clinically negative axilla when
local recurrence is 2.5-11x more likely to occur in a fully
irradiated breast than in partially irradiated ipsilateral axilla
(3).
SLNB with OSNA, using a TTL cut-off of 15,000
copies/µl, is better than AUSB for assessing nodal involvement in
patients naïve to breast surgery and radiotherapy, predicting ≥2
nodal macro-metastases and facilitating the decision to perform
ALND or adopt axillary conservation as defined by ACOSOG Z0011 and
the National Comprehensive Cancer Network guidelines (4,43). The
argument that AUSB+ can avoid unnecessary SLNB for patients
requiring ALND is no longer relevant as 51% of AUSB+ patients had
≤2 positive nodes and were potentially over-treated with ALND. The
remaining 49% of these patients may benefit from ALND, but there is
little evidence to support the notion that they all will (3,44-46).
Where OSNA is available, the present study recommends that patients
who present with cT1-2 cN0 breast carcinoma, should continue to
receive AUS as part of their routine follow-up, but only
selectively include AUSB if there appear to ≥3 abnormal nodes on
imaging. Otherwise, axillary staging is more relevant and practical
using SLNB with OSNA. Additionally, for AUSB+ patients, where ≤2
suspicious nodes are seen on AUS, automatic progression to ALND
should be challenged by using further intraoperative quantitative
assessment with SLNB and OSNA. The decision to proceed with ALND,
or adopt axillary conservation, can be determined by OSNA TTL
quantification.
In conclusion, where available, OSNA should replace
AUSB as the primary and preferred method for axillary assessment in
cT1-2 cN0 patients with ≤2 indeterminate nodes on AUS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request. The data may be subject to institutional permission for
release.
Authors' contributions
BI and SK conceived and designed the study, and
contributed to data acquisition, analysis and interpretation, and
writing and revising the manuscript. VF collected and analysed the
OSNA data. NAS was involved in data analysis and interpretation,
and in writing and revising the manuscript. SH performed axillary
ultrasound examinations, was involved in writing and revising the
manuscript, and in acquisition of the ultrasound-guided biopsy
data. OH performed axillary ultrasound examinations and was
involved in acquisition of axillary ultrasound biopsy data. PS
performed axillary ultrasound examinations and contributed to
radiology data acquisition, analysis and interpretation of the
data, and in writing and revising the manuscript. GJZ was a major
contributor of OSNA data acquisition and interpretation, and in
writing and revising the manuscript. NRW contributed to statistical
analysis, data interpretation and contributed to writing and
revising the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Patient data were anonymised, and collected
retrospectively, without influence on patient therapy. This study
was approved by the Clinical Effectiveness Unit of the Sheffield
Teaching Hospitals Trust (approval no. 7137). Consent for
participation from patients was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schipper RJ, van Roozendaal LM, de Vries
B, Pijnappel RM, Beets-Tan RG, Lobbes MB and Smidt ML: Axillary
ultrasound for preoperative nodal staging in breast cancer
patients: Is it of added value? Breast. 22:1108–1113.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Humphrey KL, Saksena MA, Freer PE, Smith
BL and Rafferty EA: To do or not to do: Axillary nodal evaluation
after ACOSOG Z0011 trial. Radiographics. 34:1807–1816.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Giuliano AE, Ballman K, McCall L, Beitsch
P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Morrow M and
Hunt KK: Locoregional recurrence after sentinel lymph node
dissection with or without axillary dissection in patients with
sentinel lymph node metastases: Long-term follow-up from the
american college of surgeons oncology group (Alliance) ACOSOG Z0011
randomized trial. Ann Surg. 264:413–420. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Giuliano AE, Ballman KV, McCall L, Beitsch
PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW,
Blumencranz PW, et al: Effect of axillary dissection vs. no
axillary dissection on 10-year overall survival among women with
invasive breast cancer and sentinel node metastasis. The ACOSOG
Z0011 (Alliance) randomized clinical trial. JAMA. 318:918–926.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee J, Choi JE, Kim SJ, Lee SB, Seong MK,
Jeong J, Yoon CS, Kim BK and Sun WY: Korean Breast Cancer Society.
Comparative study between sentinel lymph node biopsy and axillary
dissection in patients with one or two lymph node metastases. J
Breast Cancer. 21:306–314. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tsujimoto M, Nakabayashi K, Yoshidome K,
Kaneko T, Iwase T, Akiyama F, Kato Y, Tsuda H, Ueda S, Sato K, et
al: One-step nucleic acid amplification for intraoperative
detection of lymph node metastasis in breast cancer patients. Clin
Cancer Res. 13:4807–4816. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Di Filippo F, Giannarelli D, Bouteille C,
Bernet L, Cano R, Cunnick G and Sapino A: Elaboration of a nomogram
to predict non sentinel node status in breast cancer patients with
positive sentinel node, intra-operatively assessed with o nucleic
acid amplification method. J Exp Clin Cancer Res.
34(136)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Peg V, Espinosa-Bravo M, Vieites B,
Vilardell F, Antúnez JR, de Salas MS, Delgado-Sánchez JJ, Pinto W,
Gozalbo F, Petit A, et al: Intraoperative molecular analysis of
total tumor load in sentinel lymph node: A new predictor of
axillary status in early breast cancer patients. Breast Cancer Res
Treat. 139:87–93. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Koca B, Kuru B, Ozen N, Yoruker S and Bek
Y: A breast cancer nomogram for prediction of non-sentinel node
metastasis-validation of fourteen existing models. Asian Pac J
Cancer Prev. 15:1481–1488. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fung V, Kohlhardt S, Vergani P, Zardin GJ
and Williams NR: Intraoperative prediction of the two axillary
lymph node macrometastases threshold in patients with breast cancer
using a one-step nucleic acid cytokeratin-19 amplification assay.
Mol Clin Oncol. 7:755–762. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Peg V, Sansano I, Vieites B, Bernet L,
Cano R, Córdoba A, Sancho M, Martín MD, Vilardell F, Cazorla A, et
al: Role of total tumour load of sentinel lymph node on survival in
early breast cancer patients. Breast. 33:8–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Intraoperative tests (RD-100i OSNA system
and Metasin test) for detecting sentinel lymph node metastases in
breast cancer. NICE diagnostics guidance 8, 2013. Available from:
http://www.nice.org.uk/dg8.
|
|
13
|
Raia-Barjat T, Trombert B, Khaddage A,
Douchet C, Seffert P, Peoc'h M, Falk AT, Magné N and Chauleur C:
OSNA (one-step nucleic acid amplification) sentinel lymph node
intraoperative molecular analysis in breast cancer: A cost-benefit
analysis. Med Oncol. 31(322)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Brambilla T, Fiamengo B, Tinterri C,
Testori A, Grassi MM, Sciarra A, Abbate T, Gatzemeier W, Roncalli M
and Di Tommaso L: One-step nucleic acid amplification in breast
cancer sentinel lymph node: A single institutional experience and a
short review. Front Med (Lausanne). 2(37)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stachs A, Thi AT, Dieterich M, Stubert J,
Hartmann S, Glass Ä, Reimer T and Gerber B: Assessment of
ultrasound features predicting axillary nodal metastasis in breast
cancer: The impact of cortical thickness. Ultrasound Int Open.
1:E19–E24. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cho N, Moon WK, Han W, Park IA, Cho J and
Noh DY: Preoperative sonographic classification of axillary lymph
nodes in patients with breast cancer: Node-to-node correlation with
surgical histology and sentinel node biopsy results. AJR Am J
Roentgenol. 193:1731–1737. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bedi DG, Krishnamurthy R, Krishnamurthy S,
Edeiken BS, Le-Petross H, Fornage BD, Bassett RL Jr and Hunt KK:
Cortical morphologic features of axillary lymph nodes as a
predictor of metastasis in breast cancer: In vitro sonographic
study. AJR Am J Roentgenol. 191:646–652. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang WT, Chang J and Metreweli CL:
Patients with breast cancer: Differences in color doppler flow and
gray-scale US features of benign and malignant axillary lymph
nodes. Radiology. 215:568–573. 2000.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mansel RE, MacNeill F, Horgan K, Goyal A,
Britten A, Townson J, Clarke D, Newcombe R and Keshtgar M:
Guildford Breast Surgeons et al. Results of a national
training programme in sentinel lymph node biopsy for breast cancer.
Br J Surg. 100:654–661. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
NHS Cancer Screening Programmes/Royal
College of Pathologists: Pathology Reporting of Breast Disease.
NHSBSP publication no. 58, 2005. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/541521/pathology_reporting_of_breast_disease.pdf.
Accessed May 21, 2016.
|
|
21
|
Galimberti V, Cole BF, Zurrida S, Viale G,
Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, et
al: Axillary dissection versus no axillary dissection in patients
with sentinel-node micrometastases (IBCSG 23-01): A phase 3
randomised controlled trial. Lancet Oncol. 14:297–305.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Glechner A, Wöckel A, Gartlehner G, Thaler
K, Strobelberger M, Griebler U and Kreienberg R: Sentinel lymph
node dissection only versus complete axillary lymph node dissection
in early invasive breast cancer: A systematic review and
meta-analy. Eur J Cancer. 49:812–825. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Straver ME, Meijnen P, van Tienhoven G,
van de Velde CJ, Mansel RE, Bogaerts J, Demonty G, Duez N,
Cataliotti L, Klinkenbijl J, et al: Role of axillary clearance
after a tumor-positive sentinel node in the administration of
adjuvant therapy in early breast cancer. J Clin Oncol. 28:731–737.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Caudle AS, Hunt KK, Tucker SL, Hoffman K,
Gainer SM, Lucci A, Kuerer HM, Meric-Bernstam F, Shah R, Babiera
GV, et al: American college of surgeons oncology group (ACOSOG)
Z0011: Impact on surgeon practice patterns. Ann Surg Oncol.
19:3144–3151. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gainer SM, Hunt KK, Beitsch P, Caudle AS,
Mittendorf EA and Lucci A: Changing behavior in clinical practice
in response to the ACOSOG Z0011 trial: A survey of the American
society of breast surgeons. Ann Surg Oncol. 19:3152–3158.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Houssami N, Ciatto S, Turner RM, Cody HS
III and Macaskill P: Preoperative ultrasound-guided needle biopsy
of axillary nodes in invasive breast cancer: Meta-analysis of its
accuracy and utility in staging the axilla. Ann Surg. 254:243–251.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Caudle AS, Kuerer HM, Le-petross HT, Yang
W, Yi M, Bedrosian I, Krishnamurthy S, Fornage BD, Hunt KK and
Mittendorf EA: Predicting the extent of nodal disease in
early-stage breast cancer. Ann Surg Oncol. 21:3440–3447.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pilewskie M, Mautner SK, Stempel M, Eaton
A and Morrow M: Does a positive axillary lymph node needle biopsy
result predict the need for an axillary lymph node dissection in
clinically node-negative breast cancer patients in the ACOSOG Z0011
era? Ann Surg Oncol. 23:1123–1128. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ahmed M, Jozsa F, Baker R, Rubio IT,
Benson J and Douek M: Meta-analysis of tumour burden in
pre-operative axillary ultrasound positive and negative breast
cancer patients. Breast Cancer Res Treat. 166:329–336.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hieken TJ, Trull BC, Boughey JC, Jones KN,
Reynolds CA, Shah SS and Glazebrook KN: Preoperative axillary
imaging with percutaneous lymph node biopsy is valuable in the
contemporary management of patients with breast cancer. Surgery.
154:831–840. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Harris CK, Tran HT, Lee K, Mylander C,
Pack D, Rosman M, Tafra L, Umbricht CB, Andrade R, Liang W and
Jackson RS: Positive ultrasound-guided lymph node needle biopsy in
breast cancer may not mandate axillary lymph node dissection. Ann
Surg Oncol. 24:3004–3010. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Stachs A, Göde K, Hartmann S, Stengel B,
Nierling U, Dieterich M, Reimer T and Gerber B: Accuracy of
axillary ultrasound in preoperative nodal staging of breast
cancer-size of metastases as limiting factor. Springerplus.
2(350)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee B, Lim AK, Krell J, Satchithananda K,
Coombes RC, Lewis JS and Stebbing J: The efficacy of axillary
ultrasound in the detection of nodal metastasis in breast cancer.
Am J Roentgenol. 200:314–320. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Moorman AM, Bourez RL, Heijmans HJ and
Kouwenhoven EA: Axillary ultrasonography in breast cancer patients
helps in identifying patients preoperatively with limited disease
of the axilla. Ann Surg Oncol. 21:2904–2910. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Verheuvel NC, van den Hoven I, Ooms HW,
Voogd AC and Roumen RM: The role of ultrasound-guided lymph node
biopsy in axillary staging of invasive breast cancer in the
post-ACOSOG Z0011 trial era. Ann Surg Oncol. 22:409–415.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Goyal A, Newcombe RG and Mansel RE:
Axillary Lymphatic Mapping Against Nodal Axillary Clearance
(ALMANAC) Trialists Group. Clinical relevance of multiple sentinel
nodes in patients with breast cancer. Br J Surg. 92:438–442.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Goyal A, Newcombe RG, Chhabra A and Mansel
RE: ALMANAC Trialists Group. Factors affecting failed localisation
and false-negative rates of sentinel node biopsy in breast
cancer-results of the ALMANAC validation phase. Breast Cancer Res
Treat. 99:203–208. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Donker M, van Tienhoven G, Straver ME,
Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH,
Klinkenbijl JH, Orzalesi L, et al: Radiotherapy or surgery of the
axilla after a positive sentinel node in breast cancer (EORTC
10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3
non-inferiority trial. Lancet Oncol. 15:1303–1310. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Krag DN, Anderson SJ, Julian TB, Brown AM,
Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP,
Jalovec LM, et al: Sentinel-lymph-node resection compared with
conventional axillary-lymph-node dissection in clinically
node-negative patients with breast cancer: Overall survival
findings from the NSABP B-32 randomised phase 3 trial. Lancet
Oncol. 11:927–933. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kohrt HE, Olshen RA, Bermas HR, Goodson
WH, Wood DJ, Henry S, Rouse RV, Bailey L, Philben VJ, Dirbas FM, et
al: New models and online calculator for predicting non-sentinel
lymph node status in sentinel lymph node positive breast cancer
patients. BMC Cancer. 8(66)2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fujisue M, Nishimura R, Okumura Y, Tashima
R, Nishiyama Y, Osako T, Toyozumi Y and Arima N: Clinical
significance of CK19 negative breast cancer. Cancers (Basel).
5:1–11. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bergkvist L, de Boniface J, Jönsson PE,
Ingvar C, Liljegren G and Frisell J: Swedish Society of Breast
Surgeons. Axillary recurrence rate after negative sentinel node
biopsy in breast cancer: Three year follow-up of the Swedish
multicenter cohort study. Ann Surg. 247:150–156. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
National Comprehensive Cancer Network.
Breast Cancer (Version 4.2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
Accessed May 11, 2020.
|
|
44
|
International Breast Cancer Study Group.
Rudenstam CM, Zahrieh D, Forbes JF, Crivellari D, Holmberg SB, Rey
P, Dent D, Campbell I, Bernhard J, et al: Randomized trial
comparing axillary clearance versus no axillary clearance in older
patients with breast cancer: First results of international breast
cancer study group trial 10-93. J Clin Oncol. 24:337–344.
2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Martelli G, Boracchi P, Ardonino I, Lozza
L, Bohm S, Vetrella G and Agresti R: Axillary dissection versus no
axillary dissection in older patients with T1N0 breast cancer:
15-year results of a randomized controlled trial. Ann Surg.
256:920–924. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Agresti R, Martelli G, Sandri M, Tagliabue
E, Carcangiu ML, Maugeri I, Pellitteri C, Ferraris C, Capri G,
Moliterni A, et al: Axillary lymph node dissection versus no
dissection in patients with T1N0 breast cancer: A randomized
clinical trial (INT09/98). Cancer. 120:885–893. 2014.PubMed/NCBI View Article : Google Scholar
|















