Introduction
Carcinoids are carcinomas arising from the
neuroendocrine system and are composed of cells that have features
of both nerve cells and endocrine cells. Thymic carcinoids are
exceedingly rare malignant tumors accounting for approximately 2 to
5% of all thymic tumors and 0.4% of all carcinoid tumors with an
incidence rate of approximately 0.2 per 1,000,000 population per
year (1). This type of tumor occurs
over a wide age range (median age, 54 years) and has a male
predilection ratio of 3:1(2).
According to the WHO classification, two main
carcinoid subtypes have been established: Typical and atypical. The
subtypes can be distinguished based on morphology, mitosis count
and the presence or absence of necrosis (3). Thymic atypical carcinoids (TACs) are
considered to be well differentiated neuroendocrine carcinomas
(4). The term ‘atypical’ is applied
to those tumors observed histologically to have carcinoid
architecture, increased mitotic activity, and necrosis. Compared
with typical carcinoids, primary TACs are rare and more aggressive,
often connected with a poorer prognosis (5); the 5-year survival rate of TAC is
~80%, while that of a typical carcinoid is 100% (6).
Although occurrences of TACs have been previously
documented, a TAC with multiple skull metastases, including
metastases to the wall of the orbital cavities, the suprasellar
region, clivus, bilateral petrous apex and cervical vertebrae, has
not been previously reported. This patient only survived 89 days
after being diagnosed, indicating the extreme malignancy of
TACs.
Case report
A 54-year-old Chinese male was admitted to Wuhan
Union Hospital in November, 2019 (P.R. China) with complaints of
lower back pain for over 1 year, which had worsened since the
previous week, and not accompanied by cough, fever, headache,
expiratory dyspnea, thoracalgia, hoarseness or dysphagia. Physical
examination revealed elevated blood pressure of 146/95 mmHg. No
cutaneous masses or ulcerations were identified. The range of
motion activity of his waist and legs was limited because of the
stiffness of his lumbar vertebrae. Beyond that, the patient felt no
numbness or pain in his legs and demonstrated grade 5 muscle
strength. The patient exhibited normal physiological reflexes,
bowel movements and urine and had not lost or gained weight.
Initial laboratory findings showed elevated levels
of serum carbohydrate antigen (CA) 125, CA 199, ferritin and
neuron-specific enolase (NSE), which were marked as tumor
biomarkers (Table I). The levels of
plasma D-dimer and fibrinogen were also increased, suggesting
increased blood viscosity (Table
I). No blood chemistry abnormalities including β-2
microglobulin, lactate dehydrogenase and calcium concentrations
were observed. In addition, the basal endocrinological examination
revealed normal levels of serum cortisol, plasma
adrenocorticotropic hormone (ACTH), follicle-stimulating hormone,
luteinizing hormone, thyrotropin, parathyroid hormone, renin
activity, aldosterone, epinephrine, norepinephrine and dopamine.
The HIV antibody tested negative.
 | Table ILaboratory findings at admission. |
Table I
Laboratory findings at admission.
| Parameters | Value | Normal range |
|---|
| Serum tumor
marker | | |
|
AFP,
µg/l | 0.03 | 0.89-8.78 |
|
CEA,
µg/l | 3.34 | <5.0 |
|
CA125,
U/ml | 78.93a | <35.0 |
|
CA19-9,
U/ml | 120.55a | <37.0 |
|
CA15-3,
U/ml | 15.34 | <31.3 |
|
FERR,
µg/l | 767.11a | 21.8-275 |
|
fPSA,
µg/l | 0.04 | <0.93 |
|
PSA,
µg/l | 0.02 | <4.00 |
|
SCC,
ng/ml | 1.3 | <1.5 |
|
CYFRA21-1,
ng/ml | 1.2 | <2.5 |
|
NSE,
µg/l | 34.55a | <16.3 |
| Blood coagulation
function | | |
|
TT, sec | 19.1 | 14.0-21.0 |
|
FIB,
g/l | 7.14a | 2.0-4.0 |
|
APTT,
sec | 36.7 | 28.0-43.5 |
|
INR | 1.07 | 0.80-1.31 |
|
PT, sec | 13.7 | 11.0-16.0 |
|
D-D,
mg/l | 3.03a | <0.5 |
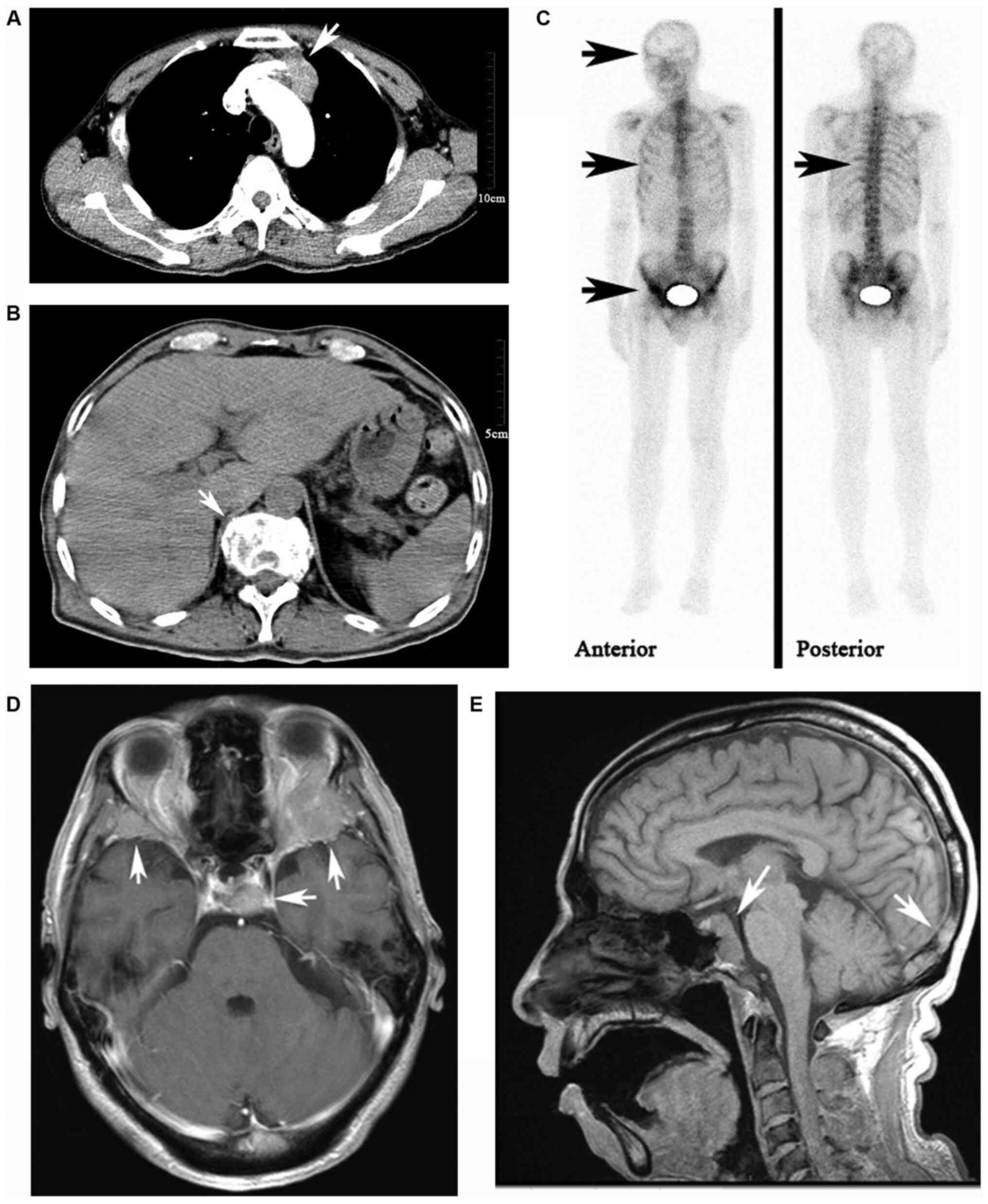
Contrast-enhanced computed tomography (CT) of the
thorax revealed that after contrast agent was administered, a
mildly enhanced soft tissue mass measuring 2.1x3.5x3.2 cm with a
sharp border in the left upper mediastinum next to the ascending
aorta was observed, with necrosis within it. Multiple ribs and
thoracic vertebrae were damaged, but no enlarged lymph nodes or
lung metastases were found (Fig.
1A). Contrast-enhanced CT of the abdomen did not reveal any
metastatic nodules in the liver, spleen or pancreas, nor did any
enlarged lymph node in the retroperitoneum. However, multiple
lumbar vertebrae damages were observed (Fig. 1B). Single-photon emission computed
tomography (SPECT) revealed increased uptake of imaging agent in
multiple bones, including bilateral rib bones, thoracic vertebrae,
lumbar vertebrae as well as the skull, which indicated multiple
bone metastases (Fig. 1C).
For diagnostic purposes, the patient underwent a
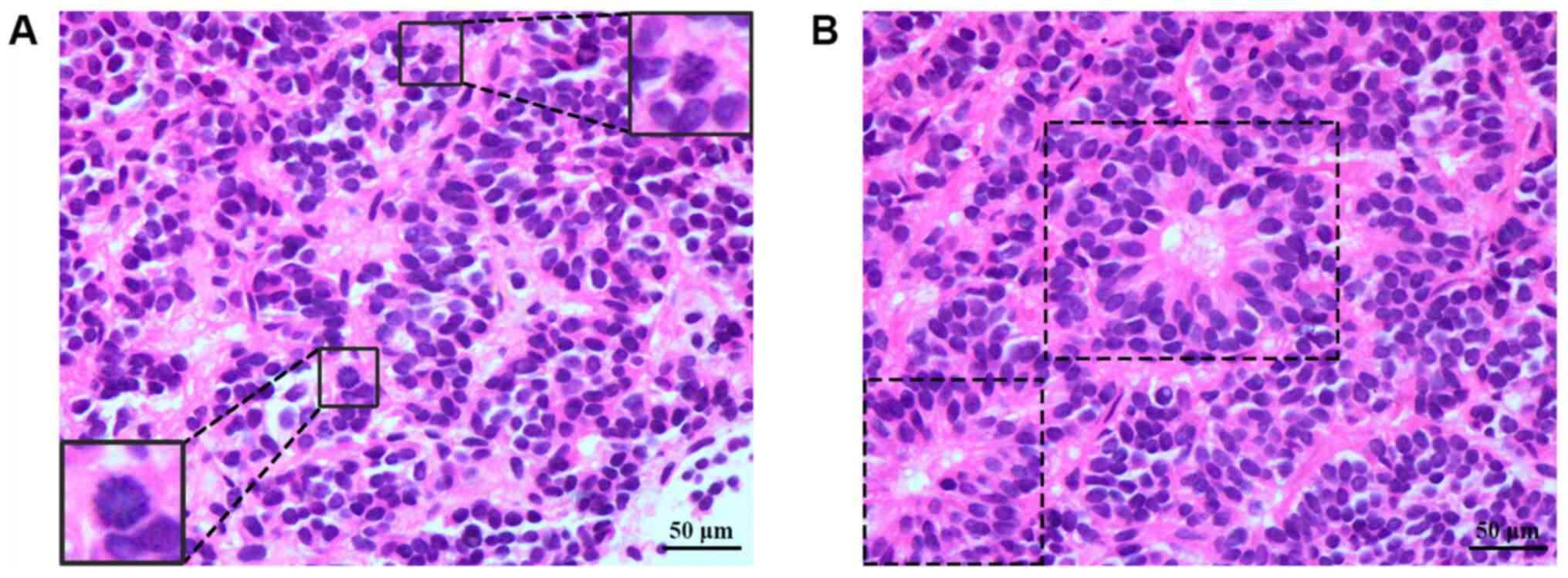
CT-guided, percutaneous tissue biopsy. High-magnification optical
microscopy showed a clustered growth pattern with 2 mitoses/10
high-power fields (HPFs) (Fig. 2).
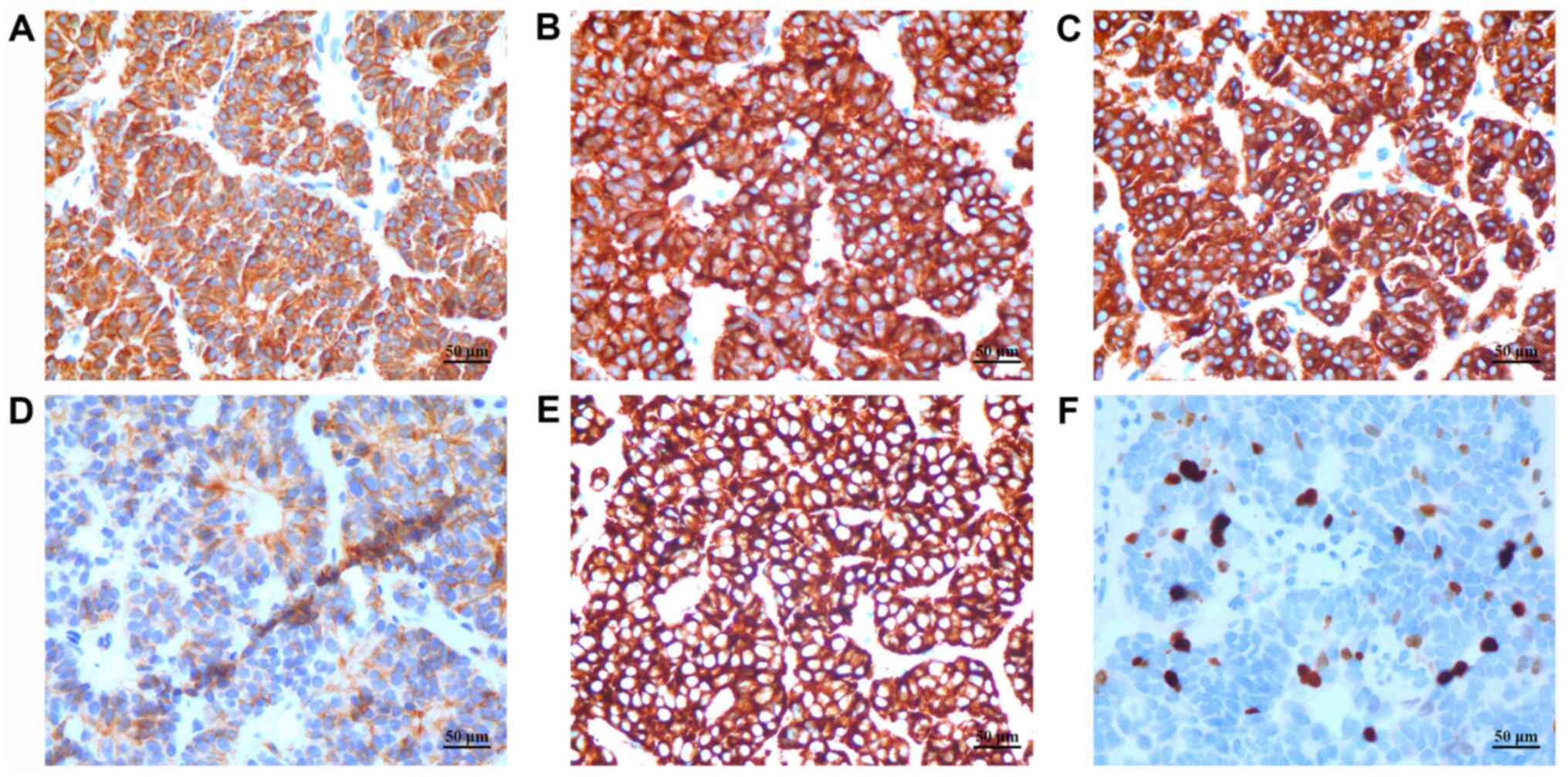
Immunohistochemically (Fig. 3),
most tumor cells were positive for pan-cytokeratin (PCK),
cytokeratin 8/18 (CK8/18), epithelial membrane antigen (EMA),
cluster of differentiation 56 (CD56), and synaptophysin (Syn), and
the cells had a Ki-67 labeling index (LI) of 5%. The tissue was
negative for chromogranin A, leukocyte common antigen (LCA),
thyroid transcription factor-1 (TTF-1), S-100 and CD99.
Four days after the biopsy, the patient complained
of a headache, which was alleviated by mannitol and dexamethasone.
Contrast-enhanced magnetic resonance imaging (MRI) (Fig. 1D) scans of the head revealed
bilateral damage to the parietal bones, outer wall of the orbital
cavities, and petrous apex, as well as damage to the suprasellar
region, clivus, and cervical vertebrae. Damages were also exhibited
on multiple cranial bones (Fig.
1E). However, no meningeal or parenchymal metastases were
observed.
The patient was admitted to the hospital with a pain
score of 8-9. During the hospitalization, the patient was first
given 200 mg celecoxib capsules (Celebrex) orally every 12 h, but
his pain was not relived. Thus, the patient was instead given
oxycodone and acetaminophen tablets (Tylenol 1#) orally every 6 h;
however, the pain was still unbearable. Finally, the patient's pain
was controlled when 30 mg oxycodone hydrochloride prolonged-release
tablets (OxyContin) were administered orally every 12 h with 200 mg
Celebrex capsules, and 7.5 mg dexamethasone pills were administered
orally daily.
After the examinations were completed, the patient
was diagnosed with stage IVb TAC according to Masaoka staging.
Considering the lymphatic and extensive bone metastases,
chemotherapy and radiotherapy were recommended to this patient.
However, the patient refused to take any chemotherapy or
radiotherapy and only received alleviative treatment until he died
on February 20th, 2020, 89 days after he was diagnosed.
Discussion
Thymic neuroendocrine tumors (NETs) are rare tumors,
In SEER database, the incidence of thymic NETs was 0.02/100,000
population per year (7).
Consequently, most data about thymic NETs are shown in case
reports. The prevalence of thymic NETs is ~3% of the total number
of NETs at all sites (8). Despite
the benign behavior that the name suggests, thymic NETs are known
to be more aggressive than NETs in other parts of the body,
frequent metastases, and occurrence as a component of multiple
endocrine neoplasia-1 (MEN-1) in ~25% of cases (9).
Thymic NETs are derived from Kulchitsky cells, which
exist in normal thymic tissue and are part of the amine precursor
uptake and decarboxylation (APUD) system. These cells originate
from neural crest cells, are readily stained with silver and are
one source of foregut carcinoids (10). Thymic NETs can be categorized as
typical carcinoids, atypical carcinoids, large cell neuroendocrine
carcinomas, and small cell neuroendocrine carcinomas according to
the WHO guidelines for bronchopulmonary NETs and thymic NETs
(4), and can be further defined as
well or poorly differentiated, or as type G1-4, based on the
observed mitosis and Ki-67 LI. The European Society for Medical
Oncology (ESMO) clinical practice guidelines define typical
carcinoids as low-grade while atypical carcinoids as
intermediate-grade tumors featuring greater mitotic activity than
typical carcinoids (2-9/10 HPFs vs. <2/10 HPFs), as well as
focal and discrete necrosis (8).
Atypical carcinoids account for 4.6% of all thymic tumors (11). Compared with lower-grade tumors, the
malignant behavior of higher-grade tumors is more aggressive.
Atypical carcinoid tumors of the lung and thymus show a 5-year
survival rate ranging from 56 to 78%, while low-grade typical
carcinoids show a 97-100% survival rate (12,13).
Atypical carcinoid tumor is highly malignant and
invasive without specific clinical manifestations. Since
approximately one-third of patients have no obvious early symptoms
and are often identified during routine health examination due to
the discovery of an anterior mediastinal mass, many TAC patients
already have metastases when they are diagnosed (9). Growth of the tumor mass can cause
chest pain, cough or dyspnea. Patients can also be admitted with
symptoms related to endocrine abnormalities (14). However, MEN-1-related TACs have been
reported and are typically not accompanied by carcinoid syndromes
(e.g., erythema, diarrhea, and elevated 5-hydroxyindole acetic
acid), and this lack of hormonal activity probably contributes to
their late diagnosis (15). In this
case, this patient did not exhibit typical signs and symptoms
indicating TAC because the size of the tumor in this case was
relatively small and was not accompanied by endocrine
abnormalities. As there were already severe damage to multiple
bones at the time of the diagnosis, the patient had missed the
optimal treatment stage. This case suggests the importance of
routine chest X-rays or low-dose CT examinations for the early
diagnosis and treatment of this disease.
Contrast-enhanced CT scanning is the initial imaging
modality of choice for this disease, which can accurately show the
location, size, density and shape of a tumor. This technique can
also clearly illustrate the surroundings of the tumor, such as the
relationship between the tumor and pericardium, great vessels and
chest wall. Most thymic carcinoids are anterior mediastinal masses
with an average diameter of approximately 8 cm (11). The tumors appear as irregular masses
of uniform density in CT scans and show moderate to high,
inhomogeneous enhancement when contrast agents are administered.
These tumors can invade the surrounding pericardium and large
vascular structures and can form mediastinal lymph node metastases
(10,16). With the widespread use of positron
emission and computed tomography (PET/CT), the diagnosis and
staging of TACs has increasingly improved. Recent studies have
shown strong value of PET/CT for the assessment of intermediate-
and high-grade NETs and may have prognostic value (17,18).
Because TACs exhibit high glucose uptake, metastases can be more
accurately detected, including those to the lymph nodes and bones.
At present, the body bone scan (as SPECT scanning) is not
recommended in the guideline of thymic endocrine tumor. However,
bone metastasis may be involved in the relapse and prognosis of TAC
patients. A case report reported that a male TAC patient was
diagnosed with bone metastasis 5 months after the second operation
(19), suggesting that bone
metastasis may occur at various stages and be relatively hard to
discover. In our case, the patient didn't show any relative symptom
indicating bone metastasis at admission, which reminds us that the
full body bone scan should be considered to newly diagnosed TAC
patients even without any symptom indicating bone metastasis.
CT-guided core needle biopsy is crucial because the
diagnosis of TACs mainly relies on pathological examination. Upon
observation, Thymic carcinoids tumor cells are round or oval and
have large nuclei and finely punctate chromatin; mitosis is also
visible. The cells form nests or trabecular arrangements and are
distinct from fibrous tissue and blood vessels when observed using
light microscopy. Immunohistochemistry is the basic tool used to
diagnose carcinoids. The most commonly used markers for NETs are
chromogranin, Syn, NSE and CD56. In addition, EMA, CK5/6, Ki-67,
and mitotic rate are also markers frequently assessed (4).
Atypical and typical carcinoids have similar
immunohistochemistry factors. However, high mitotic counts, nucleus
polymorphisms, hyperpigmentation, disordered cell arrangement,
focal necrosis and calcification are more common in atypical
carcinoids. In our case, the immunohistochemistry results revealed
that EMA, Syn, chromogranin, and CD56 were strongly positive,
indicating this tumor had an endocrine phenotype, However, this
patient did not present with any symptom or sign of cryptorrhea at
admission, and the main characteristic of this case was severe
damage to multiple bones. The relationship between the observed
bone damage and the pathological characteristics remains to be
explored.
Dense-core granules could be observed in the
cytoplasm by electron microscopy. These neurosecretory granules
participate in ectopic hormone secretion that leads to endocrine
abnormalities, the most common being Cushing's syndrome (14). Although this patient described did
not exhibit symptoms of endocrine abnormalities or changes in
hematology upon admission, his prognosis was still extremely
poor.
So far, there are few reports of genomic tests for
TAC, but a recent study shows that the average copy number
instability (CNI) score of TAC patients is higher than that of
patients with thymic typical carcinoid, indicating that TAC has a
higher degree of genomic instability. What's more, the study
presented two cases whose primary and metastatic foci were
classified differently according to the WHO classification, which
revealed gene alterations during the metastatic process (20). In this regard, genetic factors may
be involved in the process of metastasis to specific organs such as
multiple bones in our case.
The diagnosis of TACs is implied by excluding other
diagnoses. Thymic carcinoids need to be differentiated from
numerous other types of mediastinal tumors, such as thymoma, thymic
carcinoma, lymphoma, and germ cell tumors, as well as other NETs.
Thymic carcinoids also need to be differentiated from other
metastatic malignancies, such as small-cell lung cancer.
Complete surgical resection is strongly recommended
for localized thymic carcinoids, according to the newest version of
the National Comprehensive Cancer Network (NCCN) guidelines. The
treatment guidelines include complete resection of the tumor and
sweeping of the peripheral adipose tissue, as well as selective
sweeping of the surrounding lymph nodes. For incompletely resected
tumors, postoperative adjuvant chemotherapy or radiotherapy is
recommended. However, no large-scale clinical trials have confirmed
the effectiveness of this approach. For locoregional, unresectable
disease or distant metastases, the complete surgical resection of
the primary mass and metastases remains the first choice. Once
surgery is no longer an option, ablative therapy is recommended.
Systemic chemotherapy has been described in case reports, most
frequently using temozolomide (21). At present, a phase 2 clinical trial
aimed to assess the efficacy and safety of long-acting pasireotide
and everolimus, given alone or in combination, in patients with
advanced carcinoids of the lung or thymus is ongoing, which is the
first prospective, randomized clinical trial focusing on this
specific patient group (22).
Generally, the prognosis of thymic carcinoids is
believed to be related to tumor volume, infiltration depth, growth
site, and whether the tumor is accompanied by the carcinoid
syndrome. Previous reports have indicated that a high mitotic count
can be considered the most important determinant of poor prognosis
for atypical carcinoids (23). But
in our reported case, the mitotic rate was 2/HPF, and Ki-67
remained at 5%. These results indicated that this tumor had a
relatively slow growth rate. The carcinoid was highly
neuroendocrine in nature but exhibited relatively low mitosis. The
malignant behavior of the TAC reported here did not match its
growth speed. Thus, it is not appropriate to use the mitotic rate
and Ki-67 as indicators of malignancy on this type of tumor.
Regardless of whether endocrine abnormalities are
present, once TAC patients lose the opportunity for complete tumor
resection, the overall survival time dramatically decreases.
Compared with patients who had resectable tumors, this patient had
a very short survival time.
This patient was admitted with lumbar back pain. The
contrast-enhanced chest CT revealed a tumor located in the left of
the anterior superior septum; the longest diameter of the tumor was
3.5 cm. There were no metastases to any lymph nodes or solid
organs. CT, SPECT and MRI scan all confirmed damage to multiple
bones of the spine, ribs, pelvis and skull. Bone metastasis may
occur in TAC cases, but skull metastasis is rare. This is the first
reported case of TAC without osteodynia as the initial symptom with
damage to multiple axial bones, including metastases to the
sacroiliac joint, acetabulum joint and pubis. Consequently,
examination of bones is strongly recommended in this disease, even
when there are no metastases to other organs.
It is the first report about primary TAC which had
metastases to the parietal bones, outer wall of the orbital
cavities, petrous apex, suprasellar region and the clivus. In
conclusion, more clinical evidence is urgently needed to support
the development of more accurate and effective means of diagnosing
and treating TAC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81802287; to RZ).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZ and QD made substantial contributions to the
conception and design of the current study. RZ curated the data. GW
and GP confirmed the authenticity of all the raw data. XR, WC, GW,
GP and JL performed formal analysis. XR wrote the original draft of
the manuscript. RZ, GW, GP and QD critically revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Union Hospital of Tongji Medical College, Huazhong
University of Science and Technology (approval no. 2018S369; Wuhan,
China).
Patient consent for publication
Written informed consent for publication of the
patient's data was obtained from the patient's family member.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Litvak A and Pietanza MC: Bronchial and
thymic carcinoid tumors. Hematol Oncol Clin North Am. 30:83–102.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen G, Marx A, Chen WH, Yong J, Puppe B,
Stroebel P and Mueller-Hermelink HK: New WHO histologic
classification predicts prognosis of thymic epithelial tumors.
Cancer. 95:420–429. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 world health organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Filosso PL, Yao X, Ahmad U, Zhan Y, Huang
J, Ruffini E, Travis W, Lucchi M, Rimner A, Antonicelli A, et al:
Outcome of primary neuroendocrine tumors of the thymus: A joint
analysis of the International thymic malignancy interest group and
the European society of thoracic surgeons databases. J Thorac
Cardiovasc Surg. 149:103–109.e2. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tiffet O, Nicholson AG, Ladas G, Sheppard
MN and Goldstraw P: A clinicopathologic study of 12 neuroendocrine
tumors arising in the thymus. Chest. 124:141–146. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gaur P, Leary C and Yao JC: Thymic
neuroendocrine tumors: A SEER database analysis of 160 patients.
Ann Surg. 251:1117–1121. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Öberg K, Hellman P, Ferolla P and Papotti
M: ESMO Guidelines Working Group. Neuroendocrine bronchial and
thymic tumors: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 23 (suppl 7):vii120–vii123.
2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jia R, Sulentic P, Xu JM and Grossman AB:
Thymic neuroendocrine neoplasms: Biological behaviour and therapy.
Neuroendocrinology. 105:105–114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Moran CA and Suster S: Neuroendocrine
carcinomas (carcinoid tumor) of the thymus. A clinicopathologic
analysis of 80 cases. Am J Clin Pathol. 114:100–110.
2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Valli M, Fabris G, Dewar A, Chikte S,
Fisher C, Corrin B and Sheppard MN: Atypical carcinoid tumour of
the thymus: A study of eight cases. Histopathology. 24:371–375.
1994.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Garcia-Yuste M, Matilla JM, Cueto A,
Paniagua JM, Ramos G, Canizares MA and Muguruza I: Spanish
Multi-centric Study of Neuroendocrine Tumours of the Lung for the
Spanish Society of Pneumonology and Thoracic Surgery
(EMETNE-SEPAR). Typical and atypical carcinoid tumours: Analysis of
the experience of the Spanish multi-centric study of neuroendocrine
tumours of the lung. Eur J Cardiothorac Surg. 31:192–197.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mann M, Chowdhury B, Sullivan E, Nicklas
R, Anthracite R and Meyer RJ: Serious asthma exacerbations in
asthmatics treated with high-dose formoterol. Chest. 124:70–74.
2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cardillo G, Rea F, Lucchi M, Paul MA,
Margaritora S, Carleo F, Marulli G, Mussi A, Granone P and Graziano
P: Primary neuroendocrine tumors of the thymus: A multicenter
experience of 35 patients. Ann Thorac Surg. 94:241–246.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
The BT: Thymic carcinoids in multiple
endocrine neoplasia type 1. J Intern Med. 243:501–504.
1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Araki T, Sholl LM, Hatabu H and Nishino M:
Radiological features and metastatic patterns of thymic
neuroendocrine tumours. Clin Radiol. 73:479–484. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sanli Y, Garg I, Kandathil A, Kendi T,
Zanetti MJB, Kuyumcu S and Subramaniam RM: Neuroendocrine tumor
diagnosis and management: (68)Ga-DOTATATE PET/CT. AJR Am J
Roentgenol. 211:267–277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Binderup T, Knigge U, Loft A, Federspiel B
and Kjaer A: 18F-fluorodeoxyglucose positron emission tomography
predicts survival of patients with neuroendocrine tumors. Clin
Cancer Res. 16:978–985. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhu S, Wang ZT, Liu WZ, Zong SX and Li BS:
Invasive atypical thymic carcinoid: Three case reports and
literature review. Onco Targets Ther. 9:6171–6176. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dinter H, Bohnenberger H, Beck J,
Bornemann-Kolatzki K, Schütz E, Küffer S, Klein L, Franks TJ, Roden
A, Emmert A, et al: Molecular classification of neuroendocrine
tumors of the thymus. J Thorac Oncol. 14:1472–1483. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hann CL and Forde PM: Lung and thymic
carcinoids. Endocrinol Metab Clin North Am. 47:699–709.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ferolla P, Brizzi MP, Meyer T, Mansoor W,
Mazieres J, Do Cao C, Léna H, Berruti A, Damiano V, Buikhuisen W,
et al: Efficacy and safety of long-acting pasireotide or everolimus
alone or in combination in patients with advanced carcinoids of the
lung and thymus (LUNA): An open-label, multicentre, randomised,
phase 2 trial. Lancet Oncol. 18:1652–1664. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han B, Sun JM, Ahn JS, Park K and Ahn MJ:
Clinical outcomes of atypical carcinoid tumors of the lung and
thymus: 7-year experience of a rare malignancy at single institute.
Med Oncol. 30(479)2013.PubMed/NCBI View Article : Google Scholar
|