Introduction
Approximately 9% of all breast cancers are caused by
pathological germline mutation of cancer susceptibility genes, and
~48 to 56% of these cancers have BRCA1 or BRCA2
mutations (1,2). Approximately 70% of breast cancers
caused by germline BRCA1 mutation are the triple negative subtype,
which is defined as estrogen receptor (ER) negative, progesterone
receptor (PgR) negative, and human epidermal receptor 2 (HER2)
negative (3,4). Therefore, triple-negative breast
cancer (TNBC) is listed as one of the criteria for obtaining an
evaluation for genetic risk of hereditary breast and ovarian cancer
syndrome (5).
TNBC, which accounts for 12-15.5% of breast cancers
in Japan (6,7), is characterized by rapid growth and
worse prognosis compared with other subtypes of breast cancer
(8). Recently, several poly
(ADP-ribose) polymerase (PARP) inhibitors were shown to be
effective for breast and ovarian cancers with germline
BRCA1/2 mutations, and the PARP inhibitor olaparib has been
approved for clinical use in Japan (9). A BRCA1/2 genetic testing for
breast cancer patients with high risk of hereditary breast and
ovarian cancer syndrome has been covered by the Japanese national
insurance system since April 2020. Therefore, breast cancer
patients who would like to have the genetic testing for
BRCA1/2 mutations, especially those with TNBC, will now have
better access to the test in Japan.
The prevalence of germline BRCA1/2 mutations
in TNBC varied from 9.3 to 15.4% in large (N>100) studies from
mainly Europe and North America (10,11)
and was higher in several small studies from the USA and China
(12,13). However, few studies have reported
the prevalence of germline BRCA1/2 mutations in Japanese
patients, especially those with TNBC (4,14).
Here, we report a retrospective analysis for the prevalence of
BRCA1/2 mutations among Japanese TNBC patients who had
genetic testing in a single institute. Additionally, we assessed
the risk factors for BRCA1/2 mutation positivity in the same
cohort.
Patients and methods
Target patients
Patients who were diagnosed with TNBC and underwent
genetic testing for germline BRCA1/2 mutations from October
2014 to March 2020 at Osaka International Cancer Institute
(formerly Osaka Medical Center for Cancer and Cardiovascular
Diseases) were included in our study.
Determination of breast cancer
subtypes and BRCA genetic testing
TNBC was determined as both ER and PR negativity
(<1%) and HER2 negativity and was evaluated according to the
American Society of Clinical Oncology/College of American
Pathologists guidelines. Most patients underwent genetic testing
because of a wish to participate in clinical trials of a PARP
inhibitor or an immune checkpoint inhibitor. Many patients had no
family history concerns. Genetic counseling was performed for all
patients undergoing genetic testing. Written informed consent was
obtained from all patients prior to genetic testing. Mutation
analysis and interpretation was performed by Myriad Genetics, Inc.
or FALCO Biosystems Ltd..
Retrospective analysis
Medical records and genetic counseling reports were
examined retrospectively. In patients who had bilateral TNBCs, the
age at first diagnosis was adopted. Family history was defined as
having at least one relative with breast or ovarian cancer within
the patient's third-degree relatives.
Statistical analysis
All statistical analyses were performed using EZR
ver.1.4.0 (Saitama Medical Center, Jichi Medical University,
Saitama, Japan), which is a graphical user interface for R ver.
3.6.0 (The R Foundation for Statistical Computing, Vienna, Austria)
(15). We analyzed by using
Logistic regression analysis for univariate analysis and
multivariate analysis. We performed a Mann-Whitney U test to
examine if there was any difference in age between the
BRCA-positive and BRCA-negative groups, and created a
box-and-whisker plot to visualize the bias. In addition, an F-test
was performed to examine whether there was a difference in age
variability between the two groups.
Results
Patient characteristics
The patients' characteristics are summarized in
Table I. Sixty-five TNBC patients
were evaluated in this study; all were female. Fifty-five patients
(84.6%) were 60 years old or younger, and 50 (76.9%) underwent
genetic testing for clinical trials. Thirty patients (46.2%) had a
family history of at least one relative with breast or ovarian
cancer within their third-degree relatives. One patient received
genetic counseling at another hospital before visiting our
institute for a genetic testing; therefore, her family history was
not obtained.
 | Table ICharacteristics of the patients with
triple-negative breast cancer. |
Table I
Characteristics of the patients with
triple-negative breast cancer.
| Variable | Number of patients
(n=65) |
|---|
| Age, years | |
|
<30 | 3 |
|
30-39 | 12 |
|
40-49 | 23 |
|
50-59 | 16 |
|
≥60 | 11 |
| Motives for genetic
counseling | |
|
Clinical
trial | 50 |
|
Others | 15 |
| Family
historya | |
|
Yes | 30 |
|
No | 34 |
|
Unknown | 1 |
| Genetic mutation | |
|
BRCA1
deleterious | 7 |
|
BRCA2
deleterious | 6 |
|
VUS | 1 |
Seven deleterious mutations of BRCA1, six
deleterious mutations of BRCA2, and one BRCA2 variant
of uncertain significance were found in a total of 14 patients. One
patient had mutations in both genes, which were a deleterious
BRCA1 mutation and benign BRCA2 mutation (not shown).
The prevalence of germline BRCA1/2 mutations in this cohort
was 20.0% (13/65; Table I).
Bias in age distribution of the
BRCA-positive group
The median age was 46 and 49 years for the
BRCA-positive and -negative subjects respectively, and the
logistic regression analysis showed no statistically significant
difference (Table II). No
deleterious BRCA1/2 mutations were observed among patients
older than 60 years old; the prevalence of mutations among patients
60 years old or younger was 24.1% (13/54; Table II). No deleterious mutations were
observed in patients from 30-39 years old (0/12). The Mann-Whitney
U test showed no statistically significant difference in age
between the BRCA-positive and BRCA-negative groups,
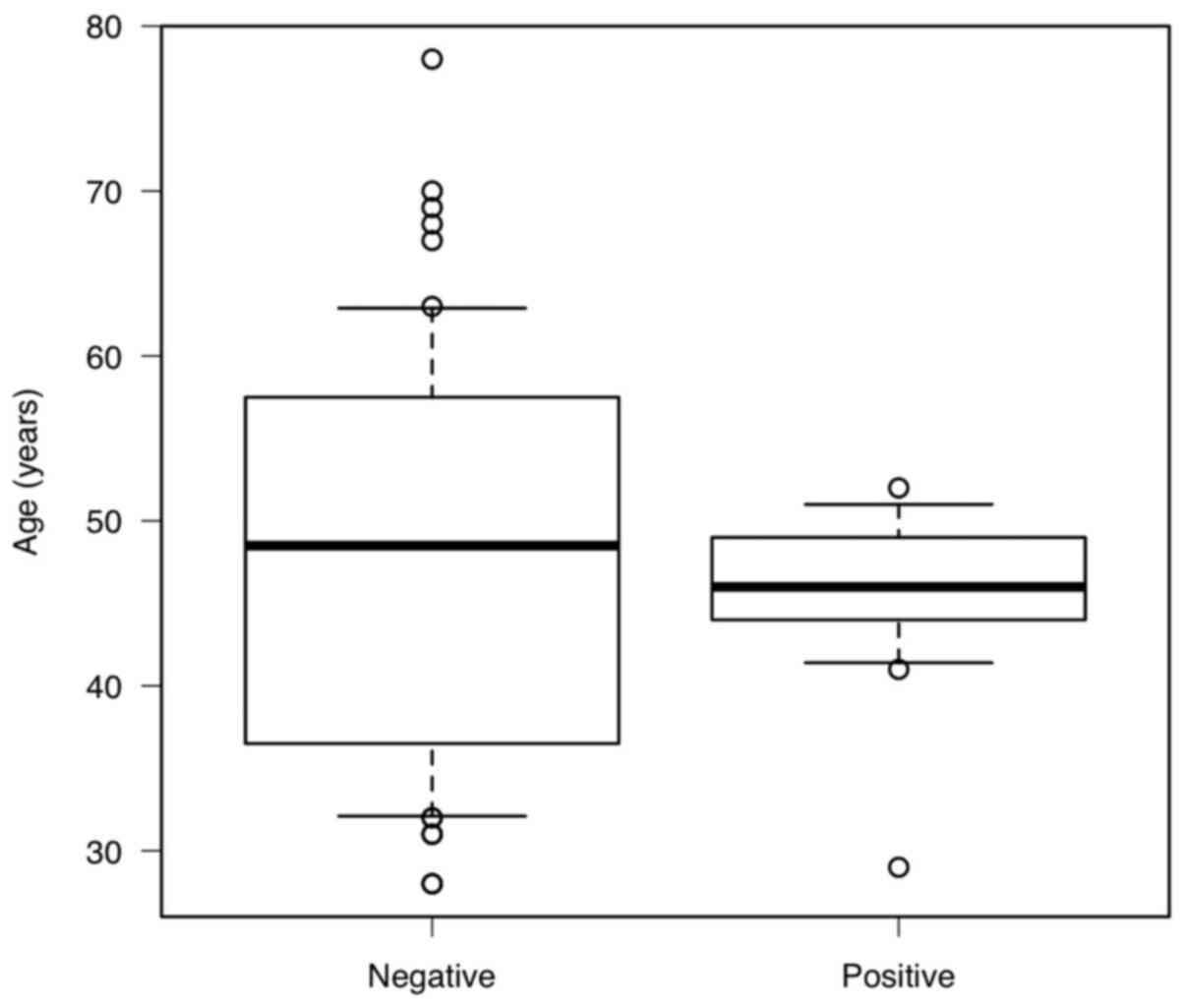
P=0.527, but the box-and-whisker plot appeared to be biased, and
when the F test was performed to verify homoscedasticity, the
variance was statistically significantly smaller (P=0.00984) and
was concentrated around 46 years of age (Fig. 1).
 | Table IIRisk factors for the presence of
BRCA1/2 deleterious mutations in patients with
triple-negative breast cancer. |
Table II
Risk factors for the presence of
BRCA1/2 deleterious mutations in patients with
triple-negative breast cancer.
| | BRCA1/2
deleterious mutation | P-valuea |
|---|
| Variable | Positive, n | Negative, n | Univariate | Multivariate |
|---|
| Age, years | | | 0.462 | 0.605 |
|
Median
(range) | 46 (29-52) | 49 (28-78) | | |
|
<30 | 1 | 2 | | |
|
30-39 | 0 | 12 | | |
|
40-49 | 9 | 14b | | |
|
50-59 | 3 | 13 | | |
|
≥60 | 0 | 11 | | |
| Family
historyc,d | | | 0.004e | 0.014e |
|
Yes | 12 | 18 | | |
|
No | 1 | 33 | | |
| Tumor size | | | 0.613 | 0.960 |
|
Tis/T1/T2 | 10 | 45 | | |
|
T3/T4 | 3 | 7 | | |
| Lymph node
metastasis | | | 0.077 | 0.853 |
|
N0/N1 | 10 | 47 | | |
|
N2/N3 | 3 | 5 | | |
| Histological
grade | | | 0.994 | 0.995 |
|
G1/G2 | 0 | 9 | | |
|
G3 | 8 | 37 | | |
|
Not
assessed | 5 | 6 | | |
Correlation with family history
Only one patient among those without family history
had a deleterious BRCA2 mutation. The prevalence of germline
BRCA1/2 mutations among patients with family history was
41.4% (12/29; Table II).
Table II shows the
result of the univariate and multivariate analysis. Univariate
analysis and multivariate analysis using logistic regression
analysis showed a significant relationship between BRCA1/2
mutations and family history (P=0.00425, P=0.0136), but did not
show a significant relationship between germline BRCA1/2
mutations and age (P=0.462, P=0.605). Tumor size, lymph node
metastasis, and histological grade were not related to
BRCA1/2 mutation.
Discussion
In 2012, the Japanese Hereditary Breast and Ovarian
Cancer Consortium was established, and a nationwide registration
system began in 2013(4). However,
the BRCA1/2 genetic testing was not initially covered by the
national insurance system and few clients underwent the test.
Therefore, few reports show the prevalence of BRCA1/2
mutation carriers in Japan, especially those with TNBC. Arai et
al (4) reported the analysis of
germline BRCA1/2 mutations among 963 Japanese individuals
who received a BRCA1/2 genetic testing and were registered
in the database mentioned above from 2012 to 2014. The ratios of
TNBC in patients with BRCA1 and BRCA2 mutations were
75.8 and 18.6%, respectively. However, the prevalence of germline
BRCA1/2 mutations among TNBC patients was not shown in their
report. In 2015, Nakamura et al (14) reported an analysis of BRCA1/2
mutations in 320 Japanese individuals with a strong family history
of breast cancer and/or ovarian cancer. The analysis included 41
TNBC patients, and 22 of these TNBC patients had deleterious
germline BRCA1/2 mutations (53.7%). However, all TNBC
patients in the study had a high-risk condition, which was a cancer
diagnosis at an age younger than 40 years old or having more than
one family member with breast and/or ovarian cancer. Therefore, the
prevalence of BRCA1/2 mutations in the general TNBC cohort
in Japan was unclear in the study. In our study, 76.9% of patients
received a genetic testing as part of clinical trials targeting
TNBC patients and 53.1% (34/65) of patients subjected to a test had
no family history. Although the patients were not consecutive
patients and comprised only a small proportion of all TNBC patients
treated in our institute during the study period, our report is the
first to show the prevalence of germline BRCA1/2 mutations
in the near general cohort of Japanese TNBC patients. The
prevalence value of 20% in our study is higher than in larger
studies (11,16). The higher prevalence of
BRCA1/2 mutations in our study may be because of the small
number of patients and the fact that patients with a strong family
history of ovarian cancer were consciously enrolled as candidates
for clinical trials, which are limitations of this study.
As for risk factors for BRCA1/2 mutations in
TNBC, the age of diagnosis is important. Emborgo et al
(11) reported that 49 out of 294
patients (16.7%) with TNBC diagnosed at age 60 years or younger
were positive for BRCA1/2 deleterious mutations. Conversely,
only 2 out of 86 patients (2.3%) with TNBC diagnosed at >60
years had BRCA1/2 mutations. In line with other reports,
these results indicate that being diagnosed with TNBC at >60
years of age was not significantly correlated with a positive
BRCA1/2 mutation. In our study, 10 patients were aged >60
years, and none had a BRCA1/2 mutation. In addition, no
patients from 30-39 years old were positive for BRCA1/2
mutation. The results of the F test also showed a bias in the age
of the positive subjects, with a statistically significantly
smaller variance than the negative group and a concentration near
the median age of 46 years. This may be related to the age at which
the BRCA mutation-positive patients develop breast cancer.
Regardless of the underlying reason, for younger breast cancer
patients, testing with a gene panel for detecting mutations
associated with hereditary cancer other than BRCA might be
considered.
Another risk factor is family history, which is one
of the criteria for a genetic testing for BRCA1/2 mutations.
However, whether family history is a risk factor for BRCA1/2
mutation in TNBC patients is unclear. Sharma et al (17) examined 207 TNBC patients who
prospectively underwent genetic testing for BRCA1/2
mutations and reported that the BRCA1/2 mutation prevalence
rates in patients with and without family history were 21.1 and
6.3% (P=0.00425), respectively. Our study also showed a significant
difference in BRCA1/2 mutation positivity between patients
with or without family history. The prevalence of BRCA1/2
mutations was 41.4% among patients with family history, while only
one patient had BRCA2 mutation in the group without family
history. This patient's mother died of a carcinoma of unknown
origin in her abdominal cavity, which might have been ovarian or
peritoneal cancer. Therefore, there may have been no patients with
BRCA1/2 mutations in the group without family history.
In conclusion, the prevalence of BRCA1/2
mutations among Japanese TNBC patients in our cohort was 20.0%,
which is similar to or slightly higher than that in reports from
Europe or North America with large cohorts. Family history is a
significant risk factor for BRCA1/2 mutation positivity in
TNBC patients. However, more prospective studies with greater
numbers of consecutive TNBC patients are needed to clarify the
accurate prevalence of BRCA1/2 mutations. Furthermore,
because young women under 30 years of age who may harbor germline
mutations in other genes such as TP53 are included in the
BRCA1/2-negative TNBC cohort, studies using multi-gene panel
tests for cancer susceptibility genes should be planned in the
future.
Acknowledgements
The authors would like to thank Dr Gabrielle White
Wolf and Dr Nicole Clarke for editing a draft of this
manuscript.
Funding
The present study was conducted with support from The Osaka
Foundation for The Prevention of Cancer and Lifestyle-related
Diseases (grant no. 24-7).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT conceived the design of the study and wrote the
outline of the manuscript. FF analyzed the data and wrote the final
manuscript. TI was responsible for genetic counseling as the
subjects underwent BRCA1/2 genetic testing. TN, TYa, NK, TYo, MN,
SM, HK and SK were involved in the treatment of their patients as
attending physicians and provided important advice for decision
making. TN, TYa, NK, TYo, MN, SM, HK and SK also performed data
curation to conduct this study and contributed to the writing of
the final manuscript. YT and TI confirm the authenticity of all the
raw data. All authors agree to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
OICI Institutional Review Board and conducted in accordance with
the ethical standards of the institutional and national research
committee and with the 1964 Helsinki Declaration and its later
amendments or comparable ethical standards. The approval number is
20044. Written informed consent was obtained from all patients
prior to genetic testing of BRCA1/2. All confirmations of consent
for research participation were described with the option to
opt-out on the institution's website.
Patient consent for publication
All confirmations of consent for publication were
described with the option to opt-out on the institution's
website.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Buys SS, Sandbach JF, Gammon A, Patel G,
Kidd J, Brown KL, Sharma L, Saam J, Lancaster J and Daly MB: A
study of over 35,000 women with breast cancer tested with a 25-gene
panel of hereditary cancer genes. Cancer. 123:1721–1730.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sun J, Meng H, Yao L, Lv M, Bai J, Zhang
J, Wang L, Ouyang T, Li J, Wang T, et al: Germline mutations in
cancer susceptibility genes in a large series of unselected breast
cancer patient. Clin Cancer Res. 23:6113–6119. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mavaddat N, Barrowdale D, Andrulis IL,
Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Spurdle A, Robson M,
Sherman M, et al: Pathology of breast and ovarian cancers among
BRCA1 and BRCA2 mutation carriers: Results from the Consortium of
Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol
Biomarkers Prev. 21:134–147. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Arai M, Yokoyama S, Watanabe C, Yoshida R,
Kita M, Okawa M, Sakurai A, Sekine M, Yotsumoto J, Nomura H, et al:
Genetic and clinical characteristics in Japanese hereditary breast
and ovarian cancer: First report after establishment of HBOC
registration system in Japan. J Hum Genet. 63:447–457.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment:
Breast, Ovarian, and Pancreatic. Version 1.2020-December 4, 2019.
NCCN org. http://www.nccn.org/professionals/. Accessed May
2020.
|
|
6
|
Iwase H, Kurebayashi J, Tsuda H, Ohta T,
Kurosumi M, Miyamoto K, Yamamoto Y and Iwase T: Clinicopathological
analyses of triple negative breast cancer using surveillance data
from the Registration Committee of the Japanese Breast Cancer
Society. Breast Cancer. 17:118–124. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shibuta K, Ueo H, Furusawa H, Komaki K,
Rai Y, Sagara Y, Kamada Y and Tamaki N: The relevance of intrinsic
subtype to clinicopathological features and prognosis in 4,266
Japanese women with breast cancer. Breast Cancer. 18:292–298.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kinoshita T, Fukui N, Anan K, Iwamoto T,
Niikura N, Kawai M, Hayashi N, Tsugawa K, Aogi K, Ishida T, et al:
Comprehensive prognostic report of the Japanese Breast Cancer
Society Registry in 2004. Breast Cancer. 23:39–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Robson M, Im SA, Senkus E, Xu B, Domchek
SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, et al:
Olaparib for metastatic breast cancer in patients with a Germline
BRCA mutation. N Engl J Med. 377:523–533. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Armstrong N, Ryder S, Forbes C, Ross J and
Quek RG: A systematic review of the international prevalence of
BRCA mutation in breast cancer. Clin Epidemiol. 11:543–561.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Emborgo TS, Saporito D, Muse KI, Brrera
AMG, Litton JK, Lu KH and Arun BK: Prospective evaluation of
universal BRCA testing for women with triple-negative breast
cancer. JNCI Cancer Spectr. 4(pkaa002)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Greenup R, Buchanan A, Lorizio W, Rhoads
K, Chan S, Leedom T, King R, McLennan J, Crawford B, Kelly Marcom
P, et al: Prevalence of BRCA mutations among women with
triple-negative breast cancer (TNBC) in a genetic counseling
cohort. Ann Surg Oncol. 20:3254–3258. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li YT, Ni D, Yang L, Zhao Q and Ou JH: The
prevalence of BRCA1/2 mutations of triple-negative breast cancer
patients in Xinjiang multiple ethnic region of China. Eur J Med
Res. 19(35)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nakamura S, Takahashi M, Tozaki M,
Nakayama T, Nomizu T, Miki Y, Murakami Y, Aoki D, Iwase T,
Nishimura S, et al: Prevalence and differentiation of hereditary
breast and ovarian cancers in Japan. Breast Cancer. 22:462–468.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Couch FJ, Hart SN, Sharma P, Toland AE,
Wang X, Miron P, Olson JE, Godwin AK, Pankratz VS, Olswold C, et
al: Inherited mutations in 17 breast cancer susceptibility genes
among a large triple-negative breast cancer cohort unselected for
family history of breast cancer. J Clin Oncol. 33:304–311.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sharma P, Klemp JR, Kimler BF, Mahnken JD,
Geier LJ, Khan QJ, Elia M, Connor CS, McGinness MK, Mammen JM, et
al: Germline BRCA mutation evaluation in a prospective
triple-negative breast cancer registry: Implications for hereditary
breast and/or ovarian cancer syndrome testing. Breast Cancer Res
Treat. 145:707–714. 2014.PubMed/NCBI View Article : Google Scholar
|