Introduction
For women with early-stage breast cancer, whole
breast radiotherapy (WBRT) after breast-conserving surgery (BCS) is
a longstanding standard practice. WBRT reduces the risk of
locoregional and distant first recurrence by 50%, and reduces
breast cancer death by ~1/6 (1,2).
However, most patients with breast cancer who undergo adjuvant
radiotherapy (RT) are affected by skin toxicities (3). During RT treatment, these skin
toxicities cause physical discomfort and emotional distress, and
therefore negatively affect the patients' quality of life (4). In consequence, previous studies
investigated factors associated with an increased risk of
developing acute skin toxicities. Dose distribution and resulting
dosimetric parameters (in particular high-dose ranges of >105,
>107 and >110% within the target volume) are RT treatment
procedure-related factors, reported to be significantly associated
with an increased risk of developing skin toxicities (5-8).
In modern RT, field-in-field (FIF) techniques are
considered the standard-of-care when three-dimensional conformal RT
(3D-CRT) treatment planning is available (5,9).
However, using the FIF for patients who have relatively small-sized
breasts results in high-dose areas remaining in the irradiation
field around the reference point. Therefore, in our institution, we
implemented a new FIF technique, the field-in-field with 2
reference points (FIF w/ 2RP) method, aimed at improving dosimetric
parameters and thus reducing high-dose ranges and subsequent skin
toxicities.
The current study sought to examine the usefulness
of this new irradiation method. We performed a comparative analysis
of dosimetric parameters of three irradiation methods including the
FIF w/ 2RP method and two other irradiation methods, the wedge (W)
method and the field-in-field without 2 reference points (FIF w/o
2RP) method, as control. Furthermore, we assessed radiation-induced
skin toxicities. We then investigated the association of each
irradiation method and skin toxicities.
Materials and methods
Patients
Between April 2008 and December 2016, 577 females
with early-stage breast cancer treated at Okayama University
Hospital with BCS and adjuvant RT were evaluated. These patients
were selected from 723 consecutive females with early-stage breast
cancer. Exclusion criteria were simultaneous bilateral breast
cancer, treatment with regional nodal irradiation and treatment
using hypo-fractionated irradiation. Furthermore, four patients
treated without the use of wedge filters or the FIF method were
excluded from this study due to insufficient sample size. Finally,
data regarding 573 patients, aged 24-82 years (mean, 55 years),
were evaluated. Patients provided written informed consent for
undergoing RT and using their anonymous data for scientific
studies. This study was conducted in accordance with the
Declaration of Helsinki, as revised in 2013.
Radiation treatment
Each patient received WBRT in 25 consecutive
fractions of 2 Gy to a total prescribed dose of 50 Gy. In total,
271 patients received a sequential boost of 10 or 16 Gy to the
tumor bed. Free-breathing computed tomography (CT) acquisition was
performed with an Asteion Super4 Edition multi-slice CT scanner
(Toshiba Corporation) and reconstructed with 2 mm-thick slices. To
set the irradiation field, radio-opaque markers were placed on
external landmarks; the sternal notch, the midline, the
mid-axillary line, 1 cm below the infra-mammary fold, surgical
scars, the nipple and the margin of palpable breast tissue; at the
acquisition of the CT scan to facilitate contouring segmentation of
the CT dataset. CT images were then transferred to the treatment
planning system CMS Xio Ver 4.3.4 (Computerized Medical Systems,
Inc.).
The planning target volume (PTV) was defined
three-dimensionally as 5 mm inside the previously set irradiation
field, excluding the part outside the patient and the first 5 mm of
tissue under the skin, and posteriorly limited no deeper to the
anterior surface of the ribs excluding the boney thorax and lung.
Breast PTV Eval (BPe) was determined according to the clinical
research protocol Radiation Therapy Oncology Group (RTOG)
1005(10) and its defined clinical
target volume (CTV) and PTV. This BPe volume was used for breast
volume evaluation. Contoured organs at risk (OARs) were the lung
and the heart. The heart and the axillary lymph nodes levels I, II
and III were countered according to the RTOG-endorsed consensus
guidelines for delineation of target and normal structures for
breast cancer (11). These target
contours were then checked and modified to be included within the
previously set irradiation field to be 3D-CRT treatments. Beam
energies were 4, 6 and 10 MV by LINEACS (Mevatron M2/6327, PRIMUS
High Energy KD2/7467 and ONCOR High Energy ONCR-K; Toshiba
Corporation).
Three irradiation methods were performed: i) W
method was used for 178 patients. The RP was set at the level of
the nipple or at the mid-level between the upper and lower edges of
the irradiation field. Physical wedges in the opposing tangential
beams were used to improve target dose distribution. For some
patients, to avoid hot spots, dose distribution was optimized by
adding an extra tangent FIF; ii) the FIF w/o 2RP method was used
for 142 patients. Two opposed tangential fields were set up without
wedges. The RP was set at the mid-level between the upper and lower
edges of the irradiation field or 2 cm apart from the deepest point
and upper edge of the irradiation field. The main field was copied
as subfields and the multileaf collimators (MLCs) were manipulated
to shield the areas of the breast receiving a high dose. However,
these MLCs were not allowed to block within 2 cm of the RP; and
iii) the FIF w/ 2RP method was used for 253 patients. Two RPs were
set for each patient; one RP for the main beam at a point 2 cm
apart from the deepest point and upper edge of the irradiation
field, and another RP for the FIF at the mid-level between the
upper and lower edges of the irradiation field. As one RP was used
in the FIF w/o 2RP method, the MLCs could not be inserted in the
range of 2 cm around the RP, which resulted in the high-dose area
remaining close to that RP. For patients who have relatively
small-sized breasts, this can be problematic, as it results in a
high-dose area around that RP. Therefore, in the FIF w/ 2RP method,
the RPs were set simultaneously at two places in the irradiation
field, and high-dose areas were sequentially shielded and
completely eliminated by the MLCs. Patients treated with the W
method and the FIF w/o 2RP method were considered as control
patients.
Dosimetric analysis
A dose distribution in the PTV and BPe of 95-107%
according to ICRU criteria was obtained while lowering OARs doses
as much as possible. The dosimetric parameters recorded for all
plans, including PTV and BPe, were mean dose, V0-95% (the volume
percentage receiving less than 95% of the prescribed dose),
V95-107% (the volume percentage receiving between 95 and 107% of
the prescribed dose), V107% (the volume percentage receiving more
than 107% of the prescribed dose) and V105% (the volume percentage
receiving 105% or more of the prescribed dose). Dose distribution
in the axillary lymph nodes was evaluated using the mean axillary
lymph nodes levels I, II and III doses. The OAR constraints used
for planning for the ipsilateral lung were the mean ipsilateral
lung dose (MLD), ipV20 (the lung volume percentage receiving ≥20
Gy) and ipV30 (the lung volume percentage receiving ≥30 Gy), while
those for the heart were the mean heart dose (MHD), V10 (the heart
volume percentage receiving ≥10 Gy), V20 (the heart volume
percentage receiving ≥20 Gy), and the maximum doses to the left
anterior descending coronary artery (LAD), the circumflex coronary
artery (CCA) and the right coronary artery (RCA).
Acute toxicity grading for skin
Patients were evaluated on a weekly basis for acute
skin toxicities. The highest grade of radiation dermatitis for each
treated breast was prospectively recorded at each on treatment
during the 5 or 6 weeks of treatment and at the follow-up visit at
the week 6 or 7 for patients who received RT without or with boost,
respectively, using the National Cancer Institute Common
Terminology Criteria for Adverse Events (NCI CTCAE) v3.0 grading
criteria (12). The NCI CTCAE
describes grade 0 as ‘no reaction’, grade 1 as ‘faint erythema or
dry desquamation’, grade 2 as ‘moderate to brisk erythema, patchy
moist desquamation mostly confined to skin folds and creases and
moderate edema’, grade 3 as ‘moist desquamation other than skin
folds or creases and bleeding induced by minor trauma or abrasion’
and grade 4 as ‘skin necrosis or ulceration of full thickness of
the dermis, spontaneous bleeding from involved site’ (3,12). To
ensure standard grading of skin toxicities for study purposes, all
information regarding toxicity grade in individual patient were
recorded and reviewed by a single experienced radiation
oncologist.
Data collection and statistical
analysis
Demographic characteristics and clinical tumor
characteristics were recorded for each patient. Tumor site was
defined according to the International Classification of Diseases
for Oncology (third edition) (13).
Each radiation treatment plan was assessed, and dosimetric
parameters were analyzed using a dose-volume histogram. For this
study, acute toxicity grading for skin within the first 5 weeks of
treatment was analyzed.
Statistical analyses were performed using Bell Curve
for Excel (Social Survey Research Information Co., Ltd.) and SPSS
software v27.0 (IBM Corp.). Analyses of differences in the baseline
characteristics, the RT characteristics, the skin toxicity grade
throughout the RT treatment and the maximum skin toxicity grade
during the 5 weeks of RT treatment according to the type of
irradiation method used were conducted using the Kruskal-Wallis
test with Dunn's test and the χ2 test. The correlation
between the irradiation methods and the dose-distribution factors
was examined to assess the impact of irradiation methods on the
dose-distribution factors, using Eta correlation ratio (η). The
correlation between the skin toxicity grade and patients' baseline
characteristics was analyzed using η and Spearman's correlation
coefficient (rs), and the correlation between the skin toxicity
grade and irradiation methods was analyzed using η. The following
proposed guidelines were used for interpreting the absolute values
of η and rs: 0.00-0.19, markedly weak; 0.20-0.39, weak; 0.40-0.59,
moderate; 0.60-0.79, strong; and 0.80-1.00, markedly strong. Data
are presented as the mean ± SD, n (%), η or rs values. P<0.05
was considered to indicate a statistically significant
difference.
Results
Baseline characteristics according to the
irradiation methods are detailed in Table I. Age, tumor site, breast volume or
separation did not show statistically significant differences
between the three different irradiation methods (Table I).
 | Table IBaseline characteristics according to
the irradiation methods. |
Table I
Baseline characteristics according to
the irradiation methods.
| A, All patients |
|---|
| | Irradiation
method | |
|---|
| Variable | All irradiation
methods | W | FIF w/o 2RP | FIF w/ 2RP | P-value |
|---|
| Patients, n | 573 | 178 | 142 | 253 | |
| Mean age ± SD,
years | 55±11 | 54±11 | 55±12 | 55±11 | P=0.38a |
| Tumor site, n
(%) | | | | | P=0.29b |
|
Upper-inner
quadrant | 161 (28.1) | 47 (26.4) | 48 (33.8) | 66 (26.1) | |
|
Lower-inner
quadrant | 50 (8.7) | 18 (10.1) | 8 (5.6) | 24 (9.5) | |
|
Upper-outer
quadrant | 278 (48.5) | 81 (45.5) | 64 (45.1) | 133 (52.6) | |
|
Lower-outer
quadrant | 55 (9.6) | 23 (12.9) | 14 (9.9) | 18 (7.1) | |
|
Central
portion | 29 (5.1) | 9 (5.1) | 8 (5.6) | 12 (4.7) | |
| Mean BPe ± SD,
cm3 | 442±254 | 457±271 | 428±244 | 439±247 | P=0.77a |
| Mean separation ±
SD, cm | 19.1±2.4 | 19.1±2.3 | 18.7±2.4 | 19.3±2.5 | P=0.07a |
| B, Left-sided
patients |
| | Irradiation
method | |
| Variable | All irradiation
methods | W | FIF w/o 2RP | FIF w/ 2RP | P-value |
| Patients, n | 296 | 89 | 70 | 137 | |
| Mean age ± SD,
years | 55±11 | 55±11 | 57±11 | 55±11 | P=0.26a |
| Tumor site, n
(%) | | | | | P=0.75b |
|
Upper-inner
quadrant | 82 (27.7) | 26 (29.2) | 23 (32.9) | 33 (24.1) | |
|
Lower-inner
quadrant | 30 (10.1) | 11 (12.4) | 4 (5.7) | 15 (10.9) | |
|
Upper-outer
quadrant | 149 (50.3) | 42 (47.2) | 34 (48.6) | 73 (53.3) | |
|
Lower-outer
quadrant | 16 (5.4) | 6 (6.7) | 4 (5.7) | 6 (4.4) | |
|
Central
portion | 19 (6.4) | 4 (4.5) | 5 (7.1) | 10 (7.3) | |
| Mean BPe ± SD,
cm3 | 442±253 | 471±255 | 430±260 | 429±249 | P=0.38a |
| Mean separation ±
SD, cm | 18.9±2.6 | 19.1±2.5 | 18.6±2.7 | 18.9±2.5 | P=0.37a |
Dosimetric analysis
RT characteristics according to the irradiation
methods are detailed in Table II.
PTV volume did not show a statistically significant difference
between the three different irradiation methods. However, a
significant impact of the irradiation method was observed on PTV
and BPe dosimetric parameters, the mean dose, V0-95%, V95-107%,
V107% and V105%. The volumes of the PTV and BPe receiving ≥105% of
the prescribed dose were the lowest when using the FIF w/ 2RP
method, with mean ± standard deviation (SD) values of 0.09±0.75 and
0.10±0.90, respectively. The mean axillary lymph nodes levels I, II
and III doses were intermediate when using the FIF w/ 2RP method,
with mean ± SD values of 3,278±822, 1,455±1,127 and 427±528,
respectively. OAR constraints showed statistically significant
differences between three different irradiation methods. The MHD,
and the maximum doses to the CCA and the RCA were the lowest when
using the FIF w/ 2RP method, with mean ± SD values of 248±76,
201±32 and 212±34, respectively. However, the heart V10 and V20,
and the maximum doses to the LAD were intermediate when using the
FIF w/ 2RP method, with mean ± SD values of 3.0±2.1, 1.6±1.5 and
3,123±1,334, respectively. Nevertheless, the MLD, ipV20 and ipV30
were the highest when using the FIF w/ 2RP method, with mean ± SD
values of 771±198, 14±5 and 11±4, respectively (Table II).
 | Table IIRadiotherapy characteristics
according to the irradiation methods. |
Table II
Radiotherapy characteristics
according to the irradiation methods.
| A, All
patients |
|---|
| | Irradiation
method | |
|---|
| Variable | W | FIF w/o 2RP | FIF w/ 2RP |
P-valuea |
|---|
| Patients, n | 178 | 142 | 253 | |
| Target | | | | |
|
PTV | | | | |
|
Volume,
cm3 | 592±324 | 573±303 | 571±304 | P=0.93 |
|
Mean
dose, cGy | 4,924±84 | 5,011±65 | 4,952±45 | P<0.001
(P<0.01c,
P<0.001b,d) |
|
V0-95,
% | 15±8 | 10±7 | 13±7 | P<0.001
(P<0.05c,
P<0.001b,d) |
|
V95-107,
% | 84±8 | 90±7 | 87±7 | P<0.001
(P<0.01c,
P<0.001b,d) |
|
V107,
% | 0.52±1.50 | 0.27±0.28 | 0.00±0.01 | P<0.001
(P<0.001b,c,d) |
|
V105,
% | 3.50±4.88 | 6.63±3.96 | 0.09±0.75 | P<0.001
(P<0.001b,c,d) |
|
Breast PTV
evaluation | | | | |
|
Mean
dose, cGy | 4,969±62 | 5,043±60 | 4,980±48 | P<0.001
(P<0.001b,d) |
|
V0-95,
% | 10±6 | 5±5 | 8±7 | P<0.001
(P<0.01c,
P<0.001b,d) |
|
V95-107,
% | 90±6 | 95±5 | 92±7 | P<0.001
(P<0.001b,c,d) |
|
V107,
% | 0.42±1.45 | 0.25±0.35 | 0.00±0.01 | P<0.001
(P<0.001b,c,d) |
|
V105,
% | 3.24±4.86 | 6.92±4.56 | 0.10±0.90 | P<0.001
(P<0.001b,c,d) |
|
Axillary
lymph node | | | | |
|
Mean
dose, cGy | | | | |
|
Level
I | 2,928±717 | 3,529±750 | 3,278±822 | P<0.001
(P<0.05d,
P<0.001b,c) |
|
Level
II | 1,356±1,029 | 1,726±1,330 | 1,455±1,127 | P=0.10 |
|
Level
III | 357±399 | 508±566 | 427±528 | P<0.05
(P<0.05b) |
| Organ at risk:
Lung | | | | |
|
ip mean dose
(cGy) | 659±177 | 655±200 | 771±198 | P<0.001
(P<0.001c,d) |
|
ipV20
(%) | 11±4 | 11±5 | 14±5 | P<0.001
(P<0.001c,d) |
|
ipV30
(%) | 8±4 | 8±4 | 11±4 | P<0.001
(P<0.001c,d) |
| B, Left-sided
patients |
| | Irradiation
method | |
| Variable | W | FIF w/o 2RP | FIF w/ 2RP |
P-valuea |
| Patients, n | 89 | 70 | 137 | |
| Target | | | | |
|
PTV | | | | |
|
Volume,
cm3 | 610±322 | 588±325 | 555±300 | P=0.45 |
| Organ at risk:
Heart | | | | |
|
Mean dose,
cGy | 300±99 | 257±90 | 248±76 | P<0.001
(P<0.01b,
P<0.001c) |
|
V10, % | 3.8±2.9 | 2.9±2.6 | 3.0±2.1 | P<0.05
(P<0.05b) |
|
V20, % | 2.0±2.1 | 1.4±1.8 | 1.6±1.5 | P=0.08 |
|
LAD max,
cGy | 3,546±1,228 | 2,931±1,490 | 3,123±1,334 | P<0.05
(P<0.05b,c) |
|
CCA max,
cGy | 239±32 | 212±31 | 201±32 | P<0.001
(P<0.001b,c) |
|
RCA max,
cGy | 262±59 | 228±39 | 212±34 | P<0.001
(P<0.01d,
P<0.001b,c) |
Table III shows
that the irradiation methods strongly affected the dose
distribution factors. There were strong associations between all
irradiation methods and the volumes of the PTV and BPe receiving
≥105% of the prescribed dose with (η=0.62, P<0.001) and (η=0.61,
P<0.001), respectively. The strongest associations were found
when comparing the FIF w/ 2RP method vs. the FIF w/o 2RP method for
PTV V105% (η=0.79, P<0.001) and BPe V105% (η=0.76, P<0.001).
Other dose distribution factors were less affected by the
irradiation methods, with markedly weak to moderate η values. For
the heart, all irradiation methods had a weak association with MHD
(η=0.26, P<0.001), and moderate associations with maximum doses
to the CCA (η=0.45, P<0.001) and to the RCA (η=0.43,
P<0.001). The strongest associations were found when comparing
the FIF w/ 2RP method vs. the W method for MHD (η=0.29,
P<0.001), maximum doses to the CCA (η=0.50, P<0.001) and to
the RCA (η=0.47, P<0.001). For the lung, all irradiation methods
had weak associations with MLD (η=0.28, P<0.001), ipV20 (η=0.31,
P<0.001) and ipV30 (η=0.34, P<0.001). The strongest
associations were found when comparing the FIF w/ 2RP method vs.
the W method for MLD (η=0.28, P<0.001), ipV20 (η=0.30,
P<0.001) and ipV30 (η=0.33, P<0.001).
 | Table IIIAssociation between irradiation
methods and dose-distribution factors. |
Table III
Association between irradiation
methods and dose-distribution factors.
| Variable | All irradiation
methods | W vs. FIF w/o
2RP | W vs. FIF w/
2RP | FIF w/o 2RP vs. FIF
w/ 2RP |
|---|
| Target | | | | |
|
High dose
region, % | | | | |
|
PTV
V107 | 0.26a | 0.11b | 0.26a | 0.61a |
|
PTV
V105 | 0.62a | 0.33a | 0.47a | 0.79a |
|
BPe
V107 | 0.21a | 0.08 (P=0.17) | 0.21a | 0.49a |
|
BPe
V105 | 0.61a | 0.36a | 0.44a | 0.76a |
|
Adequate
dose region, % | | | | |
|
PTV
V95-107 | 0.28a | 0.36a | 0.17a | 0.21a |
|
BPe
V95-107 | 0.28a | 0.40a | 0.14a | 0.23a |
|
Low dose
region, % | | | | |
|
PTV
V0-95 | 0.26a | 0.33a | 0.13b | 0.22a |
|
BPe
V0-95 | 0.27a | 0.38a | 0.11b | 0.24a |
|
Mean dose,
cGy | | | | |
|
PTV | 0.45a | 0.49a | 0.21a | 0.47a |
|
BPe | 0.47a | 0.52a | 0.10b | 0.50a |
|
Axillary
lymph node mean dose, cGy | | | | |
|
Level
I | 0.28a | 0.38a | 0.22a | 0.15b |
|
Level
II | 0.12b | 0.16b | 0.05 (P=0.35) | 0.11b |
|
Level
III | 0.11b | 0.15b | 0.07 (P=0.14) | 0.07 (P=0.16) |
| Organs at risk | | | | |
|
Lung | | | | |
|
ip
mean dose, cGy | 0.28a | 0.01 (P=0.84) | 0.28a | 0.27a |
|
ipV20,
% | 0.31a | 0.02 (P=0.75) | 0.30a | 0.30a |
|
ipV30,
% | 0.34a | 0.00 (P=0.94) | 0.33a | 0.31a |
|
Heart
(left-sided patients) | | | | |
|
Mean
dose, cGy | 0.26a | 0.22b | 0.29a | 0.05 (P=0.45) |
|
V10,
% | 0.16b | 0.17b | 0.16b | 0.03 (P=0.68) |
|
V20,
% | 0.13 (P=0.07) | 0.15 (P=0.06) | 0.12 (P=0.06) | 0.05 (P=0.50) |
|
LAD
max, cGy | 0.17b | 0.22b | 0.16b | 0.07 (P=0.35) |
|
CCA
max, cGy | 0.45a | 0.39a | 0.50a | 0.17b |
|
RCA
max, cGy | 0.43a | 0.31a | 0.47a | 0.20b |
Skin toxicity analysis
All irradiation methods were well tolerated, since
no case of treatment interruption due to acute skin toxicity
neither grade 3 skin toxicities were documented (Figs. 1 and 2).
The association between the time course of the RT
and the mean skin toxicity grade registered at weeks 1-5, and the
maximum skin toxicity grade during the 5 weeks of RT treatment for
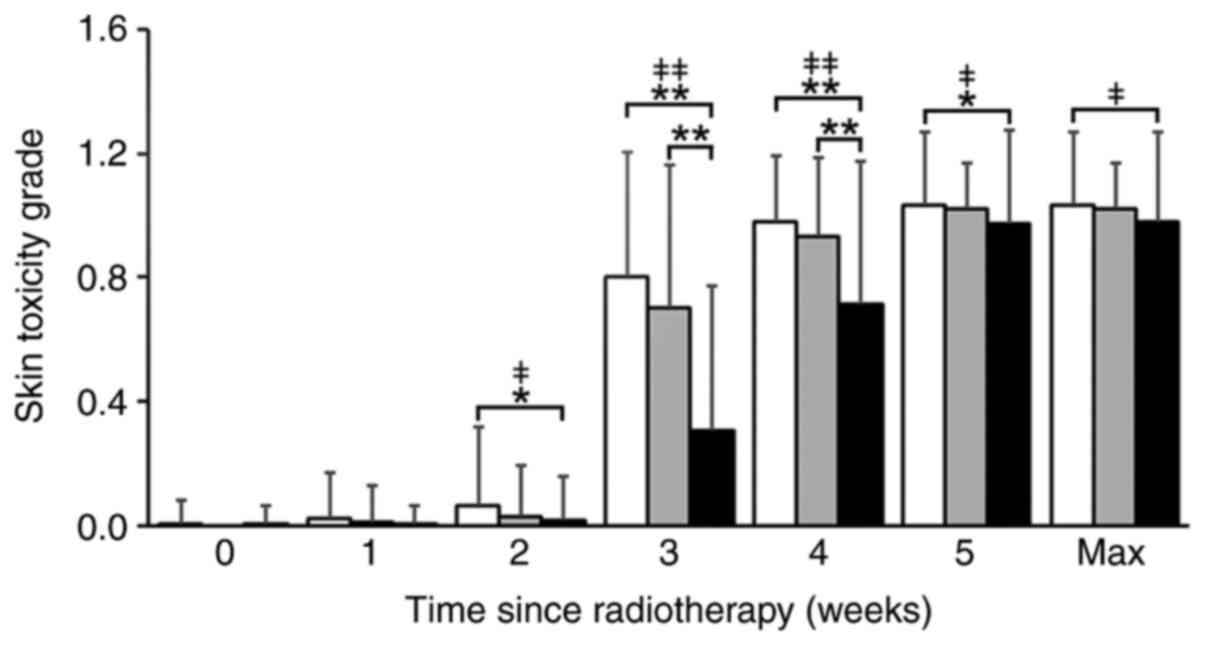
each irradiation method are shown in Fig. 1. The occurrence and mean skin
toxicity grade increased from the start of the RT for all the
irradiation methods, especially after 3 weeks of RT. In weeks 3 and
4, the FIF w/ 2RP method had a significant impact on lowering the
skin toxicity mean grade. Kruskal-Wallis test showed that there
were significant differences in the skin toxicity median grade
during weeks 2 to 5 (Fig. 1).
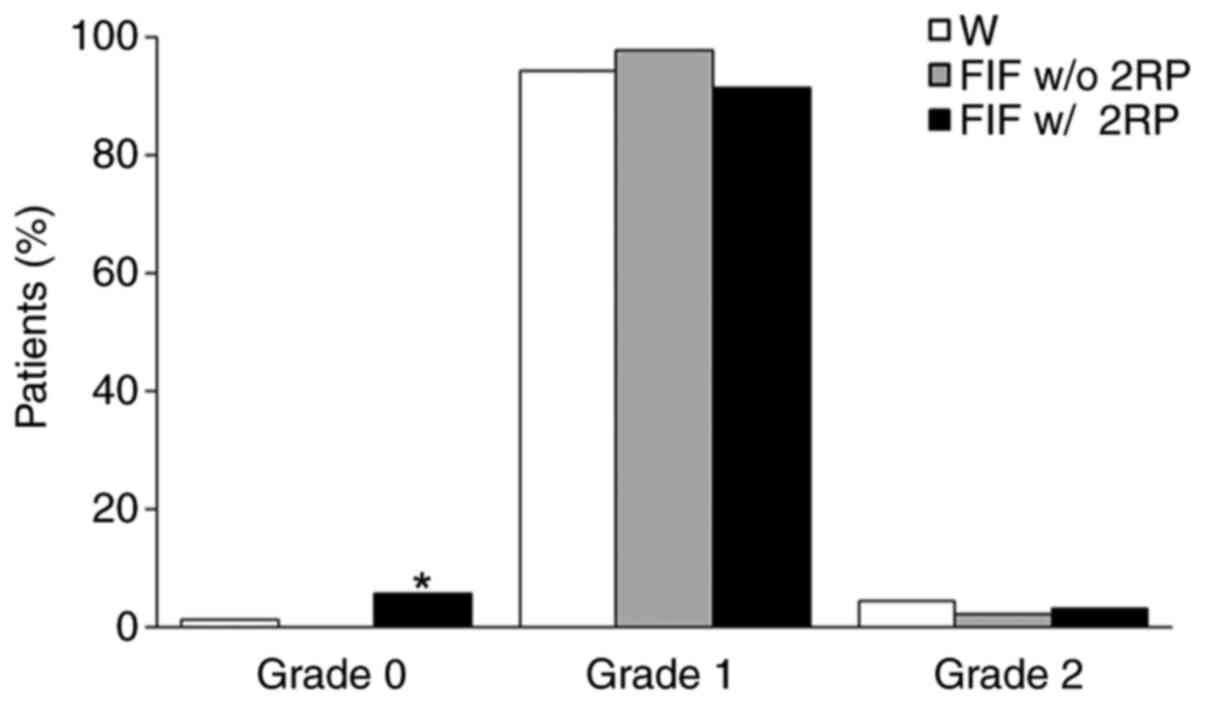
The percentage of patients according to the maximum
skin toxicity grade during the 5 weeks of RT treatment for each
irradiation method is summarized in Fig. 2. For the three irradiation methods,
the maximum skin toxicity grade recorded was grade 2. The
irradiation methods were significantly associated with the
appearance of adverse effects. The use of the FIF w/ 2RP method
increased the occurrence of grade 0 skin toxicity. Grade 0 was
observed in 2 (1.1%), 0 (0.0%) and 14 (5.5%) cases, respectively,
for the W, FIF w/o 2RP and FIF w/ 2RP methods. Grade 0 for the FIF
w/ 2RP method was significantly higher compared to the other
irradiation methods. Furthermore, the use of the FIF w/ 2RP method
decreased the occurrence of grade 1 skin toxicity. Grade 1 was
observed in 168 (94.4%), 139 (97.9%) and 231 (91.3%) cases,
respectively, for the W, FIF w/o 2RP and FIF w/ 2RP methods.
However, grade 2 skin toxicity had an intermediate percentage when
using the FIF w/ 2RP method. Grade 2 was observed in 8 (4.5%), 3
(2.1%) and 8 (3.2%) cases, respectively, for the W, FIF w/o 2RP and
FIF w/ 2RP methods.
For associations related to skin toxicity grade,
results were examined at the following three time points: Week 3,
week 4 and maximum skin toxicity grade during the 5 weeks of RT
treatment. As shown in Fig. 1, the
skin toxicity grades from the start of the RT treatment to the 2nd
week were low; moreover, the skin toxicity grade at week 5 and the
maximum grade during the 5 weeks of RT treatment were similar.
Therefore, associations of skin toxicity grade were examined at
these three time points.
The association between patients' baseline
characteristics and skin toxicity grade was analyzed for all
patients. Most significant associations were observed in the
following baseline characteristics: Breast volume factors (BPe
volume, separation and PTV volume) and weight exhibited markedly
weak positive correlations with the maximum skin toxicity grade
during the 5 weeks of RT treatment. The rs values were as follows
for BPe volume (rs=0.17, P<0.01), separation (rs=0.17,
P<0.01), PTV volume (rs=0.18, P<0.01) and weight (rs=0.17,
P<0.01). Tumor site exhibited markedly weak associations with
the skin toxicity grade at weeks 3 and 4. The η values were as
follows for tumor site at week 3 (η=0.16, P<0.05) and at week 4
(η=0.16, P<0.05).
Table IV shows the
association between irradiation methods and skin toxicity grade.
The impact of the irradiation methods differed with the time course
of the RT treatment. Associations between all irradiation methods
and the skin toxicity grade were moderate at week 3 (η=0.46,
P<0.001), weak at week 4 (η=0.33, P<0.001) and markedly weak
for the maximum skin toxicity grade during the 5 weeks of RT
treatment (η=0.11, P<0.05). Significant associations were found
at all time points in the FIF w/ 2RP method vs. the W method group,
but only at weeks 3 and 4 in the FIF w/ 2RP method vs. the FIF w/o
2RP method group.
 | Table IVAssociation between irradiation
methods and skin toxicity grade. |
Table IV
Association between irradiation
methods and skin toxicity grade.
| Variable | All irradiation
methods | W vs. FIF w/o
2RP | W vs. FIF w/
2RP | FIF w/o 2RP vs. FIF
w/ 2RP |
|---|
| Skin toxicity
grade | | | | |
|
Week 3 | 0.46a | 0.12b | 0.49a | 0.38a |
|
Week 4 | 0.33a | 0.10 (P=0.07) | 0.33a | 0.25a |
|
Max week
(0-5) | 0.11b | 0.03 (P=0.58) | 0.10b | 0.09 (P=0.09) |
Discussion
In accordance with the modern RT goal of reducing
skin toxicity and improving the quality of life of patients with
early-stage breast cancer, our study aimed to present an
irradiation method that is both clinically practical and effective
in achieving lower skin toxicity rates. This study showed that the
FIF w/ 2RP method, compared with the W and FIF w/o 2RP methods, was
advantageous in maintaining a homogeneous dose distribution while
reducing high-dose areas in the target and doses at OARs, and
consequently lowering skin toxicity occurrence and grades.
For WBRT, several RT techniques are available to
achieve a uniform and standardized dose throughout the whole
breast. The progression of RT techniques and delivery systems,
including intensity-modulated radiation therapy (IMRT), volumetric
modulated arc therapy (VMAT) and deep inspiration breath-hold
methods, improved dose homogeneity and enabled a better dose
distribution. However, the 3D-CRT treatment planning is a
widespread implemented system, and when used in adjunction to the
FIF technique, enables an improved homogeneity in the irradiated
volume and lowers high-dose areas (9,14).
Wright et al (15) evaluated 392 patients, 83% of which
were treated with conventionally fractionated scheme and 17% with a
hypofractionated scheme. Regional nodal radiation was delivered in
15% of patients, and the dose range was 45.0-50.4 Gy in 25
fractions. A boost to the lumpectomy cavity of 10-16 Gy was
received by 88% of patients. These patients had 35% of V105% and
16% of V110% (15). Giri et
al (16) compared three
techniques (VMAT, FIF and W methods) in 20 patients, and indicated
that VMAT is a superior method compared with the other two methods.
The authors reported that V105% for PTV using VMAT was 3.3±5.5%.
Our V105% using the FIF w/ 2RP method was better than this value.
Osei et al (17) conducted a
dose-volumetric analysis for the normalized PTV_eval for patients
treated with hybrid inverse planned IMRT technique (50-Gy dose in
25 fractions). The mean PTV_eval V105% was 0.81% for all patients
with a mean breast separation of 22.21 cm, whereas the mean
PTV_eval V105% was 0.37% for patients with a mean breast separation
<20 cm. Compared with these results and with the use of the FIF
w/o 2RP method, the use of the FIF w/ 2RP method resulted in values
of PTV V105% as low as 0.09%.
The impact of the irradiation method used on dose
distribution to target volumes was further elucidated by the
significantly strong associations in high-dose regions,
particularly in the FIF w/o 2RP method vs. FIF w/ 2RP method group,
confirming that our method significantly decreases high-dose region
volumes.
As for OARs, particularly dose distribution in the
heart, Duma et al (18)
assessed the heart dosimetry parameters in patients with breast
cancer using 3D-CRT by tangential fields, half beam technique. For
a group of patients with an estimated low dose to the heart, the
authors reported a median MHD to the whole heart of 2.6 Gy (0.8-3.5
Gy), V10 of 3.4% and V20 of 2.5% (18). The values reported in our study are
lower, thus improving dose distribution in the heart and lowering
the risk of late cardiac radiation injuries. Beaton et al
(19) identified all cardiovascular
deaths in women with early-stage breast cancer treated with
breast/chest wall RT. The authors found that the radiation-induced
cardiac death at 10 years was low if MHD is <3.3 Gy, maximum LAD
dose is <45.4 Gy and V25 <5% (19). For each of the three used
irradiation methods, our study calculated the percentage of
patients receiving MHD <3.3 Gy and maximum LAD dose <45.4 Gy.
It was found that the FIF w/ 2RP method had higher percentage of
cases with MHD <3.3 Gy (86.1%) and higher percentage of cases
with maximum LAD dose <45.4 Gy (86.9%) compared with those
subjected to the W and FIF w/o 2RP methods, thus suggesting that
FIF w/ 2RP might be a better method to reduce radiation-induced
cardiac death at 10 years.
For dose distribution in the lung, Chung et
al (20) reported that the
dosimetric parameter MLD using the partially wide tangent technique
is correlated with the incidence of radiation pneumonitis. There
was a statistically significant difference in the incidence of
radiation pneumonitis between patients who had an MLD of 17.9±3.2
and patients who had an MLD of 19.3±2.8. The MLD was significantly
different between total radiation pneumonitis grade 0 and grade ≥1
(P=0.042). MLD of 20.5 Gy was determined as the cut-off point for
the incidence of radiation pneumonitis. However, the average value
of V20 and V30 showed no statistically significant difference in
the incidence of radiation pneumonitis. Patients with an average
value of V20 of 36.1±7.7% and V30 of 31.3±7.1% had no radiation
pneumonitis (20). Although the FIF
w/ 2RP method had higher values than the FIF w/o 2RP method, these
values are still considerably low compared with the values reported
to have a correlation with the incidence of radiation pneumonitis.
Therefore, the difference in lung doses within the used irradiation
methods may not have clinical significance. Yang et al
(21) compared wedge and FIF IMRT
techniques in patients with small-sized breasts. The authors
suggested that the conventional FIF method has favorable dose
conformity and is an optimal method in patients with breast volume
≤350 cm3. Using the conventional FIF method, the authors
reported a lung V20% of 14.42±2.61% and a heart V25% of 2.44±1.07%
(21). Although our patients had a
mean breast volume of 493±247 cc using the FIF w/ 2RP method, our
results indicated a similar value for the lung V20% of 14±5% and a
lower value for the heart V20% of 1.6±1.5%. Morganti et al
(22), using a forward FIF IMRT
technique for 201 patients with a mean breast volume of 528 cc,
reported that V107% of the irradiated volume was 2.4%, which is
higher than the 0% value observed in our patients treated with the
FIF w/ 2RP method. The authors also reported MLD and MHD values of
9.1 and 3.4 Gy, respectively, whereas our FIF w/ 2RP method could
reduce the MLD and MHD to 7.7 and 2.5 Gy, respectively.
The existence of a high-dose range in the
irradiation field and its association to skin toxicities is
reported in the literature (6-8).
Chen et al (7) reported
that, for patients treated with BCS, higher volume receiving
PTV-V107% >31.5% and treated volume TV-V110% >4.37% were
associated with higher incidence of acute radiation dermatitis.
These reported values are significantly higher than 0% of V107% for
PTV in our patients treated with FIF w/ 2RP. According to Vicini
et al (6), the breast volume
V105% and V110% are significantly associated with increasing skin
toxicities. Using 3D treatment planning and intensity modulation
with an MLCs technique, the authors found a significant association
between median breast volume V105% of 11% and increasing skin
toxicities. In our study, using the FIF w/ 2RP method, the mean
V105% for breast volume was reduced to 0.1%.
Considering the reported association between
high-dose range and skin toxicities, lowering high-dose areas is
likely to reduce the occurrence of radiation dermatitis and its
severity. In view of the comparison of the FIF w/ 2RP method with
the W and FIF w/o 2RP methods and literature reports, our method
proved to reduce high-dose areas, notably V105% of the target and
doses at OARs. Therefore, the FIF w/ 2RP method is expected to
diminish skin toxicities.
The awareness of appearance and timing of skin
toxicities is crucial to ensure an effective management of
patients. The acute phase of radiation dermatitis typically occurs
within 30 to 90 days of RT treatment (9). Our study evaluated the association
between the time course of RT and skin toxicity grade. Although the
FIF w/ 2RP method had a less significant impact on skin toxicity
grade by the end of the RT treatment compared with that of the
other two irradiation methods, it had a substantial impact during
the 3rd and 4th weeks. This is particularly beneficial for the
patient, since the appearance of radiation dermatitis can lead to
treatment interruptions or cessation (23).
To further assess the impact of the FIF w/ 2RP
method on skin toxicities, the maximum skin toxicity grade during
the 5 weeks of RT treatment was examined for the three irradiation
methods used, in addition to the skin toxicity grades reported in
the literature. Tortorelli et al (8) reported, for patients who underwent
irradiation with conventional fractionation followed by an electron
tumor bed boost, the maximum acute toxicity during or after
completion of RT using the RTOG Acute Morbidity Scale. Grades 1, 2
and 3 acute skin toxicities were registered in 45.2, 42 and 13% of
patients, respectively. Borm et al (5) evaluated 255 patients treated with
tangential 3D-CRT and prescribed doses of 50.4 or 50.0 Gy, followed
by a sequential boost to the tumor bed for 92.5% of the patients,
using the CTCAE V.4.0 scale. By the end of the treatment, skin
toxicity grade 1 was observed in 42.4% of patients, grade 2 in
55.7% and grade 3 in 2%. Wright et al (15) reported that, in patients treated
only with conventionally fractionated irradiation, 42% developed
CTCAE grade 0-1 skin toxicity and 58% developed grade 2-3. Vicini
et al (6) reported that,
using the MLCs IMRT technique, a prescribed dose of 45 Gy followed
by a supplemental boost to the tumor bed of 16 Gy, a total of 56%
of patients experienced RTOG grade 0 or 1 acute skin toxicity,
while 43% experienced grade 2 acute skin toxicity and only 1%
experienced grade 3 toxicity. In our study, compared with the
observations in the W and FIF w/o 2RP methods, for patients treated
with the FIF w/ 2RP method, the skin toxicity was reduced, since
5.5% experienced grade 0, 91.3% grade 1, 3.2% grade 2 and none
grade 3. This clinical outcome supports that FIF w/ 2RP is a useful
method to reduce both high-dose areas and acute skin toxicity.
Previous studies identified predictive factors for
acute skin toxicities and categorized them into patient-related
factors and treatment procedure-related factors. Our study, through
the evaluation of association between these factors and skin
toxicity, demonstrated that the irradiation methods have a greater
impact on skin toxicity compared with the patients' baseline
characteristics. Breast size was reported as a predictive factor
for acute skin toxicity (9). In our
study, the correlations between breast volume and skin toxicity
grade throughout RT treatment, although markedly weak, they were
significant. Separation, PTV volume and weight also had a markedly
weak, but significant impact on the skin toxicity maximum grade
during the 5 weeks of RT treatment, whereas tumor site had
significant impact at weeks 3 and 4. Overall, the effect of the
patients' baseline characteristics on skin toxicity was limited,
while the irradiation methods effect, particularly that of the FIF
w/ 2RP method, was comparatively substantial. Associations with the
skin toxicity grade were significant when comparing the FIF w/ 2RP
method vs. the W or the FIF w/o 2RP methods, indicating that a
significant decrease in skin toxicity grade might be expected when
using the FIF w/ 2RP method. The utmost benefit of our method can
be perceptible during the RT treatment at weeks 3 and 4, as skin
toxicity grade is significantly improved compared with that of the
W and to the FIF w/o 2RP methods. This decline in skin toxicities
during the treatment course minimizes risks of interruption or
cessation and provides patient comfort.
Assessment of skin toxicities is of a subjective
nature; however, in our institution, the skin toxicity grade was
evaluated by a single experienced radiation oncologist with a
standardized approach, which reduces potential variations and
ensures a reproductible quantification. The FIF w/ 2RP method is a
clinically practical and achievable method; nevertheless, it is
more time consuming than conventional irradiation methods.
Furthermore, the impact of this method is more considerable in
patients with small-sized breasts; therefore, this aspect should be
taken into consideration when selecting the appropriate irradiation
method.
In conclusion, our study confirmed that the FIF w/
2RP method decreased the high-dose range V105% of the target to 0%,
while maintaining a homogeneous dose distribution across the breast
tissue. This decrease in high-dose range was in conjunction with a
decrease in the occurrence and grade of skin adverse events.
Therefore, the FIF w/ 2RP could be advised as an optimal method in
clinical practice for patients with early stage breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NT and MK conceived and designed the study,
processed the data and wrote the manuscript. MK and HIs assessed
the authenticity of the raw data. KKa, SS, KW, KY, NK, TO and HIh
were involved in the clinical studies and in the collection and
assembly of the data. HIs, MB, KH, KKo, KS, SK, AK and JA were
involved in data analysis and interpretation. NT, MK, HIs, AK, SK
and JA edited the article. All authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Okayama University Graduate School of Medicine,
Dentistry and Pharmaceutical Sciences, and Okayama University
Hospital (approval no. 1907-027). Patients provided written
informed consent for undergoing RT and using their anonymous data
for scientific studies. The institutional informed consent forms
for treatment included consent for the use of patient data and
materials for research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106.
2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Darby S, McGale P, Correa C, Taylor
C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, et al:
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: Meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 378:1707–1716. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Keenan LG, Lavan N, Dunne M and McArdle O:
Modifiable risk factors for acute skin toxicity in adjuvant breast
radiotherapy: Dosimetric analysis and review of the literature. Med
Dosim. 44:51–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schnur JB, Ouellette SC, Dilorenzo TA,
Green S and Montgomery GH: A qualitative analysis of acute skin
toxicity among breast cancer radiotherapy patients. Psychooncology.
20:260–268. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Borm KJ, Loos M, Oechsner M, Mayinger MC,
Paepke D, Kiechle MB, Combs SE and Duma MN: Acute radiodermatitis
in modern adjuvant 3D conformal radiotherapy for breast cancer-the
impact of dose distribution and patient related factors. Radiat
Oncol. 13(218)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vicini FA, Sharpe M, Kestin L, Martinez A,
Mitchell CK, Wallace MF, Matter R and Wong J: Optimizing breast
cancer treatment efficacy with intensity-modulated radiotherapy.
Int J Radiat Oncol Biol Phys. 54:1336–1344. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen MF, Chen WC, Lai CH, Hung CH, Liu KC
and Cheng YH: Predictive factors of radiation-induced skin toxicity
in breast cancer patients. BMC Cancer. 10(508)2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tortorelli G, Di Murro L, Barbarino R,
Cicchetti S, di Cristino D, Falco MD, Fedele D, Ingrosso G,
Janniello D, Morelli P, et al: Standard or hypofractionated
radiotherapy in the postoperative treatment of breast cancer: A
retrospective analysis of acute skin toxicity and dose
inhomogeneities. BMC Cancer. 13(230)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kole AJ, Kole L and Moran MS: Acute
radiation dermatitis in breast cancer patients: Challenges and
solutions. Breast Cancer (Dove Med Press). 9:313–323.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
ClinicalTrials: Radiation Therapy Oncology
Group: NRG oncology RTOG 1005. A phase III trial of accelerated
whole breast irradiation with hypofractionation plus concurrent
boost versus standard whole breast irradiation plus sequential
boost for early-stage breast cancer. Available from: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?action=openFile&FileID=9366.
|
|
11
|
Radiation Therapy Oncology Group: Breast
Cancer Atlas. Available from: https://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx.
|
|
12
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events v3.0 (CTCAE). Available
from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
|
|
13
|
International Classification of Diseases
for Oncology. Available from: https://apps.who.int/iris/bitstream/handle/10665/96612/9789241548496_eng.pdf.
|
|
14
|
Sheng Y, Li T, Yoo S, Yin FF, Blitzblau R,
Horton JK, Ge Y and Wu QJ: Automatic planning of whole breast
radiation therapy using machine learning models. Front Oncol.
9(750)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wright JL, Takita C, Reis IM, Zhao W, Lee
E, Nelson OL and Hu JJ: Prospective evaluation of radiation-induced
skin toxicity in a race/ethnically diverse breast cancer
population. Cancer Med. 5:454–464. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Giri UK, Sarkar B, Jassal K, Munshi A,
Ganesh T, Mohanti B and Pradhan A: Left-sided breast radiotherapy
after conservative surgery: Comparison of techniques between
volumetric modulated arc therapy, forward-planning
intensity-modulated radiotherapy and conventional technique. J
Radiother Pract. 16:1–8. 2016.
|
|
17
|
Osei E, Darko J, Fleck A, White J, Kiciak
A, Redekop R and Gopaul D: Dosimetric evaluation of whole-breast
radiation therapy: Clinical experience. Med Dosim. 40:355–365.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Duma MN, Herr AC, Borm KJ, Trott KR, Molls
M, Oechsner M and Combs SE: Tangential field radiotherapy for
breast cancer-the dose to the heart and heart subvolumes: What
structures must be contoured in future clinical trials? Front
Oncol. 7(130)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Beaton L, Bergman A, Nichol A, Aparicio M,
Wong G, Gondara L, Speers C, Weir L, Davis M and Tyldesley S:
Cardiac death after breast radiotherapy and the QUANTEC cardiac
guidelines. Clin Transl Radiat Oncol. 19:39–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chung Y, Yoon HI, Kim YB, Ahn SK, Keum KC
and Suh CO: Radiation pneumonitis in breast cancer patients who
received radiotherapy using the partially wide tangent technique
after breast conserving surgery. J Breast Cancer. 15:337–343.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang DS, Lee JA, Yoon WS, Chung SY, Lee S,
Kim CY, Park YJ and Son GS: Whole breast irradiation for
small-sized breasts after conserving surgery: Is the field-in-field
technique optimal? Breast Cancer. 21:162–169. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Morganti AG, Cilla S, de Gaetano A,
Panunzi S, Digesù C, Macchia G, Massaccesi M, Deodato F, Ferrandina
G, Cellini N, et al: Forward planned intensity modulated
radiotherapy (IMRT) for whole breast postoperative radiotherapy. Is
it useful? When? J Appl Clin Med Phys. 12(3451)2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Drost L, Li N, Vesprini D, Sangha A, Lee
J, Leung E, Rakovitch E, Yee C, Chow E and Ruschin M: Prospective
study of breast radiation dermatitis. Clin Breast Cancer.
18:e789–e795. 2018.PubMed/NCBI View Article : Google Scholar
|