Introduction
Sequencing of DNA to identify polymorphisms has
catalyzed the quest for protein kinase ‘driver’ mutations, which
contribute to the transformation of a normal cell to a
proliferating cancerous cell. Approximately one-third of patients
in Asian populations with non-small cell lung cancer (NSCLC) harbor
epidermal growth factor receptor (EGFR) mutations.
For these patients, EGFR-targeted tyrosine kinase inhibitors (TKIs)
have shown improved efficacy and longer progression-free survival
(PFS) than standard chemotherapies (1). Currently, TKI treatment is considered
the standard of care for EGFR-mutated NSCLC. However, most
patients eventually develop resistance with a PFS from 10 months to
18.9 months (1,2).
For NSCLC, recent alternative treatments include
immune checkpoint inhibitors (ICIs) such as programmed cell death 1
(PD-1) antibody (nivolumab or pembrolizumab) or a programmed cell
death 1 ligand (PDL-1) antibody (atezolizumab or durvalumab). ICI
monotherapy with nivolumab or pembrolizumab is efficacious for
NSCLC, achieving response rates of ~20%, with a 5-year survival
rate of ~15%. However, EGFR-mutated NSCLC is insensitive to ICIs
(3,4).
Previous researchers, Coley W and Maruyama C,
experienced immune responses to human malignant tumors by
erysipelas and tuberculosis, respectively, indicating there might
be a relationship between infection and cancer immunity (5,6).
Previously, the effect of erysipelas was partially explained via
the production of interleukin (IL)-12 and tumor necrosis factor
(7). Regarding tuberculosis,
Maruyama (6) developed Specific
Substance Maruyama (SSM), a hot-water extract from human bacillus
tuberculosis containing polysaccharides including arabinomannan and
mannan (8). SSM is an
immunomodulatory agent and its carcinostatic potential was reported
in 1966(6). Later studies
indicated that SSM acts as an immune adjuvant, resulting in a
change from innate immunity to adaptive immunity (9-12).
No report has described ICI and immunomodulatory
arabinomannan extracted from Mycobacterium tuberculosis in
humans.
Material and methods
The two cases presented below gave oral consents as
for presenting this case-report, and written consents were also
obtained from the families. This manuscript followed a Japanese
law, Act on the Protection of Personal Information.
To produce a hot-water extract from human bacillus
tuberculosis, Mycobacterium tuberculosis, Aoyama B strain,
grown on the surface of Sauton's media for 5 weeks at 37 degrees
Celsius is diluted in distilled water. To extract polysaccharides,
it is kept at 100˚C for 120 min, and, after filtering it to delete
Mycobacterium tuberculosis, the filtrate is adjusted at the
following concentrations (8).
There are three types of hot water extracts from human
Mycobacterium tuberculosis strain Aoyama B: SSM A at a dose
of 2 mg as D-arabinose, SSM B at a dose of 0.2 mg as D-arabinose,
and Ancer®, 1 ml ampoule containing Z-100 for
subcutaneous injection, at a dose of 20 mg as D-arabinose.
Ancer® has been approved in Japan as a
granulocyte-macrophage (GM)-colony stimulating factor (CSF) under
radiation therapy. SSM and Ancer® are supplied by Zeria
Pharmaceutical Co. Ltd.
The cases were treated in clinical practice, and
computed tomography (CT), positron emission tomography (PET),
magnetic resonance imaging (MRI), and measurement of
carcinoembryonic antigen (CEA) by a kit of CEA-ABOTT JAPAN were
performed when needed in clinical practice. CT was performed by
Light Speed VCT (GE, USA), and Biograph mCT 16 (Siemens Healthcare,
Erlangen, Germany) was used for PET-CT using 193.8MBq of F-18
FDG.
Case reports
Case one
In 2016, a 67-year-old woman was diagnosed as NSCLC
(adenocarcinoma) harboring a sensitive EGFR mutation, exon
19 deletion of the right lower lung. TNM and staging were cT1bN3M1c
(lymph nodes metastasis, bone metastases, and adrenal metastasis)
and Stage IVb disease. Thus, an EGFR-TKI, erlotinib, was started in
February 2016, and achieved a partial response (PR). However, her
disease progressed 10 months after starting the treatment. Second
line treatment with carboplatin and pemetrexed with bevacizumab was
immediately started, and achieved a PR. As disease progressed again
and her tumor had no T790M mutation, erlotinib plus
bevacizumab were administered from July 2017, and this maintained
stable disease (SD). However, in December 2017, the disease
progressed to the neck lymph nodes and left axillary lymph nodes
with multiple bone metastases, and bilateral adrenal metastases. At
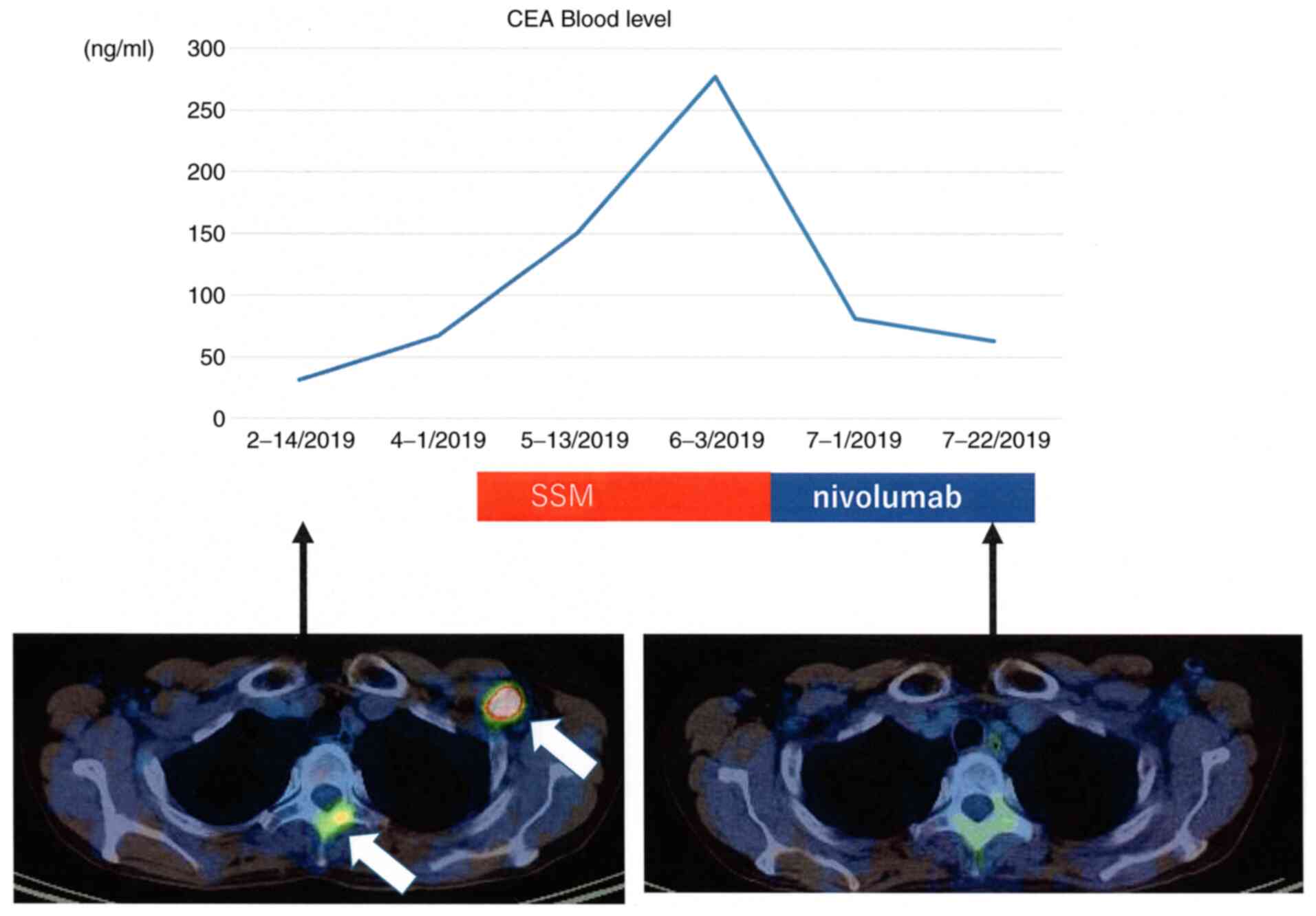
this point, the patient requested to be treated with SSM of her own
free will. Between March and May 2018, SSM at doses of 0.2 and 2 mg
as D-arabinose were alternatively administered every other day, but
the disease progressed further and the SSM treatment stopped.
Immediately after it, she was treated with nivolumab as fifth line
treatment from June 2018. Nivolumab after SSM treatment achieved a
marked PR, resulting in the disappearance of the neck lymph nodes
and the left axillary lymph nodes, and decreased the size of the
primary site and bone metastases (Fig.
1). Nivolumab treatment was continued for 10 cycles until
December 2018. Then, interstitial lung disease of Grade 2 common
toxicity criteria occurred, and nivolumab treatment was terminated.
Its efficacy continued until February 2019, and then the disease
progressed. She was treated by osimertinib due to having T790M,
followed by nab-paclitaxel, and died in November 2020.
Case two
A 57-year-old male with a history of smoking was
diagnosed with pulmonary adenocarcinoma harboring an EGFR mutation,
exon 19 deletion, in the left upper lobe (TNM and staging:
cT2aN2M1c (brain metastasis and bone metastases) and Stage IVb
disease, respectively) in 2015. Afatinib was initiated in November
2015, and achieved PR. Tumor recurrence and a T790M mutation
were found in December 2016; therefore, osimertinib was
administered. Six months after its initiation, progressive disease
was observed. Then cisplatin plus pemetrexed was administered, and
he also received erlotinib plus bevacizumab. However, apparent
disease progression was observed in September 2017, and cancer
histology by bronchial rebiopsy was changed from adenocarcinoma to
undifferentiated carcinoma. Nivolumab was administered for the
recurrent disease. Although he experienced tumor remission,
regrowth of the primary tumor was observed in December 2019. Adding
celecoxib (400 mg/day) due to lumbar pain produced a tentative
effect and chest radiography revealed a shrinkage of the primary
site (13). However, his disease
progressed again in both the primary site and distant metastases in
October 2020. He also felt pain and disturbance of motility in the
bilateral legs, and it was later diagnosed as leptomeningeal
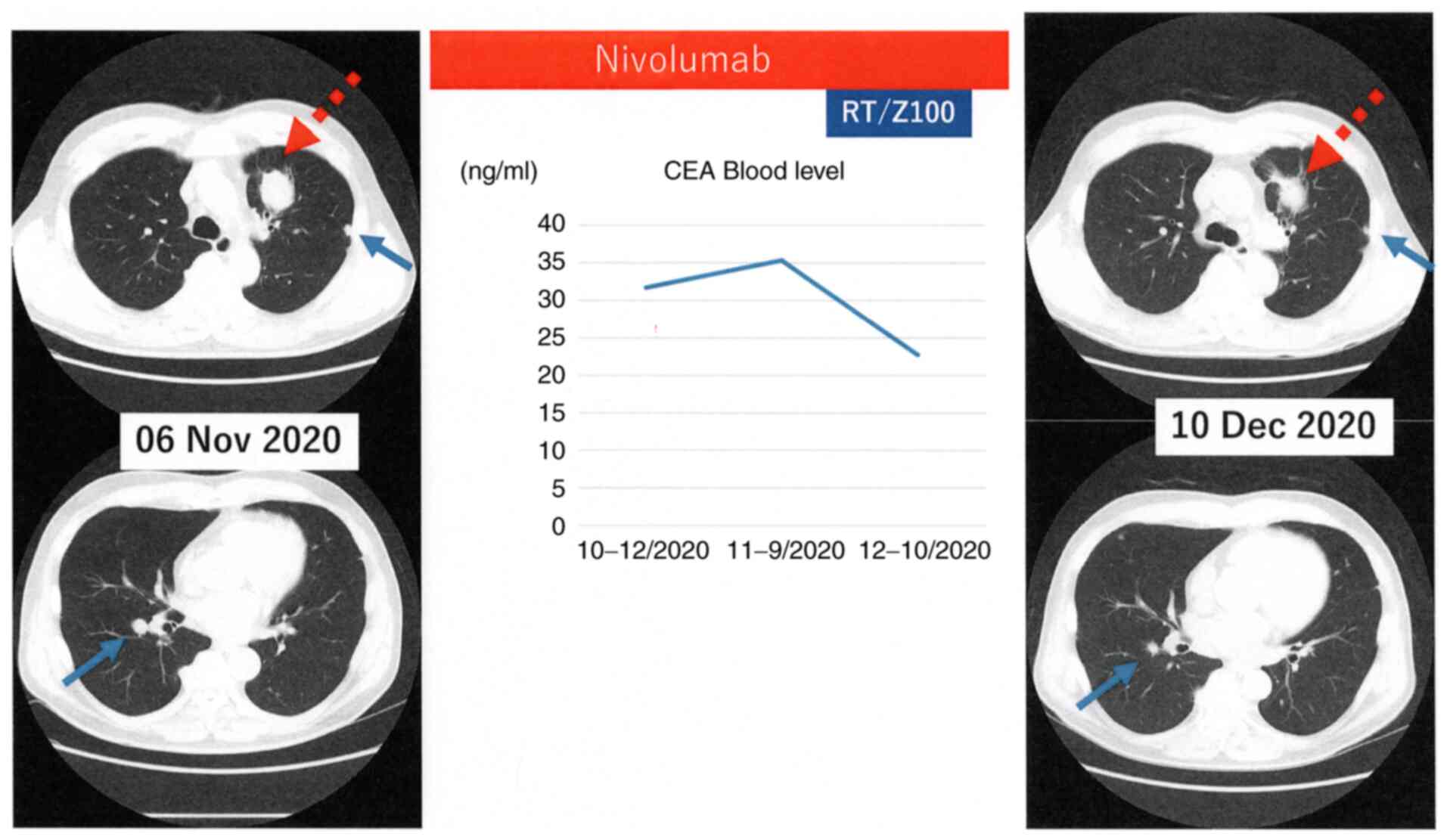
metastases by MRI. Under continuing nivolumab, he was treated with
palliative irradiation to the right pelvic bone metastasis at a
total dose of 20 Gy in 4 fractions and left adrenal metastasis at a
total dose of 30 Gy in 10 fractions with a subcutaneous injection
of Ancer® for two months. In December 2020, a chest
computed tomography scan showed a reduction in the sizes of the
primary site and pulmonary metastases, which were not irradiated,
with a decreasing trend in carcinoma embryonic antigen (CEA) blood
levels (Fig. 2). However, active
treatments stopped and he moved another hospital for palliative
care because of progressing leptomeningeal metastases, which made
him bed rest in January 2021 and died in late January 2021,
indicating that the patient with leptomeningeal metastases could
survive for 3 months.
Discussion
We experienced two lung cancer patients harboring
EGFR mutations in whom the immunomodulatory arabinomannan
extracted from Mycobacterium tuberculosis might act as an
immune adjuvant under ICI treatment. Although these cases might be
considered random or serendipitous, we think these cases provide
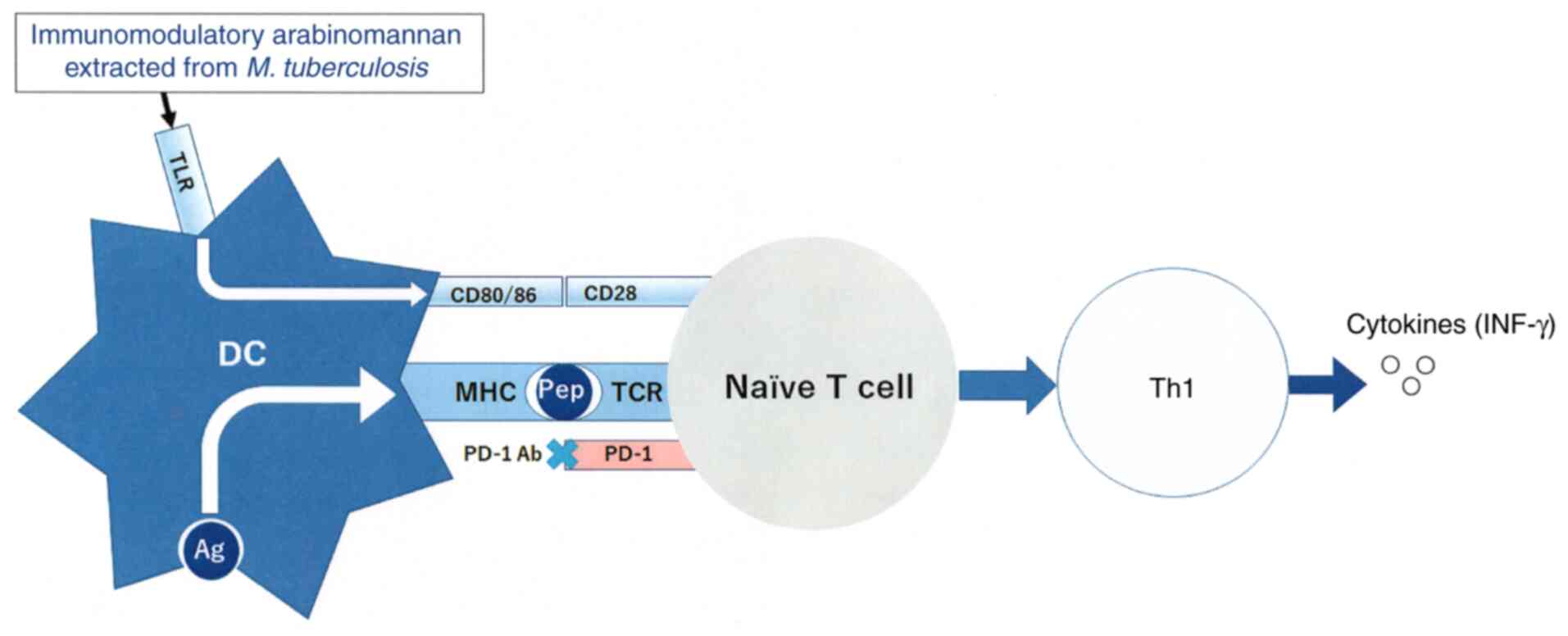
interesting information. Possible mechanisms to the events
described below are shown in Fig.
3.
Polymannan of tuberculosis is an immunogenic ligand
for Toll-like receptors (TLRs) (14). Although signaling pathways in DCs
by arabinomannan extracted from Mycobacterium tuberculosis
is unknown at present, TLR ligands act as adjuvants in adaptive
immune responses (15,16). It was reported that SSM induced
cluster of differentiation (CD)80, CD86, and major
histocompatibility complex (MHC) class II expression on bone
marrow-derived dendritic cells (DCs), and that SSM treatment of
mice increased the number of activated DCs in target lesions
(9-12).
Z-100 promoted a change in helper T-cell responses from a type 2
dominant state to a type 1 dominant state via the upregulation of
interferon-γ and IL-12 production (9-11).
These reports support that arabinomannan extracted from
Mycobacterium tuberculosis acts as an immune adjuvant for
TLRs on DCs in cancer immunity.
Although cancer cells of EGFR-mutated NCSLC
often express PDL-1 on their cell-surface, ICIs have a poor
therapeutic effect (3,4). This PDL-1 expression is induced
directly by activated EGFR signaling, not by tumor immunity
(17). Therefore, PDL-1 on cancer
cells harboring EGFR mutations is not exposed to any
cytokines (18). Type 1 cytokine
(IL-2, IFN-γ) production was increased in tumor-bearing mice
treated with arabinomannan extracted from Mycobacterium
tuberculosis (9,10). In case one, in addition to SSM
acting as an immunologic adjuvant, pretreatment with SSM caused
tumor cells to be exposed to cytokines.
It was reported that novel proteins were generated
in response to γ-irradiation, resulting in new peptides presented
by MHC molecules expressed by DCs (19). Formenti et al hypothesized
that ‘abscopal response’ might be related to the radiation-induced
exposure of immunogenic mutations to the immune system (20). To produce the abscopal response,
both antigen presentation by MHC and co-stimulation by an
immunologic adjuvant are critical. A previous study reported the
administration of Z-100 in combination with radiation showed the
inhibitory action of pulmonary metastasis in tumor-bearing mice
model, and prolonged survival time (11). In humans, a phase III
placebo-controlled double-blind randomized trial of radiotherapy
for stages IIB-IVA cervical cancer with or without Z-100 was
reported a trend in the improvement of overall survival (OS) in
locally advanced cervical cancer (21). The 5-year OS rate was 75.7% with
Z-100 and 65.8% without Z-100 (hazard ratio: 0.65, P=0.07). In our
second case receiving nivolumab treatment, palliative irradiation
to distant metastases was administered with Ancer®, and
effects on the primary site and pulmonary metastases, which were
not irradiated, were observed.
In conclusion, these two cases might indicate
immunomodulatory arabinomannan extracted from Mycobacterium
tuberculosis has immune adjuvant effects under PD-1 antibody
treatment. This treatment strategy should be validated by
prospective clinical studies, and the NEJ 046A trial is currently
underway.
Acknowledgements
The authors are especially grateful to Dr Hiroyuki
Tajima and Dr Kenji Fukushima (Saitama Medical University, Saitama,
Japan) for performing the CT/PET imaging.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KuK provided the clinical data included in the text
and wrote the manuscript draft. KuK, KyK, HI, SS, KH, YM, AS, FN
and HK treated the two patients and interpreted the PET-CT, CT
imaging and the laboratory test results. KyK critically revised the
manuscript and modified the text. KuK and KyK confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the present case report
were in accordance with the ethical standards of Saitama Medical
University International Medical Center and with the 1964
Declaration of Helsinki and its later amendments. This manuscript
followed a Japanese law, Act on the Protection of Personal
Information. The two patients gave oral consent for presenting this
case-report, and written consent was also obtained from the
families.
Patient consent for publication
Written consent for publication was provided by the
patients' families.
Competing interests
KuK received research grants from AstraZeneca Co.,
and Zeria Pharmaceutical Co., and received personal fees from
AstraZeneca Co. KyK received personal fees from Ono Pharmaceutical
Co., Boehringer Ingelheim Co., Chugai Pharmaceutical Co., Taiho
Pharmaceutical Co., Eli Lilly Japan Co., Nihon Medi-Physics Co.,
and AstraZeneca Co. HK received research grants from AstraZeneca
Co., and received personal fees from AstraZeneca Co. All other
authors declare that they have no competing interests.
References
|
1
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in Untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
Non-Small-Cell lung cancer. N Engl J Med. 375:1823–1833.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Busch W: Einfluβ von Erysipel. Berliner
Klin Wschr. 3:245–246. 1866.(In German).
|
|
6
|
Maruyama C: On the treatment of malignant
tumor with an extract from tubercle bacilli. Jpn J Dermatol.
76:399–404. 1966.(In Japanese).
|
|
7
|
Tsung K and Norton JA: Lessons from
Coley's Toxin. Surg Oncol. 15:25–28. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kobatake H, Suekane T, Murakami Y, Niwa S,
Okahira A and Kushida H: Studies on hot water extract of
Mycobacterium tuberculosis. I. Structural analyses of
polysaccharides (author's transl). Yakugaku Zasshi. 101:713–722.
1981.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
9
|
Oka H, Shiraishi Y, Sasaki H, Yoshinaga K,
Emori Y and Takei M: Antimetastatic effect of an immunomodulatory
arabinomannan extracted from Mycobacterium tuberculosis strain
Aoyama B, Z-100, through the production of interleukin-12. Biol
Pharm Bull. 26:1336–1341. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Oka H, Emori Y, Sasaki H, Shiraishi Y,
Yoshinaga K and Kurimoto T: Anti-tumor mechanism of Z-100, an
immunomodulatory Arabinomannan extracted from Mycobacterium
tuberculosis strain Aoyama B, on pulmonary metastases of B16F10
melanoma: Restoration of helper T cell responses via suppression of
glucocorticoid-genesis. Microbiol Immunol. 46:343–351.
2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oka H, Sasaki H, Shiraishi Y, Emori Y,
Yoshinaga K and Takei M: Z-100, an immunomodulatory arabinomannan
extracted from Mycobacterium tuberculosis strain Aoyama B, augments
anti-tumor activities of X-ray irradiation against B16 melanoma in
association with the improvement of type 1 T cell responses. Biol
Pharm Bull. 27:82–88. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mitsuishi T, Kabashima K, Tanizaki H,
Ohsawa I, Oda F, Yamada Y, Halifu Y, Kawana S, Kato T and Iida K:
Specific substance of Maruyama (SSM) suppresses immune responses in
atopic dermatitis-like skin lesions in DS-Nh mice by modulating
dendritic cell functions. J Dermatol Sci. 63:184–190.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kobayashi K, Kaira K and Kagamu H:
Recovery of the sensitivity to Anti-PD-1 antibody by celecoxib in
lung cancer. Anticancer Res. 40:5309–5311. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Murphy K and Weaver C: Chapters: 3-5. In:
Janeway's Immunobiology. 9th edition. W.W. Norton & Company,
Inc., New York, 2017.
|
|
15
|
Shah RR, Hassett KJ and Brito LA: Overview
of vaccine adjuvants: Introduction, history, and current status.
Methods Mol Biol. 1494:1–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Reed SG, Orr MT and Fox CB: Key roles of
adjuvants in modern vaccines. Nat Med. 19:1597–608. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Hsu PC, Jablons DM, Yang CT and You L:
Epidermal Growth Factor Receptor (EGFR) Pathway, Yes-Associated
Protein (YAP) and the Regulation of Programmed Death-Ligand 1
(PD-L1) in Non-Small Cell Lung Cancer (NSCLC). Int J Mol Sci.
20(E3821)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Teng MW, Ngiow SF, Ribas A and Smyth MJ:
Classifying cancers based on T-cell infiltration and PD-L1. Cancer
Res. 75:2139–2145. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Reits EA, Hodge JW, Herberts CA, Groothuis
TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH,
Neijssen J, et al: Radiation modulates the peptide repertoire,
enhances MHC class I expression, and induces successful antitumor
immunotherapy. J Exp Med. 203:1259–1271. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Formenti SC, Rudqvist NP, Golden E, Cooper
B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari
de Andrade L, Wucherpfennig KW, et al: Radiotherapy induces
responses of lung cancer to CTLA-4 blockade. Nat Med. 24:1845–1851.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sugiyama T, Fujiwara K, Ohashi Y, Yokota
H, Hatae M, Ohno T, Nagai Y, Mitsuhashi N, Ochiai K and Noda K:
Phase III placebo-controlled double-blind randomized trial of
radiotherapy for stage IIB-IVA cervical cancer with or without
immunomodulator Z-100: A JGOG study. Ann Oncol. 25:1011–1017.
2014.PubMed/NCBI View Article : Google Scholar
|