Introduction
Hepatocellular carcinoma (HCC) is the most common
primary liver cancer and the third leading cause of cancer-related
death worldwide (1). The incidence
and mortality of HCC are reportedly increasing in North America and
several European regions and decreasing in traditionally high-risk
regions, including Asian countries (2). Nevertheless, the prevalence of HCC
remains a critical global health issue (3).
Telomerase reverse transcriptase (TERT) is the
catalytic protein subunit of telomerase and is responsible for
maintaining chromosomal integrity and genomic stability through the
addition of telomeres to the ends of chromosomes (4). In somatic cells, telomere loss occurs
during each round of cell division, resulting in cellular aging
(5). In contrast, telomerase is
reactivated to prevent critical telomere shortening in cancer
cells, thereby enabling cancer cells to acquire replicative
immortality (6). Promoter mutations
lead to an enhancement of TERT transcription and the acquisition of
immortality in cancer cells.
Telomere shortening and reactivation of telomerase
are described in a broad range of human cancers, including liver
cancer (7,8). Telomerase reactivation is associated
with the alteration of transcriptional regulators of the TERT
promotor in cancer, TERT promotor mutations or rearrangements and
DNA copy number amplifications (9).
It has been reported that reactivation of the telomerase enzymes
was shown in more than 80% of HCCs, and TERT promotor somatic
mutations was found in 59% of the HCCs (8,9).
Regarding the relationship between liver disease and
TERT promoter mutation of HCC, the mechanisms of the mutation have
been reported in HBV- and HCV-related HCC (9). In HBV related HCC, HBV directly
integrates to TERT promoter region and activated TERT transcription
(10). On the other hand, TERT
promoter mutation of HCV-related HCC is more frequent compared as
that of HBV (10). Although HCV is
not able to integrate into host genome unlike HBV, it is considered
that the core proteins of HCV directly affect TERT promoter
(11). However, the intensity of
TERT expression in HCC was not differed by TERT promoter mutation
(12). It is skeptical whether TERT
expression is associated with the outcome of HCC patients.
Actually, a few reports have shown the relationship
between the outcome of HCC patients and the intercellular
distribution of TERT protein expression. The TERT protein has been
reported to be mainly distributed in the nuclei of glioblastoma
cells, renal cell carcinoma cells, and lung cancer, oral cancer,
colorectal cancer, and thyroid cancer cells (13-18).
Conversely, two previous studies have shown that TERT protein is
mainly distributed in the cytoplasm of HCC tumor cells (12,19).
Since telomeres are present in the nucleus, the predominant
distribution of TERT in the cytoplasm of HCC tumor cells is
difficult to understand. Additionally, the means by which the
intracellular TERT distribution affects the clinical
characteristics and outcomes of HCC patients undergoing surgery
remains unknown.
In this study, we performed immunohistochemistry
using two types of antibodies for TERT and evaluated TERT
expression in resected HCC tumor tissues. We confirmed the
significant of cytoplasmic expression of TERT in HCC cells from
patients who had undergone liver resection. Additionally, we
investigated the relationship between the intracellular
distribution of TERT in HCC tumor tissues and the expression of
DNA-protein kinase catalytic subunit (DNA-PKcs), which our group
previously reported as a novel predictor in HCC patients (20), as well as expression of
8-hydroxyganosine (8-OHdG), a surrogate of oxidative stress and DNA
damage.
Materials and methods
Patients
This study was conducted with the approval of the
Ethical Review Board of the Dokkyo Medical University Hospital (ID
number: 28110), in compliance with the Ethical Guidelines for
Clinical Research published by the Ministry of Health, Labor and
Welfare, Japan. We provided the enrolled patients with the
opportunity to opt out on our website (www2.dokkyomed.ac.jp/dep-m/surg2/pg334.html).
From January 2012 to October 2019, 455 liver
resections were performed for HCC at the Second Department of
Surgery, Dokkyo Medical University Hospital. Among these, 135 HCC
patients with good-quality pathological samples were
retrospectively enrolled in this study. The 135 patients included
103 males and 32 females, with a mean age ± standard deviation of
68.6±9.0 years (range, 33-91 years). Thirty-seven patients were
positive for hepatitis B surface antigen (HBsAg), 37 were positive
for anti-hepatitis C virus antibody (HCVAb), 2 were positive for
both HBsAg and HCVAb, and 59 were negative for both.
Routine postoperative surveillance was usually
performed every 3 months for patients who had undergone surgery. To
detect HCC recurrences, the levels of tumor markers, including
α-fetoprotein (AFP) and protein induced by vitamin K antagonist II
(PIVKA-II), were measured every 3 months after surgery. In
addition, helical dynamic computed tomography was also performed
every 6 months or when the tumor marker levels exceeded the normal
range. Beginning at 3 years after the surgery, the HDCT interval
was extended from 6 to 12 months. HDCT was performed whenever an
elevation in the tumor marker levels was observed.
Primary antibody and
immunohistochemistry
The methods of immunohistochemistry have been
described previously (20). In
brief, resected liver specimens were fixed in 10% v/v formalin, cut
into blocks, and embedded in paraffin. The blocks were sliced into
4 µm-thick sections and stained with hematoxylin and eosin or used
for immunohistochemical analysis. The TERT antibodies used for the
analysis were a rabbit monoclonal antibody (ab32020; Abcam) and a
mouse monoclonal antibody (sc-393013; Santa Cruz Biotechnology,
Inc.). The antibody for DNA-PKcs used was a mouse monoclonal
antibody (#12311; Cell Signaling Technology, Inc). The 8-OHdG
antibody used for the analysis were a mouse monoclonal antibody
(ab48508; Abcam). The sections were subjected to dewaxing,
heat-induced epitope retrieval with citrate buffer, antibody
incubation (TERT: dilution 1:50, 15 min; DNA-PKcs: Dilution 1:30,
45 min; 8-OHdG: Dilution 1:100, 45 min) and counterstaining on a
BOND Max immunostainer using Bond Epitope Retrieval Solution 2
(pH-9.0, 20 min) for TERT and DNA-PKcs, Bond Epitope Retrieval
Solution 1 (pH-6.0, 10 min) for 8-OHdG and the Bond Polymer Refine
Detection kit (Menarini). The TERT expression was judged as
positive when staining of the cytoplasm or the nucleus was observed
in more than 30% of the tumor cells. The DNA-PKcs expression was
judged as positive when staining of the nuclei was observed at
tumor edge in more than 30% of the tumor cells. The 8-OHdG
expression was judged as positive when staining of the nuclei was
observed in more than 30% of the tumor cells.
Statistical analysis
The correlation between the intercellular
distribution of TERT expression and various clinical and
pathological characteristics were analyzed using the chi-square
test, Fisher's exact test, or Mann-Whitney U test, as appropriate.
The following clinical and pathological characteristics were
examined: Patient age (years), sex (male/female), HBsAg
(positive/negative), HCVAb (positive/negative), Child-Pugh class
(B/A), liver cirrhosis (yes/no), poor tumor differentiation
(yes/no), serum levels of AFP (ng/ml) and PIVKA-II (mAU/ml), size
of the largest tumor nodule (cm), tumor number (≥2/1), portal vein
invasion (yes/no), tumor-node-metastasis (TNM) stage in accordance
with the Union for International Cancer Control (UICC)
classification, 8th edition (21),
and DNA-PKcs expression (positive/negative). The Kaplan-Meier
method and the log-rank test were performed to investigate the
relationship between TERT expression and the postoperative outcomes
of HCC patients. All the statistical analyses were performed using
IBM SPSS statistics version 25.0 software for Windows (IBM Co.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
TERT expression in HCC
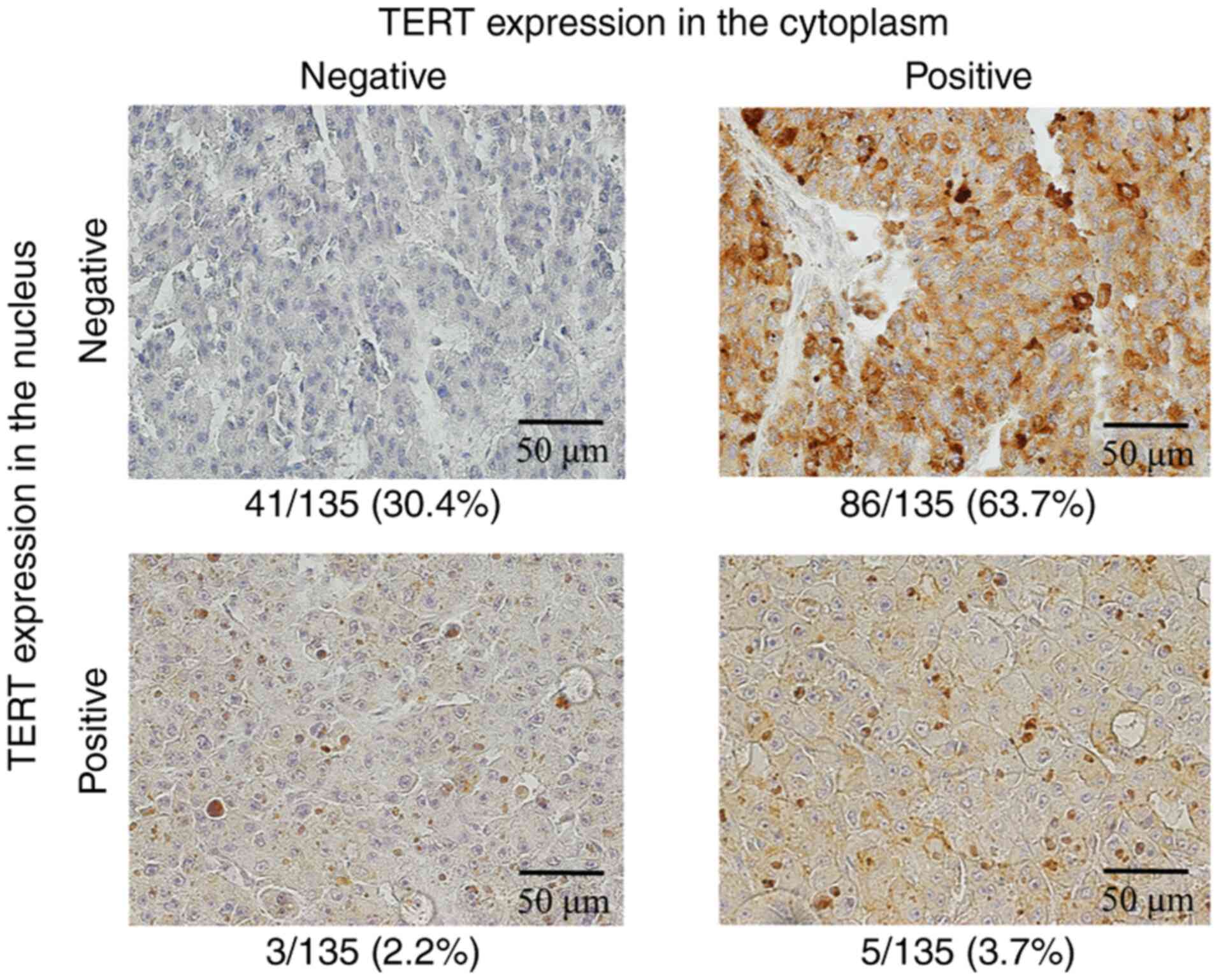
First, 135 HCCs were stained using a rabbit
monoclonal TERT antibody. TERT expression was positive only in the
cytoplasm in 86 tumors (63.7%), was positive only in the nucleus in
3 tumors (2.2%), was positive in both the cytoplasm and the nucleus
in 5 tumors (3.7%), and was negative in 41 tumors (30.4%),
respectively (Fig. 1). In total,
TERT expression in the HCC tissues was positive in the cytoplasm in
91 tumors (67.4%) and was positive in the nucleus in 8 tumors
(5.9%).
In the tumors with TERT expression in both the
cytoplasm and the nucleus, most tumor cells were positive in both
the cytoplasm and the nucleus, and the proportion of the tumor
cells which were positive only in the cytoplasm or in the nucleus
was low.
Results of repeated IHC using another
primary antibody for TERT
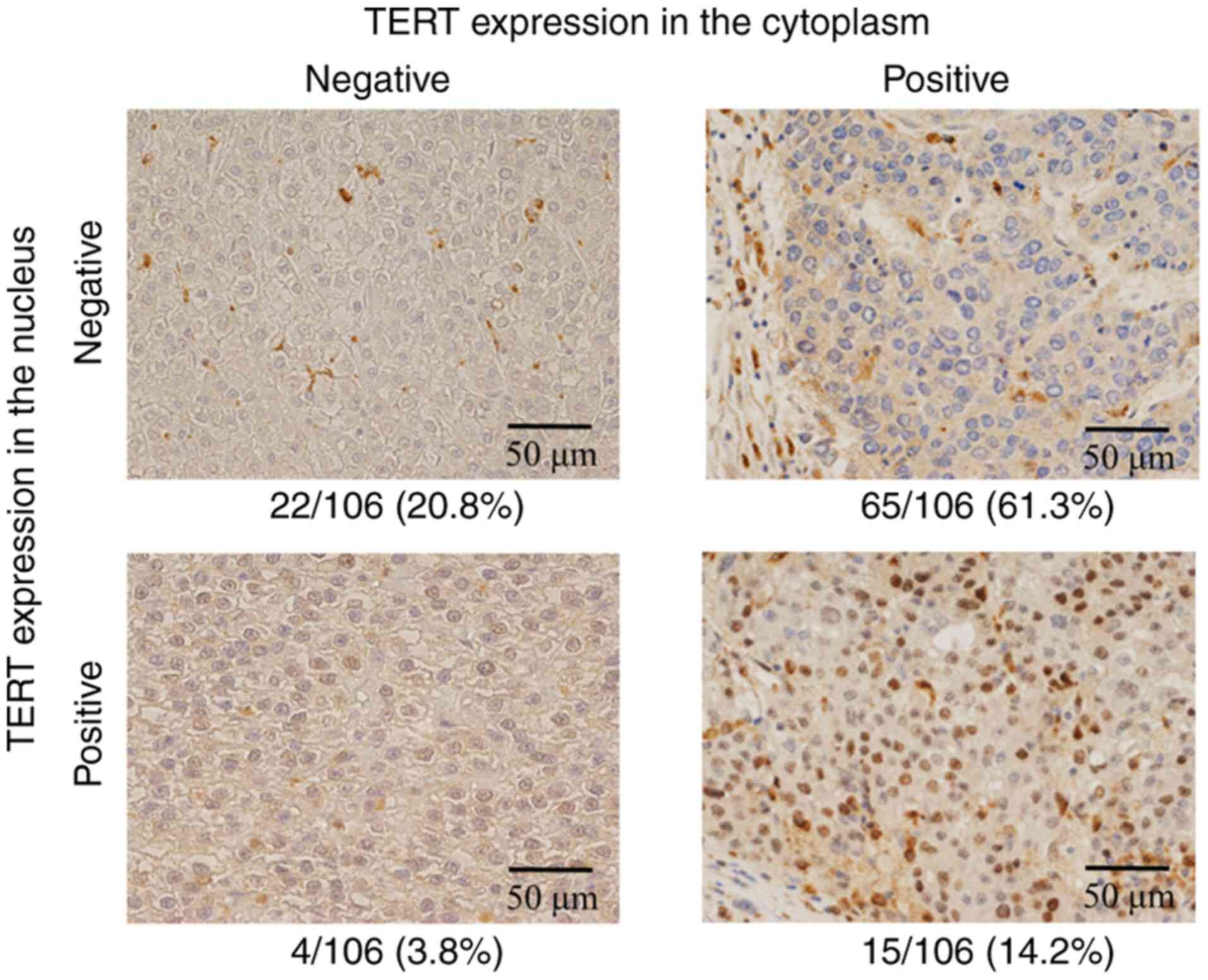
Because the intracellular distribution of TERT in
HCC tissues apparently differed from that found in other
malignancies, repeated immunohistochemistry using a mouse
monoclonal antibody was performed for 85 HCCs (Fig. 2). As shown in Table I, the results of the two
immunohistochemical series were consistent for 69 of the HCC tumors
(81.1%, 69/85, expression shown by gray-colored cells). The results
of the two types of TERT antibodies were shown to be significantly
consistent (P<0.001).
 | Table IResults of immunohistochemistry using
two types of primary antibodies for TERT. |
Table I
Results of immunohistochemistry using
two types of primary antibodies for TERT.
| | Mouse mAb |
|---|
| Rabbit mAb | Cytoplasm (-)/nucleus
(-), n | Cytoplasm (+)/nucleus
(-), n | Cytoplasm (-)/nucleus
(+), n | Cytoplasm (+)/nucleus
(+), n |
|---|
| Cytoplasm (-)/nucleus
(-) | 17 | 10 | 1 | 4 |
| Cytoplasm (+)/nucleus
(-) | 3 | 39 | 1 | 4 |
| Cytoplasm (-)/nucleus
(+) | 0 | 1 | 0 | 1 |
| Cytoplasm (+)/nucleus
(+) | 0 | 1 | 0 | 3 |
Correlation between TERT expression in
the cytoplasm and clinical and pathological characteristics
Table II shows the
clinical and pathological characteristics of the patients
stratified according to TERT expression in the cytoplasm of the HCC
tissues. The analyses revealed significant intergroup differences
in the HBsAg (positive/negative), poor tumor differentiation
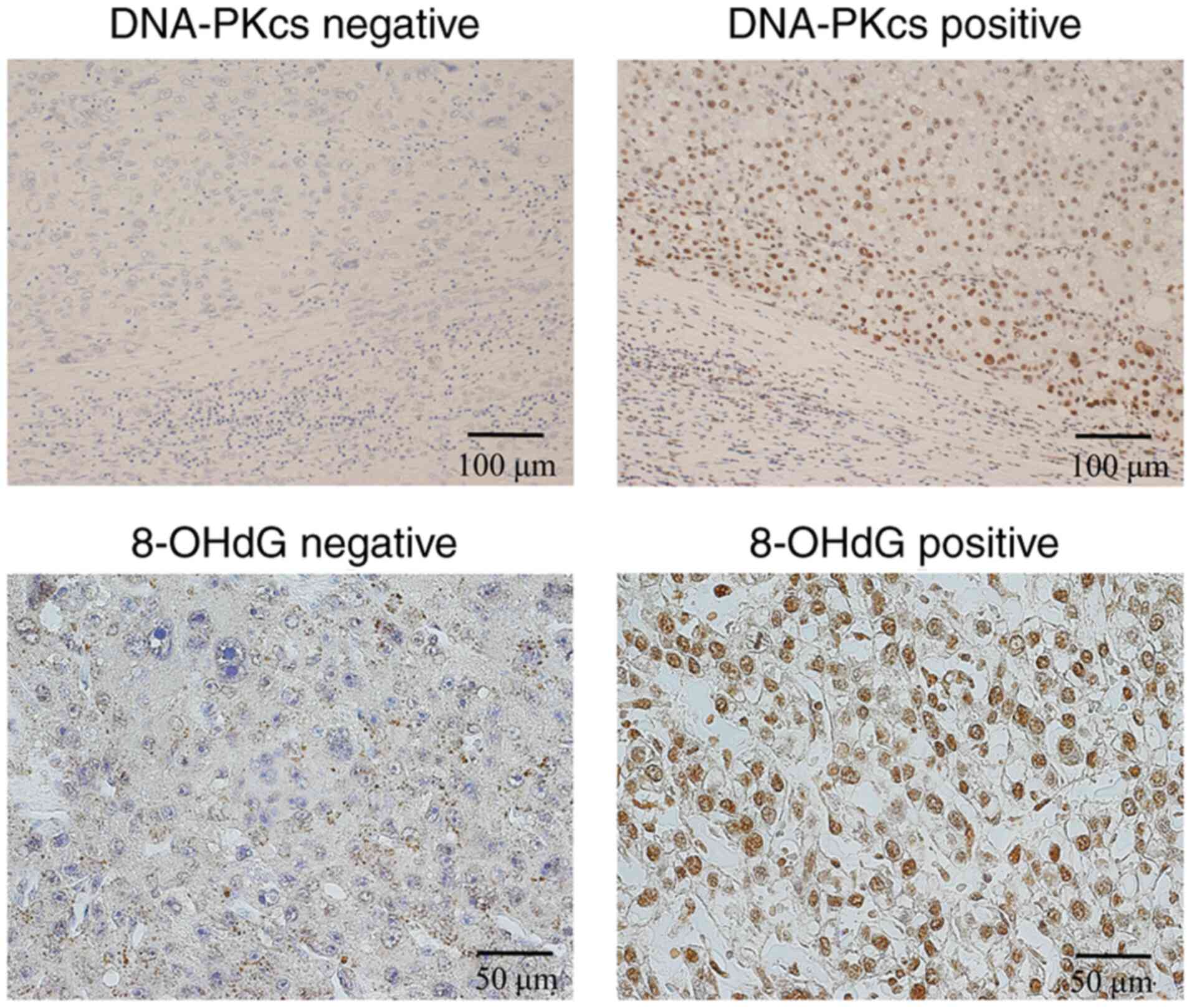
(yes/no), DNA-PKcs expression (positive/negative), and 8-OHdG
expression (positive/negative) (Fig.
3).
 | Table IIAssociation between clinical
characteristics and cytoplasmic TERT expression in patients with
hepatocellular carcinoma undergoing surgery. |
Table II
Association between clinical
characteristics and cytoplasmic TERT expression in patients with
hepatocellular carcinoma undergoing surgery.
| Variable | Cytoplasmic TERT
(-) (n=44) | Cytoplasmic TERT
(+) (n=91) | P-value |
|---|
| Agea, years | 71 (67-75) | 69 (63-75) | 0.279 |
| Sexb, n | | | 0.805 |
|
Male | 33 | 70 | |
|
Female | 11 | 21 | |
| HBs antigen
positiveb, n | | | 0.007 |
|
Yes | 6 | 33 | |
|
No | 38 | 58 | |
| HCV antibody
positiveb, n | | | 0.354 |
|
Yes | 15 | 24 | |
|
No | 29 | 67 | |
| Child-Pugh
classc, n | | | 0.576 |
|
A | 36 | 77 | |
|
B | 8 | 13 | |
|
Not
available | 0 | 1 | |
| Liver
cirrhosisc, n | | | 0.101 |
|
Yes | 12 | 38 | |
|
No | 31 | 51 | |
|
Not
available | 1 | 2 | |
| Poor tumor
differentiationc,
n | | | 0.043 |
|
Yes | 3 | 19 | |
|
No | 40 | 72 | |
|
Not
available | 1 | 0 | |
| AFPa, ng/ml | 4 (3-58) | 10 (4-92) | 0.849 |
|
PIVKA-IIa, mAU/ml | 38 (18-146) | 79 (27-587) | 0.239 |
| Size of largest
tumor nodulea, cm | 3.2 (2.2-5.3) | 3.0 (2.0-5.6) | 0.135 |
| Tumor
numberb, n | | | 0.973 |
|
≥2 | 11 | 23 | |
|
1 | 33 | 67 | |
| Portal vein
invasionb, n | | | 0.417 |
|
Yes | 13 | 21 | |
|
No | 31 | 70 | |
| TNM
stageb, n | | | 0.741 |
|
I | 21 | 42 | |
|
II | 13 | 23 | |
|
III | 10 | 26 | |
|
DNA-PKcsb, n | | | 0.042 |
|
Positive | 16 | 37 | |
|
Negative | 14 | 12 | |
|
Not
available | 14 | 42 | |
Correlation between tumor TERT
expression in the nucleus and clinical and pathological
characteristics
Table III shows
the clinical and pathological characteristics of the patients
stratified according to TERT expression in the nucleus of the HCC
tissues. Analyses revealed no significant intergroup differences in
the clinical and pathological characteristics. The clinical and
pathological characteristics of the patients without TERT
expression in the cytoplasm or in the nucleus were also
investigated, and the inter-group analysis showed significant
differences in liver cirrhosis (yes/no/NA) and DNA-PKcs expression
(positive/negative) (Table
SI).
 | Table IIIAssociation between clinical
characteristics and nucleus TERT expression in patients with
hepatocellular carcinoma undergoing surgery. |
Table III
Association between clinical
characteristics and nucleus TERT expression in patients with
hepatocellular carcinoma undergoing surgery.
| Variable | Nucleus TERT (-)
(n=127) | Nucleus TERT (+)
(n=8) | P-value |
|---|
| Agea, years | 70 (65-75) | 70 (66-72) | 0.733 |
| Sexb, n | | | 0.358 |
|
Male | 96 | 7 | |
|
Female | 31 | 1 | |
| HBs antigen
positiveb, n | | | 0.490 |
|
Yes | 37 | 2 | |
|
No | 90 | 6 | |
| HCV antibody
positiveb, n | | | 0.207 |
|
Yes | 38 | 1 | |
|
No | 89 | 7 | |
| Child-Pugh
classc, n | | | 0.573 |
|
A | 106 | 7 | |
|
B | 20 | 1 | |
|
Not
available | 1 | 0 | |
| Liver
cirrhosisc, n | | | 0.354 |
|
Yes | 46 | 4 | |
|
No | 79 | 3 | |
|
Not
available | 2 | 1 | |
| Poor tumor
differentiationc,
n | | | 0.453 |
|
Yes | 20 | 2 | |
|
No | 106 | 6 | |
|
Not
available | 1 | 0 | |
| AFPa, ng/ml | 7 (3-74) | 3 (2-18) | 0.103 |
|
PIVKA-IIa, mAU/ml | 71 (23-444) | 41 (22-80) | 0.187 |
| Size of largest
tumor nodulea,
cm | 3.1 (2.1-5.9) | 2.9 (1.9-4.2) | 0.363 |
| Tumor
numberb, n | | | 0.287 |
|
≥2 | 33 | 1 | |
|
1 | 94 | 7 | |
| Portal vein
invasionb, n | | | 0.595 |
|
Yes | 33 | 1 | |
|
No | 94 | 7 | |
| TNM
stageb, n | | | 0.549 |
|
I | 58 | 5 | |
|
II | 33 | 3 | |
|
III | 35 | 1 | |
|
DNA-PKcsb, n | | | 0.251 |
|
Positive | 50 | 3 | |
|
Negative | 25 | 1 | |
|
Not
available | 52 | 4 | |
Correlation between DNA-PKcs and
8-OHdG expressions
The examination of the association between DNA-PKcs
and 8-OHdG expressions showed no significant relationship between
the two factors (P=0.667).
Relationship between intracellular
distribution of TERT expression and postoperative outcomes
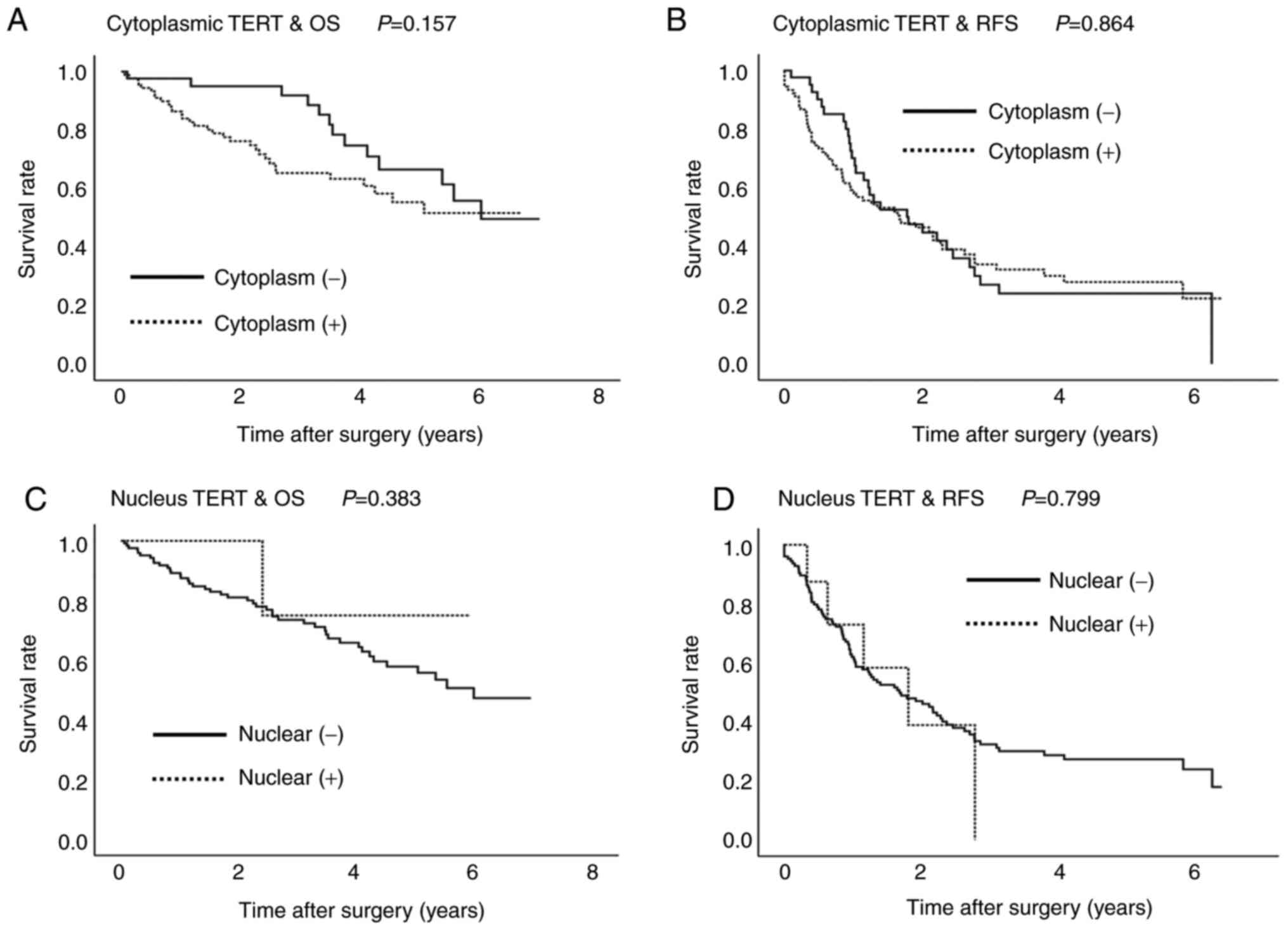
The median follow-up period was 1,089 days (range,
12-2,562 days). During the observation period, 54 patients died;
the cause of death was cancer recurrence in 35 patients; liver
failure in 9 patients; pneumonia in 4 patients; renal failure,
sepsis, ureteral cancer, gastrointestinal perforation in one
patient each; and unknown in 2 patients. TERT expression in the
cytoplasm or in the nucleus was not significantly associated with
the overall and recurrence-free survival periods (Fig. 4).
Discussion
The present results showed that TERT was mainly
expressed in the cytoplasm of HCC tumor tissues. The present
results were confirmed using another TERT antibody. These findings
suggest that TERT protein might be transferred from the nucleus to
the cytoplasm of HCC tumor cells, since TERT protein is originally
expressed in the nucleus (13-18).
Overall, 67.4% (91/135) of the HCC tumors had positive TERT
expression in the cytoplasm, and TERT expression in the nuclei was
negative in most of the cases. The previous reports have shown that
over 90% of the tumor cells had positive TERT expression in their
cytoplasm, and about half of the cells had positive TERT expression
in their nucleus (12,19). The differences in the positive
ratios between our results and those reported in the previous
articles may be ascribed to the difference in the patient cohort
and the difference in the positive decision criteria.
As mentioned above, the canonical function of TERT
is to maintain telomere length and genomic stability at telomeres.
On the other hand, previous reports have suggested that telomerase
show various non-canonical functions in the nucleus, cytoplasm, and
mitochondria. These functions include activation of intracellular
signaling pathways associated with inflammation, epithelial to
mesenchymal transition and tumor cell invasion and metastasis,
especially interaction with Wnt/β-catenin signaling pathway as well
as NF-κB signaling pathway (22-24).
In addition, mitochondrial TERT has various non-canonical functions
such as protection against reactive oxygen species and influence on
mitochondrial DNA damage and mitochondrial integrity (22,23).
Our present results may suggest that TERT exhibit its non-canonical
function in the cytoplasm, contributing to tumor development.
Interestingly, oxidative stress reportedly induces
TERT translocation from the nucleus to the cytoplasm, and
translocation from the nucleus to the mitochondria (22,23,25).
Previously, we reported that NRF2, a transcription factor that
responds to oxidative stress, frequently accumulates in the nucleus
in approximately 70% of HCC tumors (26). These observations support the
speculation that TERT is transferred from the nucleus to the
cytoplasm in HCC tumor cells as a result of oxidative stress. In
the present study, we investigated the nuclear expression of
8-OHdG, a surrogate of oxidative stress and DNA damage, in 85 HCC
specimens. The results clearly showed the positive relationship
between cytoplasmic TERT expression and nuclear 8-OHdG expression.
The present results also support the hypothesis that oxidative
stress is involved in the translocation of TERT. In detail,
H2O2-induced nuclear export of TERT is
reportedly triggered via Src kinase family-dependent
phosphorylation of tyrosine 707(25). We also examined the relationship
between the DNA-PKcs and 8-OHdG expressions, but the result was
insignificant. We have no clear explanation for the result; we
speculate that this negative result may be ascribed to the small
number of samples.
Our results showed that cytoplasmic TERT expression
was significantly associated with HBsAg. Previous studies have
revealed a relationship between HBV infection and TERT expression
(10,12,19).
TERT overexpression in HBV-associated HCC tumors has been ascribed
to TERT promoter mutations or HBV integration into the genetic
locus of TERT (10,12,19).
However, the relationship between HBV infection and cytoplasmic
TERT expression remains unclear. Further investigation is needed to
clarify the relationship between the HBV infection and altered
subcellular expression of TERT.
Our results showed that cytoplasmic TERT expression
was significantly associated with DNA-PKcs expression. DNA-PKcs is
reportedly a host protein of HBV-RNA and is significantly
associated with HBV infection and the postoperative outcome of HCC
patients undergoing surgery (20,27).
Because HBsAg and HBV-RNA are transcribed by covalently closed
circular DNA (cccDNA), TERT and DNA-PKcs expression in HBV-related
HCC might be influenced by the presence of cccDNA. However, the
current anti-viral drugs for HBV are unable to treat cccDNA;
consequently, the development of treatments for cccDNA is needed to
improve the outcomes of patients with HBV infection.
Although our results showed that cytoplasmic TERT
expression was significantly associated with poor tumor
differentiation, a previous study reported that higher cytoplasmic
TERT expression was associated with well-differentiated HCC tumors
(19). On the other hand, another
report showed that telomerase activation was significantly related
to the poor tumor differentiation of HCC tumors (28). In thyroid cancer, TERT promoter
mutation was frequently observed in poorly differentiated tumors
(29). The role of TERT is not only
the acquisition of tumor immortality, but also interactions with
several oncogenic pathways such as the Wnt/β-catenin signal pathway
and p53 suppression (30,31). Although the relationship between
cytoplasmic TERT expression and tumor differentiation remains
unclear, the present evidence suggests that cytoplasmic TERT
expression might affect the tumor dedifferentiation of
malignancies, including HCC.
Previous studies have reported that TERT promoter
mutation and TERT expression are associated with unfavorable
outcomes in HCC patients (10,12,19).
On the other hand, our results showed that TERT expression in the
cytoplasm or in the nucleus was not closely associated with the
postoperative outcomes of HCC patients. Our results suggested that
TERT expression is associated with neither tumor progression nor
metastasis. Because the TERT mutation already occurs in
premalignant liver tumor cells (9,12), the
role of TERT in HCC probably does not involve tumor progression,
but rather involves liver carcinogenesis and tumor
dedifferentiation. Recently, Ako et al (32) evaluated the human TERT promotor
mutations detected in the serum of the HCC patients using modified
droplet digital polymerase chain reaction and found that positive
TERT promotor mutation was significantly associated with shorter
recurrence-free survival period after surgery. Clinical impact of
TERT gene promotor mutation as well as overexpression of TERT
protein on tumor biology and patient outcomes is to be clarified by
various aspects of investigations.
A potential limitation of the present study was that
this was a retrospective study performed at a single institution
and with a small cohort of patients. Therefore, we could not
exclude the influence of bias provided by the retrospective design
of the study, and this may explain the inconsistencies with
previous reports.
In conclusion, unlike its expression in other
malignancies, TERT is mainly expressed in the cytoplasm of HCC
tumor tissues. Cytoplasmic TERT expression was significantly
associated with HBsAg, poor tumor differentiation, DNA-PKcs
expression, and 8-OHdG expression.
Supplementary Material
Relationships between clinical
characteristics and negative TERT expression in patients with
hepatocellular carcinoma undergoing surgery.
Acknowledgements
The authors would like to thank Professor Hajime
Kuroda (Department of Pathology, Dokkyo Medical University, Mibu,
Japan) for providing helpful comments and suggestions regarding the
pathological evaluations.
Funding
This research was supported by the Research Program on Hepatitis
from the Japan Agency for Medical Research and Development (AMED;
grant no. JP18fk0210014).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TA conceived the study. YN, TShim and TA searched
the published works. YN, TShim, TA, SS, TM, TShir, YS, SM, YI and
KK performed liver resections and were involved in acquisition of
data. YN, TShim, TA, and KK performed the data analyses and
interpreted the data. TShim, TM and MI performed the statistical
analyses. TA and TShim confirm the authenticity of all the raw
data. YN wrote the first draft of the report. TA and KK performed
critical review of the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted with the approval of
the Ethical Review Board of the Dokkyo Medical University Hospital
(ID number: 28110; Mibu, Tochigi, Japan), in compliance with the
Ethical Guidelines for Clinical Research published by the Ministry
of Health, Labor and Welfare, Japan. We provided the enrolled
patients with the opportunity to opt out on our website (www2.dokkyomed.ac.jp/dep-m/surg2/pg334.html).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Altekruse SF, Henley SJ, Cucinelli JE and
McGlynn KA: Changing hepatocellular carcinoma incidence and liver
cancer mortality rates in the United States. Am J Gastroenterol.
109:542–553. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kulik L and El-Serag HB: Epidemiology and
management of hepatocellular carcinoma. Gastroenterology.
156:477–491.e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Singal AG and El-Serag HB: Hepatocellular
carcinoma from epidemiology to prevention: Translating knowledge
into practice. Clin Gastroenterol Hepatol. 13:2140–2151.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Moyzis RK, Buckingham JM, Cram LS, Dani M,
Deaven LL, Jones MD, Meyne J, Ratliff RL and Wu JR: A highly
conserved repetitive DNA sequence, (TTAGGG)n, present at the
telomeres of human chromosomes. Proc Natl Acad Sci USA.
85:6622–6626. 1988.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Blasco MA: Telomeres and human disease:
Ageing, cancer and beyond. Nat Rev Genet. 6:611–622.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda
C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL,
Giovanella BC, et al: TERT promoter mutations occur frequently in
gliomas and a subset of tumors derived from cells with low rates of
self-renewal. Proc Natl Acad Sci USA. 110:6021–6026.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
In der Stroth L, Tharehalli U, Günes C and
Lechel A: Telomeres and telomerase in the development of liver
cancer. Cancers (Basel). 12(2048)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nault JC, Mallet M, Pilati C, Calderaro J,
Bioulac-Sage P, Laurent C, Laurent A, Cherqui D, Balabaud C and
Zucman-Rossi J: High frequency of telomerase reverse-transcriptase
promoter somatic mutations in hepatocellular carcinoma and
preneoplastic lesions. Nat Commun. 4(2218)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kawai-Kitahata F, Asahina Y, Tanaka S,
Kakinuma S, Murakawa M, Nitta S, Watanabe T, Otani S, Taniguchi M,
Goto F, et al: Comprehensive analyses of mutations and hepatitis B
virus integration in hepatocellular carcinoma with
clinicopathological features. J Gastroenterol. 51:473–486.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Donaires FS, Scatena NF, Alves-Paiva RM,
Podlevsky JD, Logeswaran D, Santana BA, Teixeira AC, Chen JJ,
Calado RT and Martinelli ALC: Telomere biology and telomerase
mutations in cirrhotic patients with hepatocellular carcinoma. PLoS
One. 12(e0183287)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang X, Guo X, Chen Y, Chen G, Ma Y, Huang
K, Zhang Y, Zhao Q, Winkler CA, An P and Lyu J: Telomerase reverse
transcriptase promoter mutations in hepatitis B virus-associated
hepatocellular carcinoma. Oncotarget. 7:27838–27847.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schjolberg AR, Clausen OP, Burum-Auensen E
and De Angelis PM: Aneuploidy is associated with TP53 expression
but not with BRCA1 or TERT expression in sporadic colorectal
cancer. Anticancer Res. 29:4381–4387. 2009.PubMed/NCBI
|
|
14
|
Palani J, Lakshminarayanan V and Kannan R:
Immunohistochemical detection of human telomerase reverse
transcriptase in oral cancer and pre-cancer. Indian J Dent Res.
22(362)2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qin Y, Chen W, Xiao Y, Yu W, Cai X, Dai M,
Xu T, Huang W, Guo W, Deng W and Wu T: RFPL3 and CBP
synergistically upregulate hTERT activity and promote Lung cancer
growth. Oncotarget. 6:27130–27145. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Insilla AC, Proietti A, Borrelli N,
Macerola E, Niccoli C, Vitti P, Miccoli P and Basolo F: TERT
promoter mutations and their correlation with BRAF and RAS
mutations in a consecutive cohort of 145 thyroid cancer cases.
Oncol Lett. 15:2763–2770. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Trifunovic J, Prvanovic M, Jovanovic A,
Dzamic Z, Lazic M, Ristanovic M, Radojevic-Skodric S and
Basta-Jovanovic G: Immunohistochemical expression of proliferative
markers in renal cell carcinoma. J BUON. 23:1103–1110.
2018.PubMed/NCBI
|
|
18
|
Potharaju M, Mathavan A, Mangaleswaran B,
Patil S, John R, Ghosh S, Kalavakonda C, Ghosh M and Verma RS:
Clinicopathological analysis of HIF-1alpha and TERT on survival
outcome in glioblastoma patients: A prospective, single institution
study. J Cancer. 10:2397–2406. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang W, Zhou W, Li C, Yang Y, Shang YK,
Chen C, Zhang J, Yao R, Wang P, Wen W, et al: Promoter mutations
and cellular distribution of telomerase in non-clear cell and clear
cell hepatocellular carcinoma. Oncotarget. 8:26288–26297.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shimizu T, Aoki T, Mori S, Iso Y, Kato M,
Ishizuka M and Kubota K: Tumor DNA-dependent protein kinase
catalytic subunit expression is associated with hepatitis B surface
antigen status and tumor progression in patients with
hepatocellular carcinoma. Sci Rep. 8(15019)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
James DB, Mary KG and Christian W (eds):
TNM Classification of Malignant Tumors. 8th edition. Wiley
Blackwell, Oxford, pp80-81, 2017.
|
|
22
|
Saretzki G: Extra-telomeric functions of
human telomerase: Cancer, mitochondria and oxidative stress. Curr
Pharm Des. 20:6386–6403. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thompson CAH and Wong JMY: Non-canonical
functions of telomerase reverse transcriptase: Emerging roles and
biological relevance. Curr Top Med Chem. 20:498–507.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ghareghomi S, Ahmadian S, Zarghami N and
Kahroba H: Fundamental insights into the interaction between
telomerase/TERT and intracellular signaling pathways. Biochemie.
181:12–24. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Haendeler J, Hoffmann J, Brandes RP,
Zeiher AM and Dimmeler S: Hydrogen peroxide triggers nuclear export
of telomerase reverse transcriptase via Src kinase family-dependent
phosphorylation of tyrosine 707. Mol Cell Biol. 23:4598–4610.
2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shimizu T, Inoue K, Hachiya H, Shibuya N,
Shimoda M and Kubota K: Frequent alteration of the protein
synthesis of enzymes for glucose metabolism in hepatocellular
carcinomas. J Gastroenterol. 49:1324–1332. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sekiba K, Otsuka M, Ohno M, Kishikawa T,
Yamagami M, Suzuki T, Ishibashi R, Seimiya T, Tanaka E and Koike K:
DHX9 regulates production of hepatitis B virus-derived circular RNA
and viral protein levels. Oncotarget. 9:20953–20964.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Piao YF, He M, Shi Y and Tang TY:
Relationship between microvessel density and telomerase activity in
hepatocellular carcinoma. World J Gastroenterol. 10:2147–2149.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Siraj AK, Bu R, Iqbal K, Parvathareddy SK,
Siraj N, Siraj S, Diaz MRF, Rala DR, Benito AD, Sabido MA, et al:
Telomerase reverse transcriptase promoter mutations in cancers
derived from multiple organ sites among middle eastern population.
Genomics. 112:1746–1753. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jin X, Beck S, Sohn YW, Kim JK, Kim SH,
Yin J, Pian X, Kim SC, Choi YJ and Kim H: Human telomerase
catalytic subunit (hTERT) suppresses p53-mediated anti-apoptotic
response via induction of basic fibroblast growth factor. Exp Mol
Med. 42:574–582. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y, Toh L, Lau P and Wang X: Human
telomerase reverse transcriptase (hTERT) is a novel target of the
Wnt/beta-catenin pathway in human cancer. J Biol Chem.
287:32494–32511. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ako S, Nouso K, Kinugasa H, Matsushita H,
Terasawa H, Adachi T, Wada N, Takeuchi Y, Mandai M, Onishi H, et
al: Human telomerase reverse transcriptzse gene promotor mutation
in serum of patients with hepatocellular carcinoma. Oncology.
98:311–317. 2020.PubMed/NCBI View Article : Google Scholar
|