Introduction
The risk factors correlated with recurrence of
primary thyroid carcinoma (PTC) are being >45 years, primary
tumor size >40 mm, widespread invasion, multifocality, positive
resection margin, lymph node and distant metastases at diagnosis
(1,2). The aggressive behavior of PTC has
been mainly revealed by the hematogenous occurrence of distant
metastases in ~20-25% of cases, with more frequent localizations to
the lungs and bones (1,2), while other unusual sites are
represented by skin and skull base (3-5).
In addition, the renal metastatic involvement from PTC represents a
very rare event, accounting for <5% of cases (6,7); in
fact, only 18 cases have been reported in the English literature,
although an additional 30 cases have been reported in the Japanese
literature (8-14).
The main PTC able to determine vascular invasion and metastasize to
the kidney is represented by differentiated follicular carcinoma,
followed by Hurthle cell as well as poorly differentiated
carcinomas, since papillary carcinoma usually presents a lymphatic
metastatic spread (2,8,10).
Unfortunately, distant metastases may appear as the initial symptom
of PTC with the thyroid function remaining unaltered (13-16);
hence, it is not surprising that metastases occurring in unusual
sites may be misdiagnosed as primary cancer until the post-surgical
pathological analysis has been carried out revealing the metastatic
nature from PTC. In light of these conditions, an interesting case
report is presented herein with a case of a large single renal
metastasis from thyroid follicular carcinoma with some poorly
differentiated associated foci. It is important to have a
fundamental knowledge of the characteristics of PTC, especially in
thus advanced stage, since these tumors often require
multidisciplinary management using multiple imaging and treatment
modalities.
Case report
A 69-year-old man was admitted to Department of
Human Pathology in Adulthood and Childhood ‘G. Barresi’, University
Hospital G. Martino, (Messina, Italy) in January 2021 suffering
from backache and spine discomfort and was consequently subjected
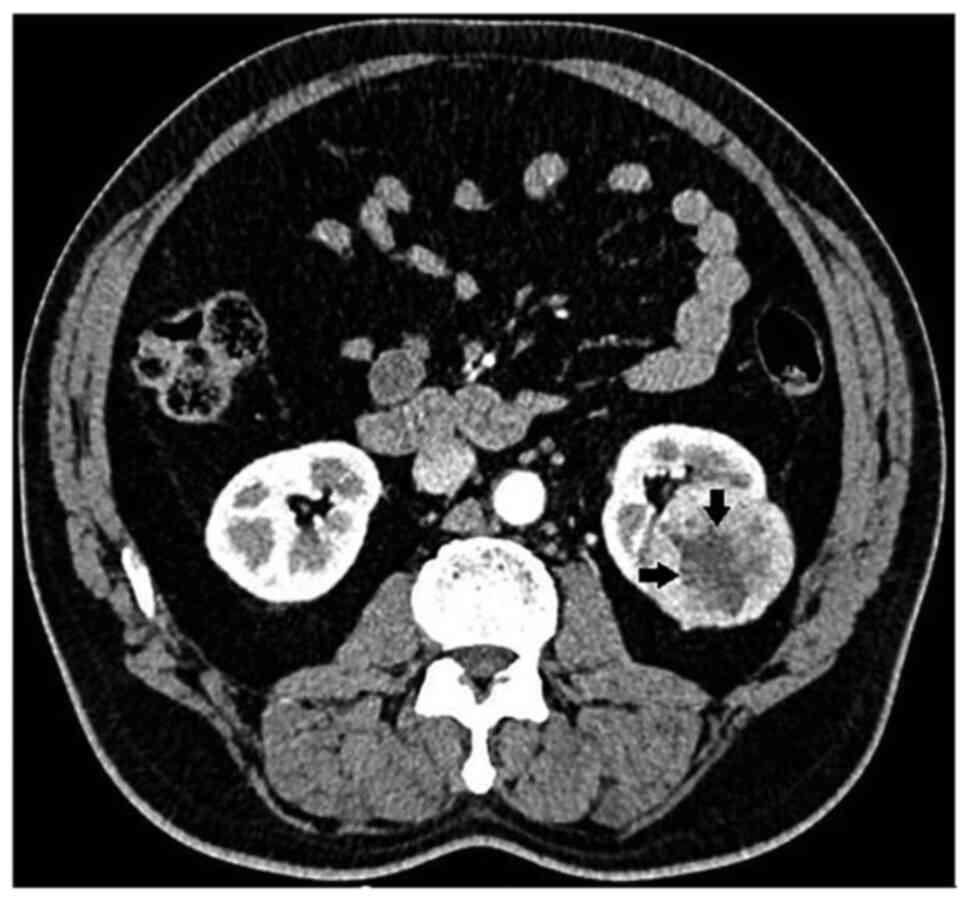
to abdominal computed tomography (CT). The CT examination
incidentally detected a parenchymal renal mass involving the
kidney, represented by a large 7.5x6.0 cm mass on the right kidney
(Fig. 1), which included some
minor not homogeneous areas referred to hemorrhage or necrosis. No
additional risk factors for renal pathology were found.
Preoperatively, the patient had a body mass index
(BMI) of 27, he was fully active and without relevant
co-morbidities (age-adjusted Charlson score=0). The maximum tumor
size was 6 cm and the PADUA nephrometry score was 10 (lesion with a
high risk of complications if partial nephrectomy is planned)
(17). Preoperative thoracic and
abdominal CT scan was negative for lymph node involvement and/or
distant metastases. Hence, the clinical stage of the patient was
determined to be cT1bN0M0. The contralateral kidney was normal.
Preoperative laboratory tests showed normal renal function; in
detail, renal function was tested using the Erythrocyte Glomerular
Filtration Rate (eGFR) starting from the creatinine level. However,
the international guidelines (EAU Guidelines) (18) do not consider it necessary to
perform a kidney scan in the preoperative evaluation of candidate
patients for partial nephrectomy. The American Society of
Anesthesiologists (ASA) score of the patient was category III
(18). According to the
international guidelines, an elective partial nephrectomy was
performed on the patient according to the guidelines on Renal Cell
Carcinoma 2021 edition (18).
In detail, a flank incision and a retroperitoneal
open approach was performed. After identification of the main renal
artery, the kidney was extensively mobilized until easy access to
the tumor from all sides was achieved. All the perirenal fat tissue
was removed except for that located on the top of the tumor. The
tumor was demarcated and then the main artery clamped (warm
ischemia). The renal capsule was incised very close to the tumor
and a plan between the healthy parenchyma and the tumor capsule was
developed performing a simple enucleation. The inner defect was
closed with a running Monocryl 4-0 suture (Johnson & Johnson
Medical N.V.) preloaded with a Absolok clip (Johnson & Johnson
Medical N.V.). The Monocryl was at the end brought outside through
the parenchyma and secured with a second Absolok clip. Through the
sliding clip technique, the right tension was brought on this
suture. Cortical renorrhaphy was performed using interrupted 2-0
(26 mm needle) polyfilament sutures placed at intervals of 1 cm
using the sliding-clip technique with Absolok® (Johnson
& Johnson Medical N.V.) clips (19). The clamp on the main artery was
removed after 14 min. The heamostatic agent Floseal®
(Baxter International) was applied on the cortical defect to
complete the hemostasis. No intraoperative complication was
observed. The estimated blood loss was 200 ml. Neither
intra-operative nor post-operative blood transfusion were needed.
No post-operative complications were recorded, and the patient was
discharged in 7 postoperative day (POD) with normal laboratory
tests.
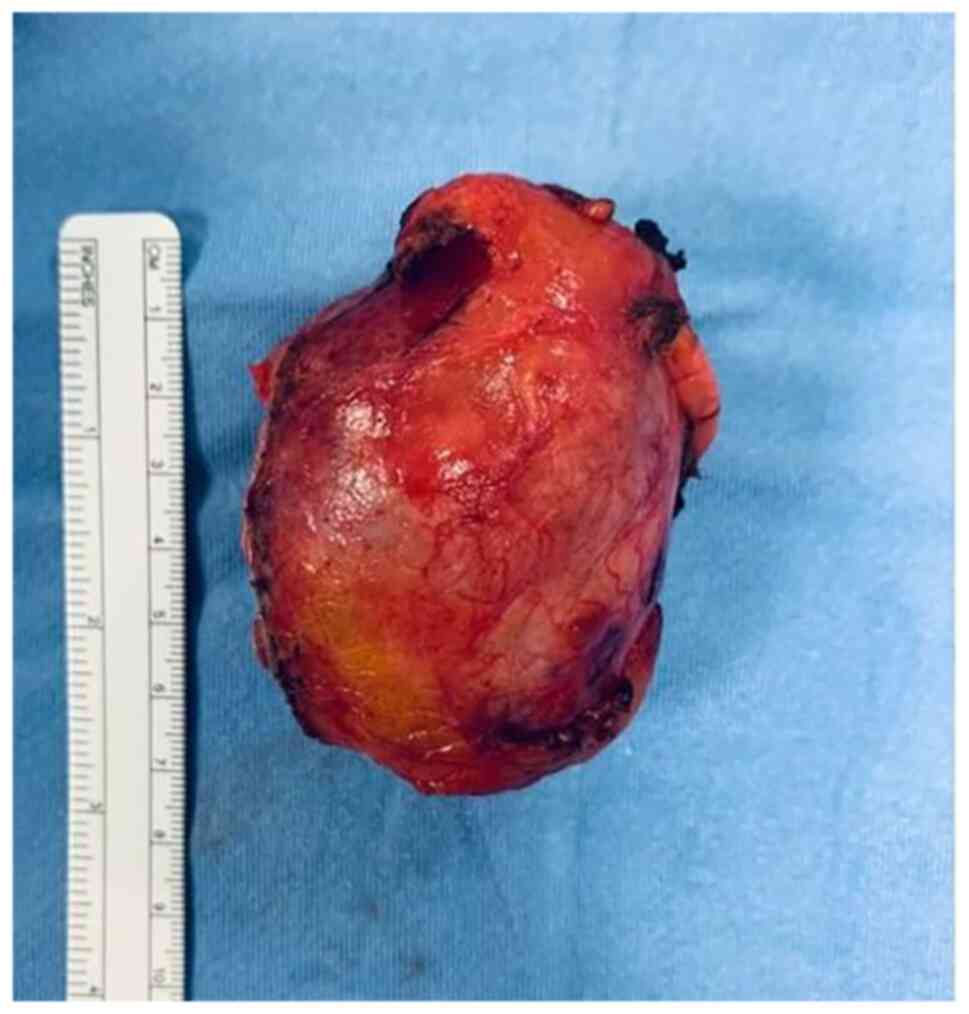
Grossly, the partial renal resection documented a
large nodular grayish mass of 70x60x35 mm diameter protruding to
the capsule (Fig. 2), but
presenting inside some regressive cystic black-reddish portions. No
invasion of perirenal adipose tissue, neither involvement of renal
vessels nor the adrenal gland were appreciable. A slight
centimetric portion of uninvolved renal tissue circumscribed the
neoplastic mass.
All samples of the renal lesion were fixed in 10%
neutral formalin for 24-36 h at room temperature, embedded in
paraffin at 56˚C, and then cut into 5-µm thick serial sections in
order to perform routine hematoxylin/eosin histological staining.
For the immunohistochemical procedure pparallel sections were cut
and mounted on silane-coated glass, then dewaxed in xylene and
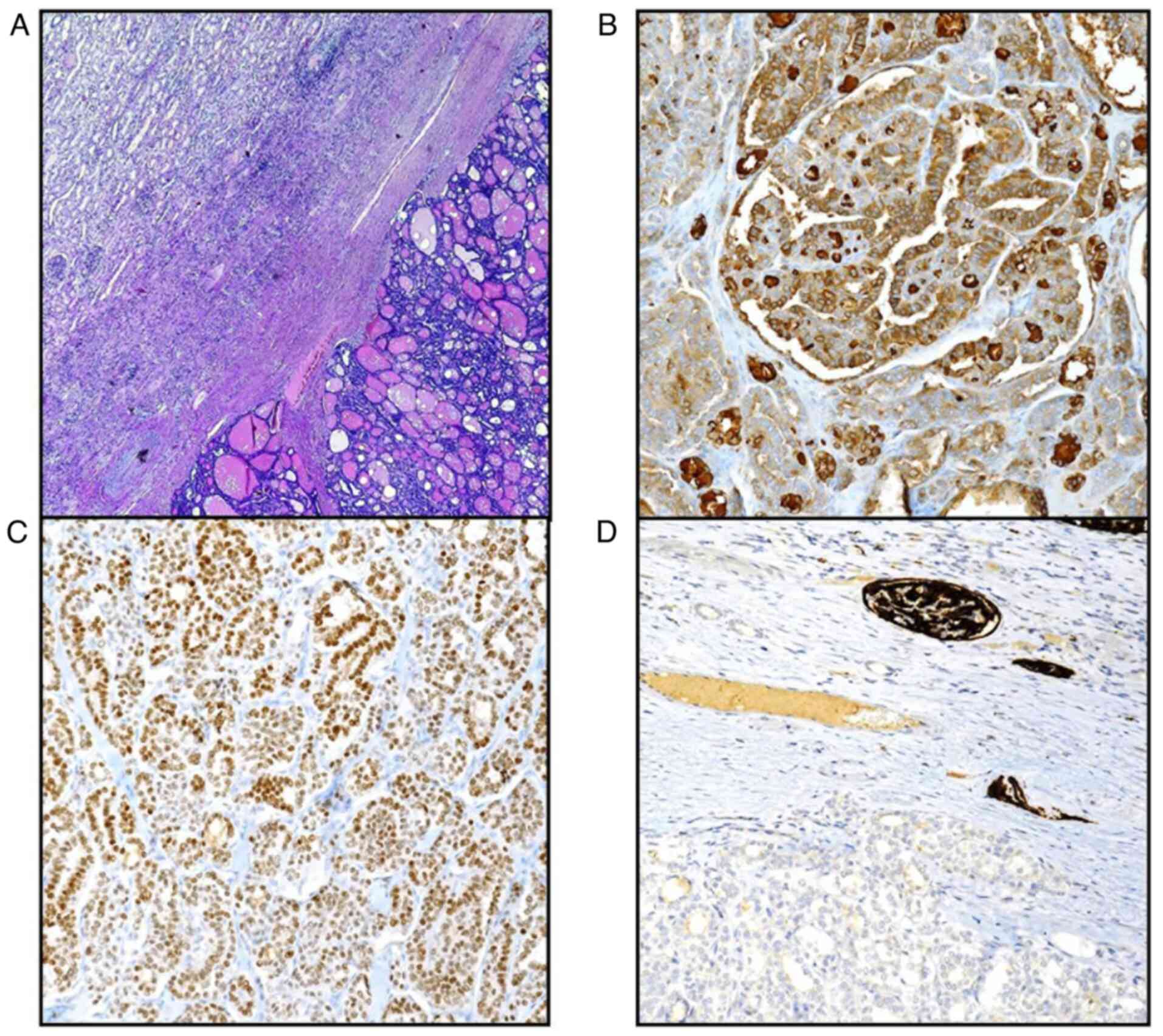
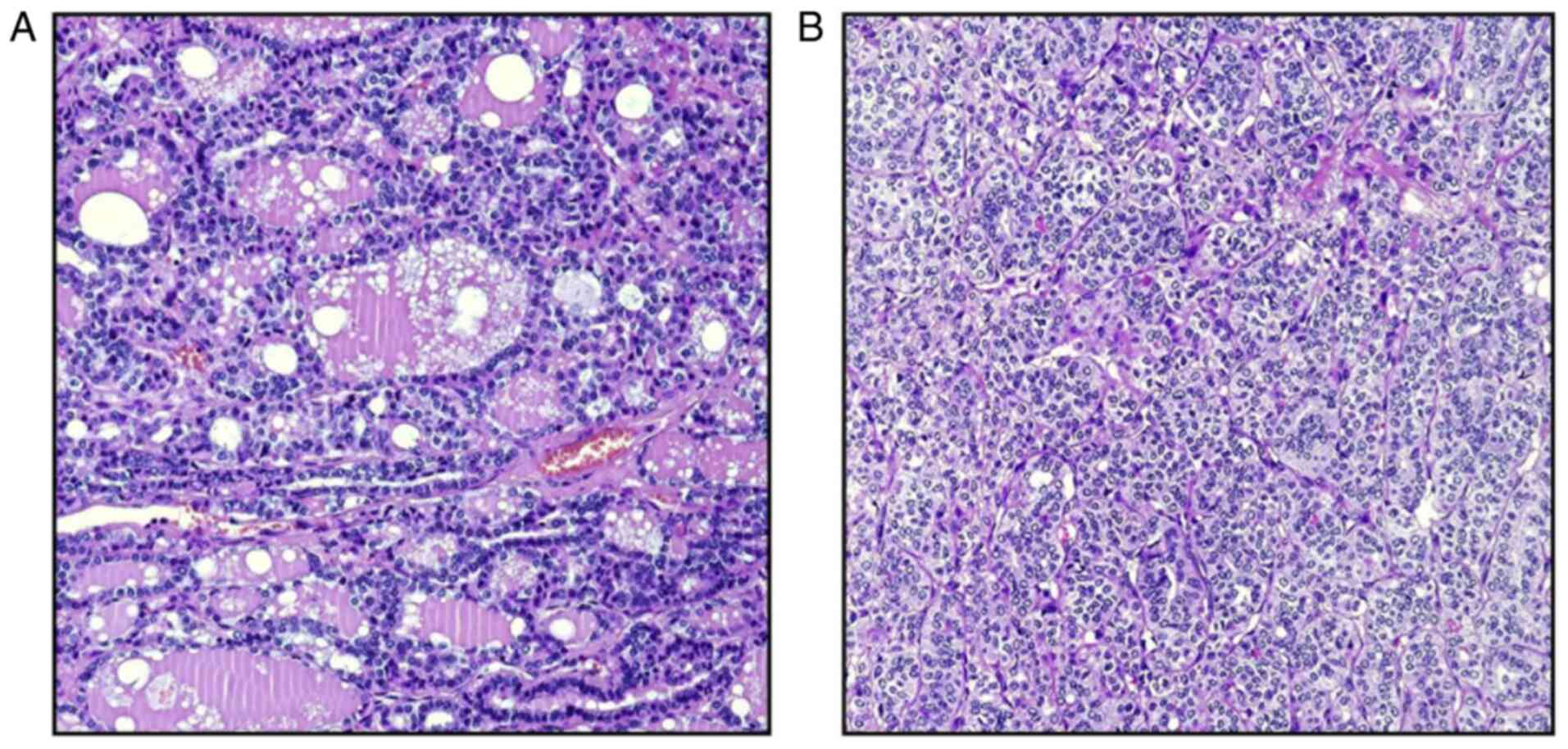
rehydrated in graded ethanol. Histologically, the neoplasia
exhibited a solid and or follicular pattern in which some
colloid-filled spaces were appreciable (Fig. 3A); the adjacent renal parenchyma
was lined by a fibrous capsule, but lymphovascular invasion was
noted without satellite neoplastic foci. Finally, surgical as well
as ureteral margins were all negative for the tumor. Taking into
account that the documented renal neoplasia could be attributable
to thyroid follicular-like renal carcinoma, as previously reported
(19), immunohistochemical
procedures were performed. Immunohistochemistry was performed using
the automated Ventana BenchMark ULTRA platform with Cell
Conditioning 1 for 64 min, pre-peroxidase inhibition and primary
antibody incubation for 16 min at 37˚C. The OptiView DAB IHC
Detection kit (Ventana Medical Systems, Inc.) was used to detect
protein expression of the following primary antibodies:
thyroglobulin (TG; 1:300; cat. no. 760-2671; Roche Diagnostics),
Thyroid transcription factor-1 (TTF-1; 1:300; cat. no 790-4398;
Roche Diagnostics), cytokeratin 7 (CK 7; 1:500; cat. no. 790-4462;
Roche Diagnostics), Paired-box gene 8 (PAX-8; 1:200; cat. no.
760-4618; Roche Diagnostics), CD10 (1:200; cat. no. 790-4506; Roche
Diagnostics), carbonic anhydrase IX (CAIX; 1:300; cat. no.
760-6080; Roche Diagnostics), α-methylacyl-CoA racemase (AMACR;
1:250; cat. no. 790-6011; Roche Diagnostics) and renal cell
carcinoma (RCC; 1:100; cat. no. 760-4273; Roche Diagnostics).
Finally, all slides were counterstained with Hematoxylin II
(Ventana Medical Systems, Inc.) and Bluing Reagent (Ventana Medical
Systems, Inc.) for 4 min at room temperature. In detail, positive
immunostaining was revealed for TG (Fig. 3B), TTF-1 (Fig. 3C), CK 7, PAX-8 while CD10 (Fig. 3D), CAIX, AMACR and RCC marker were
constantly negative.
Consequently, morphological and immunohistochemical
findings strongly supported the metastatic nature of the renal
tumor originating from a thyroid follicular carcinoma and hence,
the patient underwent an ultrasound examination. A large solitary
hyperechoic mass (5.8x4.2 cm), with some focal anechoic irregular
areas, extending from the left thyroid lobe to the isthmus was
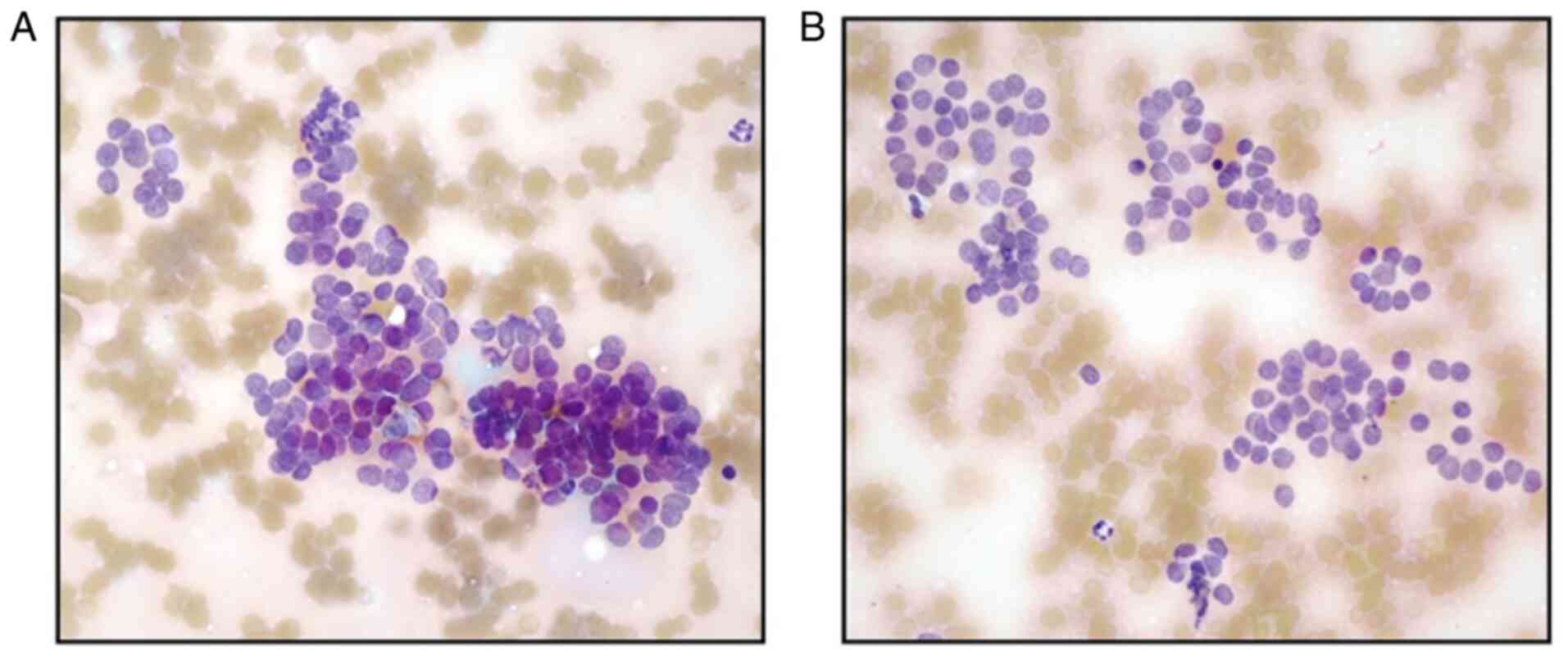
revealed. Fine needle aspiration cytology (FNAC) of the nodule,
stained with May-Grünwald-Giemsa stain, documented numerous sheets
and clusters, sometimes solid or microfollicular-patterned, with
oval or round nuclei presenting some degree of pleomorphism, but
without nuclear inclusions (Fig.
4A and B); a TIR4 according to
the Italian reporting system for thyroid cytology (20) was attributed to the lesion and the
patient was admitted to the Endocrine and Minimally Invasive
Surgery Section, University Hospital G. Martino, University of
Messina (Messina, Italy).
A total thyroidectomy was performed: the left lobe
weighed 53.3 g and it was fully substituted by a firm,
white-grayish nodule of 6x4x4 cm, joined to the isthmus of 1.0x0.7
cm with a solid sclero-calcific area; the right lobe weighed only
9.0 g and it presented a multinodular goiter appearance with
dimensions of 5.0x2.0x1.5 cm. In the perithyroidal adipose tissue,
only 2 reactive lymph nodes were isolated. The histopathological
examination of the surgical samples confirmed the neoplastic nature
of the nodule, mainly organized in microfollicular structures,
although an insular, solid and less differentiated pattern occurred
in ~30% of thyroid carcinoma (Fig.
5A and B); partial invasion of
fibrous capsule as well as of 4 invasive vessels neoplastic foci
were recorded. Immunohistochemistry confirmed the positivity for
thyroglobulin and TTF-1, with no staining for galectin-3, p53, CDX2
and BRAF V600E; the growth fraction, analyzed by Ki67 labeling
index was 15%. A final diagnosis of primitive differentiated
follicular thyroid carcinoma (pT3aN0 according to TNM/AJCC 2017)
(21), with poorly differentiated
foci (30%) infiltrating the capsule and angioinvasion was made.
On the last follow-up (July 2021) the patient was in
a good overall condition and computed tomography control revealed
no tumour recurrence in either the renal or thyroid sites.
Discussion
The prognosis of DTC is promising, with a 10-year
survival rate of 80-95%; however, this is reduced to 50% when
metastases are present (22).
Furthermore, age at diagnosis is a significant prognostic indicator
for the risk of recurrence and death, particularly in patients
>40 years of age (22). It is
well known that distant metastasis in DTC can be divided into 2
groups: i) Distant metastasis as the initial presenting diagnosis;
and ii) distant metastasis after the initial treatment of thyroid
cancer (23). The incidence of
distant metastasis after the initial treatment of DTC is between
7-23%, and the frequency of diagnosed DTC presenting initially with
metastatic disease ranges from 1-9% (23). In particular, distant metastasis in
FTC have been reported in 6-20% of cases, and the survival rates
range from 31-43% (22). In light
of these sporadic reports, the novelty of the current case is
greatly substantiated by the unspecific symptoms, focusing the
clinical attention on the lumbar region; in other words, the
present report may be defined as an ‘incidentaloma’ due to the
occurrence of renal metastatic deposit prior to the correct
diagnosis of a primitive DTC. Consequently, the morphological
definition of the secondary lesion guided further diagnostic
process towards the thyroid gland. However, due to a lack of cases
and randomized studies, there is no consensus for the management of
distant metastasis in patients with DTC, even if a complete
resection of DTC metastasis remains a good option, as has been
suggested elsewhere (24-26).
In contrast to high incidence of PTC, distant
metastases of this thyroid histotype are rarely seen and generally
presented as a single case report (14,15);
however, follicular carcinomas usually represent a neoplastic
aggressive entity able to metastasize more frequently compared with
papillary carcinoma by haematogeneous spread to distant sites, such
as lung, bone, but seldom skin and kidney (1,3-5,13,14).
Commonly, in unusual renal localization, thyroid metastases results
as multiple/or bilateral nodules, probably related to the presence
of venous vascular structures between the thyroid and kidney
(6,7). As a general rule, when thyroid tumors
metastasize to the kidney, lesions appear multifocal and bilateral
(12); nevertheless, it has been
reported previously in case reports as a solitary renal mass
(11,12). However, a renal solitary neoplastic
mass is rarely attributed to thyroid metastasis, mainly when a
general check of patients has not been performed. Although the
pathological preoperative diagnosis of renal tumors by fine needle
and core needle biopsy is not requested anymore, the presurgical
awareness of nature of the mass should be considered as a useful
approach in some selected cases. In addition, metastatic thyroid
tumor in the kidney can be difficult to diagnose on cytologic
smears since the nuclear features are easily suggestive for
diagnosis only when papillary histotype is the primary cancer.
A crucial point to define the nature of a single
renal mass, with a histological appearance of follicular carcinoma,
is to accurately differentiate the thyroid-like follicular renal
carcinoma (TLFC) which represents a peculiar pattern in the renal
tumor, firstly described 15 years ago (27); generally, the majority of TLFC
cases were low grade with of indolent course, even if renal hilar
lymph node and another widespread retroperitoneal lymph node or
lung metastases have been described (27). Histologically, the morphology of
TLFC is quite identical to the findings of the present case, with
widespread microfollicles and macrofollicles containing abundant
colloid-like material, bearing a striking resemblance to follicular
carcinoma of the thyroid gland. However, thyroidisation of the
kidney has already been reported in patients with chronic
pyelonephritis, as well as with end-stage renal disease; in this
latter entity, the renal tissue shows a thyroid-like feature
characterized by atrophic distal tubules and colloid-like hyaline
casts.
In this diagnostic challenge, a relevant important
role should be attributed to immunohistochemistry since while renal
cell follicular-like carcinomas are constantly immunostained for
CD10, RCC, vimentin, metastatic thyroid carcinoma to the kidney
exhibits a specific staining for TTF-1 and thyroglobulin, as found
in previous studies (11,20,28),
but also in the case in the present study.
Management of renal metastasis from thyroid
carcinoma, firstly includes surgical procedure consisting in
radical nephrectomy or laparoscopic partial nephrectomy (6,12,29);
these approaches should be combined with high dose radio-iodine
therapies (12). The rationale of
surgery to remove renal metastases is based on the need to perform
a neoplastic debulking, obtaining a reduction of the tumor burden
that have to be successively treated by radioactive iodine
treatment. In fact, after surgical treatment the radio metabolic
therapy may allow clinical remission and an improved 10-year
overall survival rate even in cases with additional future bone and
lung metastasis (6,30).
Hence, in conclusion, the therapeutic surgical
strategy used in the reported case represents a good and promising
approach. In fact, only the morphological analysis of metastatic
renal deposits addressed the correct investigative approach
concerning the primary neoplastic origin from the thyroid. After
the cytological diagnostic validation, total thyroidectomy with
regional lymph node dissection appears to be the most appropriate
treatment, followed by radioablative therapy to remove potential
residual disease or metastases. Finally, when neoplastic renal mass
of unidentified origin is revealed in patients with unspecific
symptoms, the presence of occult undiagnosed thyroid carcinoma
should be considered.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AI and GT designed the study and wrote the
manuscript. GA, AP, VF and GD performed the surgical procedures.
AI, GF and GT performed the morphology. AI, GF and GT wrote the
manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
D'Avanzo A, Ituarte P, Treseler P, Kebebew
E, Wu J, Wong M, Duh QY, Siperstein AE and Clark OH: Prognostic
scoring systems in patients with follicular thyroid cancer: A
comparison of different staging systems in predicting the patient
outcome. Thyroid. 14:453–458. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iwai H, Ohno Y, Ito H, Kiyokawa T and Aoki
N: Renal rupture associated with a poorly differentiated follicular
thyroid carcinoma metastasizing to the thigh muscle, lung and
kidney. Intern Med. 44:848–852. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Camacho V, Rodríguez-Revuelto A, Flotats
A, Duch J, Artigas C, Carrió I and Estorch M: Skin metastasis of
follicular thyroid carcinoma. Eur J Nucl Med Mol Imaging.
37(1237)2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Matsuno A, Katakami H, Okazaki R, Yamada
S, Sasaki M, Nakaguchi H, Yamada SM, Hoya K, Murakami M, Yamazaki
K, et al: Skull base metastasis from follicular thyroid
carcinoma-two case reports. Neurol Med Chir (Tokyo). 50:421–425.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Song HJ, Xue YL, Xu YH, Qiu ZL and Luo QY:
Rare metastases of differentiated thyroid carcinoma: Pictorial
review. Endocr Relat Cancer. 18:R165–R174. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cochetti G, Puxeddu E, Zingaro MD, D'Amico
F, Cottini E, Barillaro F and Mearini E: Laparoscopic partial
nephrectomy of thyroid cancer metastasis: Case report and review of
the literature. Onco Targets Ther. 6:355–360. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kumar A, Nadig M, Patra V, Srivastava DN,
Verma K and Bal CS: Adrenal and renal metastases from follicular
thyroid cancer. Br J Radiol. 78:1038–1041. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moudouni SM, En-Nia I, Rioux-Leclerq N,
Manunta A, Guille F and Lobel B: Follicular carcinoma of the
thyroid metastasis to the kidney nine years after resection of the
primary tumor. Ann Urol (Paris). 36:36–37. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liou MJ, Lin JD, Chung MH, Liau CT and
Hsueh C: Renal metastasis from papillary thyroid microcarcinoma.
Acta Otolaryngol. 125:438–442. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Djekidel M, Gordon M, Shah RB, Gross MD
and Avram A: Renal metastasis from Hurthle cell thyroid carcinoma
and its evaluation with hybrid imaging. Thyroid. 20:429–433.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu H, Zeng W and Tang Y: Metastatic
thyroid follicular carcinoma presenting as a primary renal tumor.
Intern Med. 51:2193–2196. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nath V, Baliga M, Lewin J, Souza F and
Akhtar I: Follicular thyroid carcinoma metastatic to the kidney:
Report of a case with cytohistologic correlation. Case Rep Pathol.
2015(701413)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cai DM, Wang HY, Jiang Y, Parajuly SS,
Tian YE, Ma BY, Li YZ, Song B and Luo Y: Primary follicular thyroid
carcinoma metastasis to the kidney and widespread dissemination: A
case report. Oncol Lett. 11:3293–3297. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou C, Urbauer DL, Fellman BM, Tamboli P,
Zhang M, Matin SF, Wood CG and Karam JA: Metastases to the kidney:
A comprehensive analysis of 151 patients from a tertiary referral
centre. BJU Int. 117:775–782. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gezer E, Selek A, Tarkun İ, Cantürk Z and
Çetinarslan B: Papillary thyroid carcinoma presenting as a primary
renal tumor with multiple pulmonary and bone metastases: A case
report. J Med Case Rep. 13(95)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sampson E, Brierley JD, Le LW, Rotstein L
and Tsang RW: Clinical management and outcome of papillary and
follicular (differentiated) thyroid cancer presenting with distant
metastasis at diagnosis. Cancer. 110:1451–1456. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ficarra V, Novara G, Secco S, Macchi V,
Porzionato A, De Caro R and Artibani W: Preoperative aspects and
dimensions used for an anatomical (PADUA) classification of renal
tumours in patients who are candidates for nephron-sparing surgery.
Eur Urol. 56:786–793. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bedke J, Albiges L, Capitanio U, Giles RH,
Hora M, Lam TB, Ljungberg B, Marconi L, Klatte T, Volpe A, et al:
The 2021 updated European association of urology guidelines on
renal cell carcinoma: Immune checkpoint inhibitor-based combination
therapies for treatment-naive metastatic clear-cell renal cell
carcinoma are standard of care. Eur Urol: May 29, 2021 (Epub ahead
of print).
|
|
19
|
Rossanese M, Crestani A, Giannarini G,
Calandriello M, Alario G, Simonato A and Ficarra V:
Absolok® versus Hem-o-Lok® clips for
renorrhaphy during partial nephrectomy for parenchymal renal
tumors. Minerva Urol Nephrol. 72:91–98. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Poller DN, Baloch ZW, Fadda G, Johnson SJ,
Bongiovanni M, Pontecorvi A and Cochand-Priollet B: Thyroid FNA:
New classifications and new interpretations. Cancer Cytopathol.
124:457–466. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC (eds), et al: American Joint Committee on Cancer Cancer
Staging Manual. 8th edition. Springer International Publishing,
Manhattan, New York, NY, 2017.
|
|
22
|
Grønlund MP, Jensen JS, Hahn CH, Grønhøj C
and von Buchwald C: Risk factors for recurrence of follicular
thyroid cancer: A systematic review. Thyroid: Jul 5, 2021 (Epub
ahead of print).
|
|
23
|
Veschi V, Verona F, Lo Iacono M, D'Accardo
C, Porcelli G, Turdo A, Gaggianesi M, Forte S, Giuffrida D, Memeo L
and Todaro M: Cancer stem cells in thyroid tumors: From the origin
to metastasis. Front Endocrinol (Lausanne). 11(566)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kunadharaju R, Goyal G, Rudraraju A and
Silberstein PT: New treatment options for metastatic thyroid
cancer. Fed Pract. 32 (Suppl 7):21S–26S. 2015.PubMed/NCBI
|
|
25
|
Pacini F, Basolo F, Bellantone R, Boni G,
Cannizzaro MA, De Palma M, Durante C, Elisei R, Fadda G, Frasoldati
A, et al: Italian consensus on diagnosis and treatment of
differentiated thyroid cancer: Joint statements of six Italian
societies. J Endocrinol Invest. 41:849–876. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Metere A, Aceti V and Giacomelli L: The
surgical management of locally advanced well-differentiated thyroid
carcinoma: Changes over the years according to the AJCC 8th edition
cancer staging manual. Thyroid Res. 12(10)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jung SJ, Chung JI, Park SH, Ayala AG and
Ro JY: Thyroid follicular carcinoma-like tumor of kidney: A case
report with morphologic, immunohistochemical, and genetic analysis.
Am J Surg Pathol. 30:411–415. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cimino-Mathews A, Sharma R and Netto GJ:
Diagnostic use of PAX8, CAIX, TTF-1, and TGB in metastatic renal
cell carcinoma of the thyroid. Am J Surg Pathol. 35:757–761.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pak H, Gourgiotis L, Chang WI, Guthrie LC,
Skarulis MC, Reynolds JC, Merino MJ, Schrump DS, Libutti SK,
Alexander HR Jr and Sarlis NJ: Role of metastasectomy in the
management of thyroid carcinoma: The NIH experience. J Surg Oncol.
82:10–18. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Durante C, Haddy N, Baudin E, Leboulleux
S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De
Vathaire F and Schlumberger M: Long-term outcome of 444 patients
with distant metastases from papillary and follicular thyroid
carcinoma: Benefits and limits of radioiodine therapy. J Clin
Endocrinol Metab. 91:2892–2899. 2006.PubMed/NCBI View Article : Google Scholar
|