Introduction
Although rare, pseudocirrhosis is an important
complication of metastatic cancer. The radiological term
pseudocirrhosis has been used to describe the development of
diffuse hepatic nodules in patients with cancer metastasis to the
liver (1). Pseudocirrhosis
presents with morphological changes similar to those of true liver
cirrhosis, including lobular hepatic contour, a retracted capsular
surface, segmental atrophy and an enlarged caudate lobe (2). Similar to cirrhosis, portal
hypertension that results in ascites and esophageal varices are
often encountered in patients with pseudocirrhosis (3-8).
Pseudocirrhosis occurs most frequently in patients with breast
cancer metastasizing to the liver (5-7,9-11),
but it is uncommon with other malignancies, although it has been
occasionally reported in association with thyroid (3), pancreatic (12), esophageal (13), small-cell lung (14), colon (15) and gastric cancer (16). We herein report the rare case of a
patient with metastatic gastric cancer who developed
pseudocirrhosis after achieving complete response to
chemotherapy.
Case report
A 72-year-old man was referred to the Gifu Municipal
Hospital (Kashimacho, Japan) in March 2019 with anorexia, feeling
of abdominal distension and general malaise. The patient was
subjected to upper gastrointestinal endoscopy and was diagnosed
with advanced gastric cancer type 2, a classification used in Japan
to grossly describe gastric cancer in which ulcer localization is
visible to the naked eye, in the greater curvature of the stomach
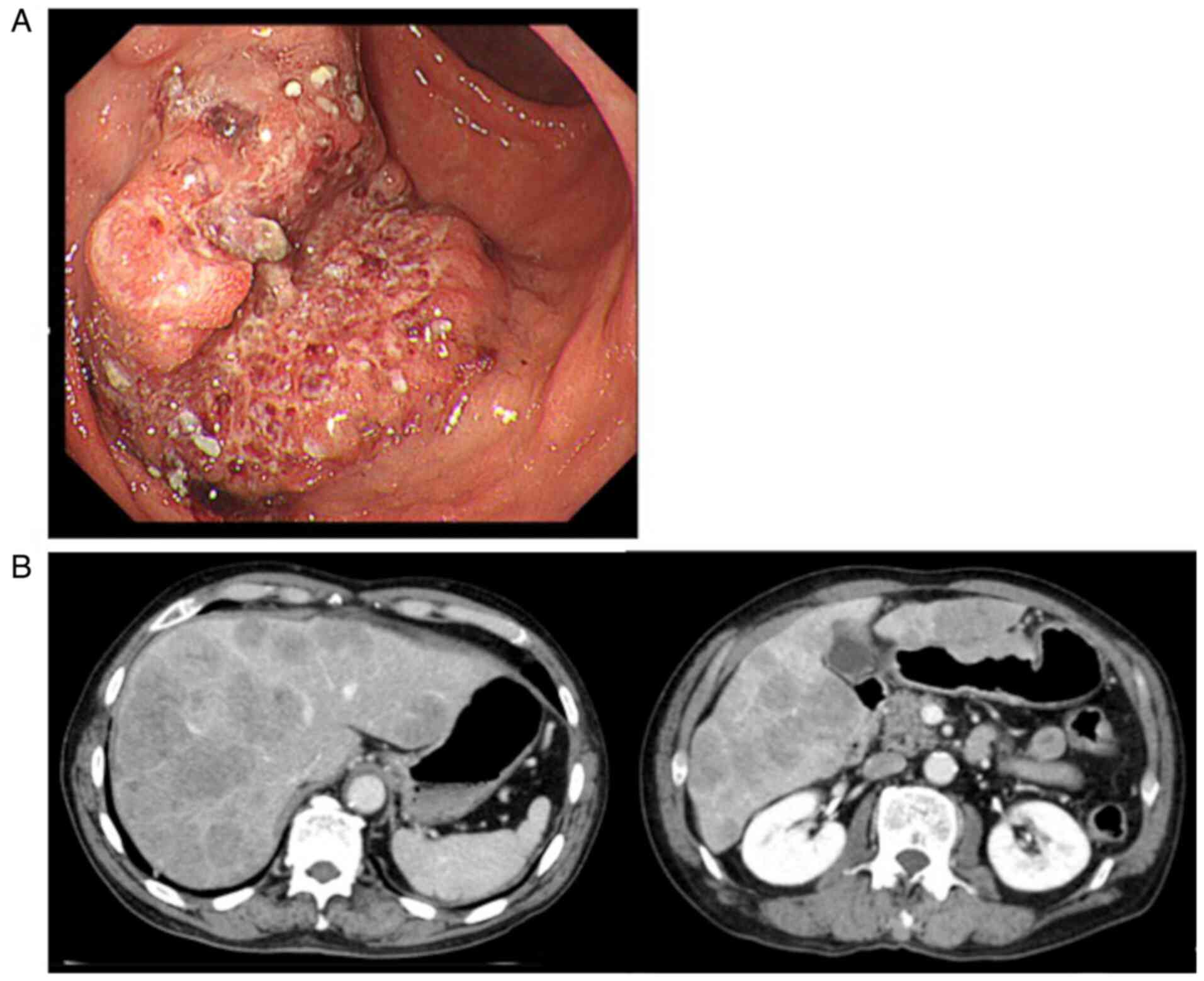
by (Fig. 1A). Histological
examination of the biopsy samples revealed well-differentiated
adenocarcinoma. Blood chemistry testing revealed the following
(Table I): Albumin 3.5 mg/dl
(normal range, 4.1-5.1 mg/dl), aspartate aminotransferase 263 IU/l
(normal range; 13-30 IU/l), alanine aminotransferase 115 IU/l
(normal range, 10-42 IU/l), lactate dehydrogenase 1769 U/l (normal
range, 124-222 U/l) and total bilirubin 1.8 mg/dl (normal range,
0.4-1.5 mg/dl). The carcinoembryonic antigen level was normal (3.7
ng/ml; normal range, 0-5 ng/ml), but that of carbohydrate antigen
19-9 (CA19-9) was highly elevated at 42.5 U/ml (normal range, 0-37
U/ml). Abdominal CT examination revealed the presence of multiple
liver metastases (Fig. 1B). The
patient's oral intake was good, and his Eastern Cooperative
Oncology Group performance status was 1. Therefore, he received
chemotherapy with S-1 (orally at 40 mg/m2 twice a day
for 2 weeks combined with 130 mg/m2 oxaliplatin
administered on day 1 every 3 weeks).
 | Table IChanges in the blood tests following
chemotherapy. |
Table I
Changes in the blood tests following
chemotherapy.
| Blood test | Prior to
treatment | After 1 treatment
cycle | After 4 treatment
cycles | 12 months after
initial treatment |
|---|
| Aspartate
aminotransferase (IU/l) | 263 | 57 | 54 | 37 |
| Alanine
aminotransferase (IU/l) | 115 | 30 | 39 | 20 |
| Lactate dehydrogenase
(IU/l) | 1,769 | - | 295 | 195 |
| Total bilirubin
(mg/dl) | 1.8 | 0.9 | 2.0 | 1.3 |
| Carcinoembryonic
antigen (ng/ml) | 3.7 | 4.4 | 7.2 | 5.6 |
| Carbohydrate antigen
19-9 (U/ml) | 42.4 | 12.4 | 11.0 | 11.1 |
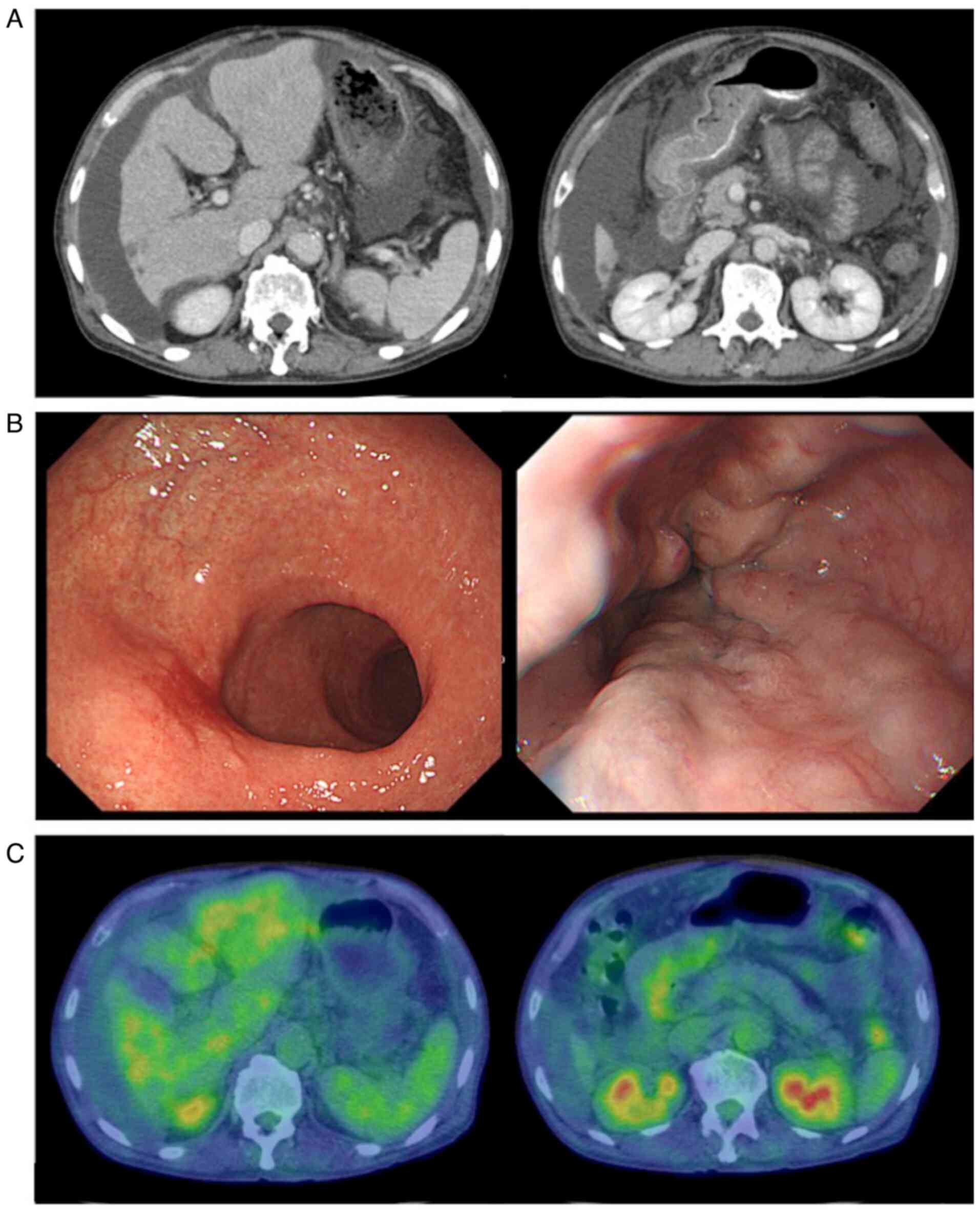
After 4 cycles of chemotherapy, the patient suddenly
developed abdominal distention. CT examination revealed a nodular
liver contour and liver volume loss, accompanied by marked
regression of the liver metastases. Massive ascites and pleural
effusion were also present (Fig.
2A). Radiologically, these findings mimicked those of liver
cirrhosis, but the liver enzyme levels were normal, and the CA19-9
level had decreased to 11.0 U/ml (Table I). The patient did not report
excessive alcohol intake, and the serological examinations
performed to investigate viral and autoimmune etiologies of
cirrhosis were negative. Cytology of the transudative ascites
revealed no malignant cells. Repeat upper gastrointestinal
endoscopy revealed esophageal varices (red color sign) and a red
scar at the site of the primary tumor (Fig. 2B); furthermore, the sample obtained
by biopsy of the lesion was free of tumor cells. Positron emission
tomography-CT revealed no abnormal fluorodeoxyglucose accumulation
in the stomach or liver (Fig. 2C).
Thus, the patient was diagnosed with pseudocirrhosis. Treatment
with abdominal paracentesis and diuretics (furosemide and
spironolactone) was initiated for his worsening abdominal
distension and peripheral edema. The patient also underwent two
sessions of endoscopic ligation of his esophageal varices with
curative intent. The patient was discharged after his ascites was
reduced to a manageable level, and chemotherapy was reinitiated
with S-1 alone at 40 mg/m2 orally twice daily for 4
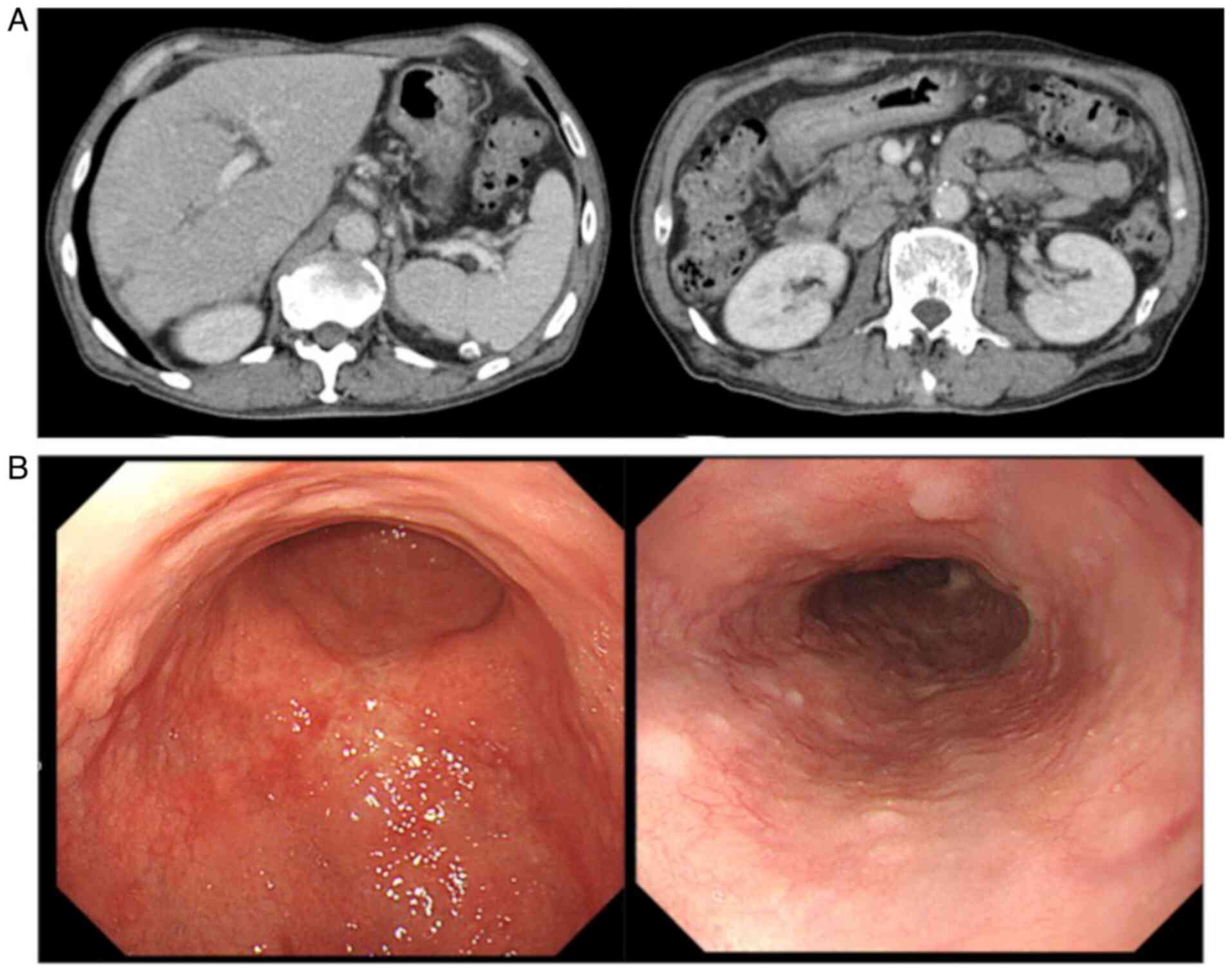
weeks. CT examination at 6 months after the initial treatment
revealed total remission of the liver metastases and disappearance
of the ascites (Fig. 3A).
Therefore, the response to treatment was deemed as complete. At 12
months after the initial treatment, CT examination revealed no
evidence of recurrence or metastasis. Another upper
gastrointestinal endoscopic examination revealed the presence of a
scar at the primary tumor site (Fig.
3B), and no tumor cells were detected following biopsy of the
lesion. The patient was maintained on S-1 monotherapy, and the
complete response was confirmed in April 2020 (12 months after the
initial chemotherapy).
Discussion
Pseudocirrhosis is a term used to describe a
complication of cancer with multiple liver metastases, and its
radiological appearance is similar to that of cirrhosis (17). However, there is no examination
that can definitively distinguish pseudocirrhosis from cirrhosis,
and the typical histopathological findings of cirrhosis are
lacking. The precise mechanism underlying the development of
pseudocirrhosis remains unclear. However, it is currently
attributed to two etiologies, either a process related to hepatic
metastases, or toxicity resulting from systemic therapy (5,6,11).
In the former, chemotherapy can induce hepatic retraction with a
lobular contour from either an increase or decrease in the size of
the subjacent tumor (2,9). In the latter, pseudocirrhosis can
occur with or without prior systemic chemotherapy. Hepatic
histology in this case may exhibit extensive tumor infiltration and
desmoplastic fibrosis (5,6,11).
The only known case to date of pseudocirrhosis
arising from metastatic gastric cancer was reported by Mitani et
al (16). The majority of
other reports on pseudocirrhosis are associated with hepatic
metastasis of breast cancer (2,4-11,18).
Furthermore, although there have been some case reports of
pseudocirrhosis in various primary cancers with liver metastases
(3,12-17,19),
these reports suggested no correlation with the specific type of
cancer.
Nodular regenerative hyperplasia, which presents as
a widespread transformation of normal hepatic parenchyma into
regenerative nodules with little or no bridging fibrosis, may also
be associated with the development of pseudocirrhosis (2). There are some reports that
pseudocirrhosis occurs when using oxaliplatin for gastric, colon,
or pancreatic cancer (12,15,16),
and oxaliplatin is well known to cause nodular regenerative
hyperplasia. However, the chemotherapeutic agents that can worsen
pseudocirrhosis remain unclear, and no chemotherapeutic agent has
yet been identified as the sole culprit (4,19).
Adike et al (18) reported abdominal distention with
ascites as the most common initial presentation of pseudocirrhosis.
In addition, certain severe complications, such as hepatic
encephalopathy and variceal bleeding, may occasionally result in a
fatal outcome (3-8),
which indicates the clinical significance of pseudocirrhosis, as
well as classic cirrhosis, and the importance of early detection
and appropriate management (16).
In conclusion, pseudocirrhosis may occur during the
achievement of a chemotherapeutic response in metastatic gastric or
breast cancer. Thus, clinicians must be aware of this entity and
recognize the onset of pseudocirrhosis in order to administer
appropriate treatment in a timely manner, even when the patients
are receiving chemotherapy for gastric cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TSh and TT analyzed and interpreted the data, wrote
the manuscript and confirm the authenticity of the raw data. TSh,
TT, TSa, MO, SF, SK, KM, KY, YS, SO and MY evaluated the patient
and participated in his therapy. All the authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of the case details and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma A, Houshyar R, Bhosale P, Choi JI,
Gulati R and Lall C: Chemotherapy induced liver abnormalities: An
imaging perspective. Clin Mol Hepatol. 20:317–326. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Young ST, Paulson EK, Washington K,
Gulliver DJ, Vredenburgh JJ and Baker ME: CT of the liver in
patients with metastatic breast carcinoma treated by chemotherapy:
Findings simulating cirrhosis. AJR Am J Roentgenol. 163:1385–1388.
1994.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Harry BL, Smith ML, Burton JR Jr, Dasari
A, Eckhardt SG and Diamond JR: Medullary thyroid cancer and
pseudocirrhosis: Case report and literature review. Curr Oncol.
19:e36–e41. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Qayyum A, Lee GK, Yeh BM, Allen JN, Venook
AP and Coakley FV: Frequency of hepatic contour abnormalities and
signs of portal hypertension at CT in patients receiving
chemotherapy for breast cancer metastatic to the liver. Clin
Imaging. 31:6–10. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sass DA, Clark K, Grzybicki D, Rabinovitz
M and Shaw-Stiffel TA: Diffuse desmoplastic metastatic breast
cancer simulating cirrhosis with severe portal hypertension: A case
of ‘pseudocirrhosis’. Dig Dis Sci. 52:749–752. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nascimento AB, Mitchell DG, Rubin R and
Weaver E: Diffuse desmoplastic breast carcinoma metastases to the
liver simulating cirrhosis at MR imaging: Report of two cases.
Radiology. 221:117–121. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chandrakar V and Isaacs C: Breast
cancer-related pseudocirrhosis and esophageal varices. Breast J.
11:301–302. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jeong WK, Choi SY and Kim J:
Pseudocirrhosis as a complication after chemotherapy for hepatic
metastasis from breast cancer. Clin Mol Hepatol. 19:190–194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fennessy FM, Mortele KJ, Kluckert T,
Gogate A, Ondategui-Parra S, Ros P and Silverman SG: Hepatic
capsular retraction in metastatic carcinoma of the breast occurring
with increase or decrease in size of subjacent metastasis. AJR Am J
Roentgenol. 182:651–655. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schreiner SA, Gorman B and Stephens DH:
Chemotherapy-related hepatotoxicity causing imaging findings
resembling cirrhosis. Mayo Clin Proc. 73:780–783. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Lee SL, Chang ED, Na SJ, Kim JS, An HJ, Ko
YH and Won HS: Pseudocirrhosis of breast cancer metastases to the
liver treated by chemotherapy. Cancer Res Treat. 46:98–103.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kang SP, Taddei T, McLennan B and Lacy J:
Pseudocirrhosis in a pancreatic cancer patient with liver
metastases: A case report of complete resolution of pseudocirrhosis
with an early recognition and management. World J Gastroenterol.
14:1622–1624. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kobashigawa C, Nakamoto M, Hokama A,
Hirata T, Kinjo F and Fujita J: Pseudocirrhosis in metastatic
esophageal cancer. South Med J. 103:488–489. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ojeda VJ: Metastatic oat cell carcinoma
simulating liver cirrhosis. N Z Med J. 86:480–481. 1977.PubMed/NCBI
|
|
15
|
Battisti S, Guida FM, Pagliara E, Tonini
G, Zobel BB and Santini D: Pseudocirrhosis after anti-EGFR-based
neoadjuvant therapy for hepatic metastasis from colon cancer: A
different point of view. Clin Colorectal Cancer. 13:e13–e15.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mitani S, Kadowaki S, Taniguchi H, Muto H
and Muro K: Pseudocirrhosis in gastric cancer with diffuse liver
metastases after a dramatic response to chemotherapy. Case Rep
Oncol. 9:106–111. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kumamoto K, Endo S, Isohata N, Nirei A,
Nemoto D, Utano K, Saito T and Togashi K: Pseudocirrhosis caused by
regorafenib in an advanced rectal cancer patient with multiple
liver metastases. Mol Clin Oncol. 6:63–66. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Adike A, Karlin N, Menias C and Carey EJ:
Pseudocirrhosis: A case series and literature review. Case Rep
Gastroenterol. 10:381–391. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ngo D, Jia JB, Green CS, Gulati AT and
Lall C: Cancer therapy related complications in the liver,
pancreas, and biliary system: An imaging perspective. Insights
Imaging. 6:665–677. 2015.PubMed/NCBI View Article : Google Scholar
|