Introduction
The recurrence rate after curative colorectal cancer
(CRC) resection ranges between 20 and 40% (1). Recurrence may develop from
microscopic residual disease or successfully implanted circulating
tumor cells released during surgery. Strong experimental (and
weaker clinical) evidence suggests that tumor growth is stimulated
during the early postoperative period (2-6).
As adjuvant therapy is not initiated for 4-6 weeks after surgery,
the postoperative period may be hazardous for patients with cancer
who harbor residual tumors. Due to these concerns, efforts have
been made to identify anticancer treatments that can be
administered perioperatively (7-9).
Several mechanisms have been proposed to explain the enhanced tumor
growth noted after surgery, including cell-mediated
immunosuppression, decreased blood levels of tumor
growth-suppressing proteins, and removal of primary
tumor-associated metastasis-suppressing factors (10-12).
Moreover, the plasma levels of at least 10 proteins
[VEGF, angiopoietin-2 (Ang-2), placental growth factor (PIGF),
soluble vascular adhesion molecule-1 (sVCAM-1), IL-8, monocyte
chemoattractant protein-1 (MCP-1), chitinase 3-like protein-1
(CHI3L1), MMP-3 and chemokine [C-X-C motif] ligand 16 (CXCL-16)]
with proangiogenic effects have been shown to be significantly
elevated over the preoperative baseline concentrations for 3-5
weeks after minimally invasive colorectal resection (MICR)
(3-5,13-17).
Postoperative plasma collected on the second and third weeks after
MICR has been shown to significantly increase endothelial cell (EC)
proliferation, invasion and migration (key components of
angiogenesis) in vitro, compared with the results obtained
with preoperative plasma (3-5,13-19).
These findings have raised concerns that the growth of residual
cancer deposits may be facilitated during the first month after
curative cancer resection via accelerated tumor angiogenesis due to
these systemic plasma protein alterations. These results have also
led to further efforts to characterize the effects of surgery on
the plasma levels of other proteins that may affect angiogenesis
and tumor growth (6,20-21).
The present study focused on one of these proteins, plasminogen
activator inhibitor-1 (PAI-1).
PAI-1 is a serine protease inhibitor (serpin) that
inhibits tissue plasminogen activator (tPA) and urokinase
plasminogen activator (uPA), thereby preventing the conversion of
plasminogen to plasmin and subsequent fibrinolysis (22). Plasmin is an antithrombotic agent
that plays a critical role in maintaining blood vessel patency and
in the proliferative phase of wound healing. Plasmin breaks down
the fibrin clots initially laid down in wounds, and facilitates
macrophage and fibroblast entry into the wounds and the subsequent
generation of collagen (23).
PAI-1 regulates fibrinolysis and is involved in the wound healing
process, and its plasma levels may be elevated during the
perioperative period. PAI-1 also affects angiogenesis via its
extracellular matrix (ECM) proteolytic and cell migration/adhesion
effects (24). PAI-1 is a
single-chain glycoprotein with a short plasma half-life, and its
plasma levels typically range between 6 and 80 ng/ml (25). Excessive PAI-1 levels and
dysregulated fibrinolysis may lead to pathogenic thrombosis.
Elevated expression levels of PAI-1 have been reported in rectal
cancer and were found to be directly associated with cancer stage
(26). Elevated plasma levels of
PAI-1 have also been associated with worse outcomes in lung,
breast, and stomach cancer, as well as CRC (27-32).
A growing body of evidence suggests that PAI-1 facilitates tumor
cell proliferation, tumor angiogenesis, and other tumorigenic
effects (33).
The effects of colorectal surgery on plasma PAI-1
levels are currently unknown. Therefore, the purpose of the present
study was to compare PAI-1 levels before and at 5 weeks after
minimally invasive surgical resection of CRC.
Materials and methods
Study population
Study patients were selected from a population of
patients with CRC undergoing MICR who had been enrolled in an
institutional review board-approved prospective perioperative
tissue and data bank at Mount Sinai West Hospital or New
York-Presbyterian Hospital between 2006 and 2015 (IRB reference no.
GCO1: 16-2619-Institutional Review Board of the Mount Sinai School
of Medicine, New York, USA; and IRB reference no.
AAAA4473-Institutional Review Board of the Columbia University
Medical Center, New York, USA). In addition to banking samples of
tumor and normal colonic mucosa, preoperative and multiple
postoperative blood samples were obtained. The blood samples were
quickly processed after collection, and the plasma was harvested,
divided into aliquots and stored at -80˚C until use. The purpose of
this protocol was to evaluate the physiological, immunological and
oncological effects of MICR. Only patients who underwent surgery
alone were eligible; those who were treated with a novel drug or
underwent other procedures were excluded. Immunosuppressed patients
and patients who received perioperative blood transfusion(s) were
also excluded, as were patients undergoing urgent or emergent
surgery. Only patients for whom blood samples had been taken
preoperatively, on postoperative day (POD) 1 or 3, and at least one
sampling point beyond POD 7, were considered as eligible. Since
post-discharge blood samples were not obtained on particular PODs,
and since the late specimens were collected over a 3-4-week period,
the late samples were bundled into 7-day blocks (POD 7-13, POD
14-20, POD 21-27 and POD 28-34) that were considered at single time
points in the data analysis. Post-hospital discharge blood sampling
was performed during follow-up hospital visits.
Blood sampling and processing
Blood samples were obtained preoperatively, at POD 1
or POD 3, and at least one late time point (POD 7-34). Blood
samples were drawn into heparin-containing blood collection tubes
and processed within 5-6 h. The plasma samples were isolated via
centrifugation (450 x g for 10 min at 6˚C) and stored in 500-µl
aliquots at -80˚C until analysis.
PAI-1 determination
Plasma PAI-1 levels were determined in duplicate
using a commercially available ELISA kit (cat no. DSE100; R&D
Systems, Inc.) according to the manufacturer's instructions. Plasma
PAI-1 concentrations are reported as ng/ml.
Statistical analysis
Continuous random variables such as age, operative
time, length of stay and surgical incision are presented as mean ±
SD, whereas categorical variables are presented as frequencies and
percentages. As previously mentioned, late samples were bundled
into 7-day blocks that were considered as single time points for
the data analysis. Since preoperative and corresponding
postoperative PAI-1 values were not normally distributed at later
time points, the comparison of PAI-1 values for the preoperative
vs. postoperative time points was performed using the
non-parametric Wilcoxon signed-rank paired test, and the data are
reported as medians and 95% confidence intervals (CIs). The data
are depicted in bar graphs expressing PAI-1 levels as medians and
75% quartile ranges. Comparisons between hand-assisted and
laparoscopic-assisted subgroups, male and female patients, and
among preoperative PAI-1 levels of stage I, II, III and IV
subgroups were performed using the non-parametric Mann-Whitney U
test. Correlation between the postoperative plasma PAI-1 levels and
the patient age and operative time were performed using Spearman's
correlation coefficient (rs). P<0.05 was considered to indicate
a statistically significant difference. All analyses were performed
using SPSS, version 15.0 (SPSS, Inc.).
Results
Clinicopathological
characteristics
A total of 91 patients with CRC who underwent MICR
were selected for the present study. The patients comprised 45 men
and 46 women, with a mean age of 67.3±13.6 years (range, 40-93
years). The breakdown of the types of resection performed is
displayed in Table I. The most
commonly performed procedures were right hemicolectomy (35%), low
anterior resection or anterior resection (20%), and sigmoid
resection (19%). Laparoscopic-assisted surgery (mean incision
length, 6.6±3.6 cm) was performed in 65% of the patients, whereas
hand-assisted laparoscopic surgery (mean incision size, 10.2±3.2
cm) was performed in 35% of the patients. The mean operative time
was 318.2±128.5 min. The mean length of hospital stay was 6.8±4.3
days. The postoperative complications included the following: Ileus
(n=5), urinary tract infection (n=5), diarrhea (n=4), atelectasis
(n=2), seroma (n=1) and phlebitis (n=1). There were no superficial,
deep, or organ-space surgical site infections, or deaths. The final
cancer stage breakdown was as follows: Stage I (n=27), II (n=26),
III (n=34) and IV (n=4).
 | Table IDemographic and clinical
characteristics of the study population. |
Table I
Demographic and clinical
characteristics of the study population.
|
Characteristics | No. (%) |
|---|
| Age, years (mean ±
SD) | 67.3±13.6 |
| Sex | |
|
Male | 45 (49.0) |
|
Female | 46 (51.0) |
| Incision length, cm
(mean ± SD) | |
|
Entire
patient population | 8.0±3.9 |
|
Leg
procedure group | 6.6±3.6 |
|
Hand
procedure group | 10.2±3.2 |
| Operative time, min
(mean ± SD) | 318.2±128.5 |
| Length of stay,
days (mean ± SD) | 6.8±4.3 |
| Type of
resection | |
|
Right | 32 (35.0) |
|
Low anterior
resection/anterior resection (16/2) | 18 (20.0) |
|
Sigmoid/rectosigmoid
(11/6) | 17 (19.0) |
|
Total/subtotal
(2/7) | 10 (9.0) |
|
Transverse | 6 (7.0) |
|
Left | 6 (7.0) |
|
Abdominoperineal
resection | 3 (3.0) |
| Surgical
method | |
|
Laparoscopic-assisted | 59 (65.0) |
|
Hand-assisted/hybrid
laparoscopic | 32 (35.0) |
Association of PAI-1 levels with
clinicopathological variables
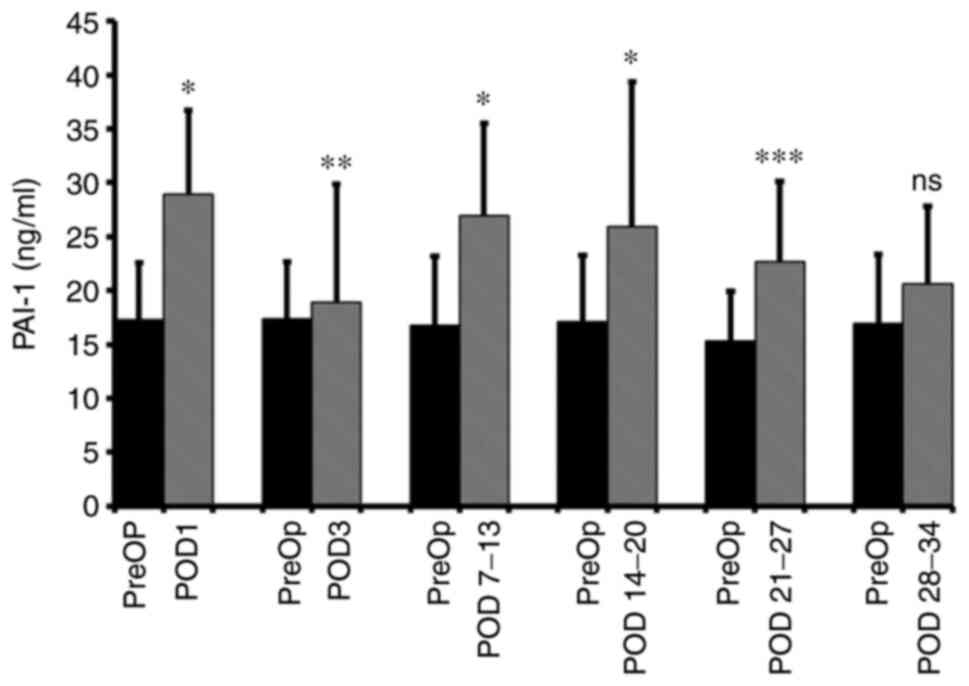
The median preoperative plasma level of PAI-1 was
17.30 ng/ml (95% CI: 15.63-19.78 ng/ml). Compared with the
preoperative baseline levels, significantly elevated median levels
were observed on POD 1 (28.86 ng/ml; 95% CI: 25.46-31.22 ng/ml;
P<0.001; n=91), POD 3 (18.87 ng/ml; 95% CI: 17.05-21.78 ng/ml;
P=0.0037; n=86), POD 7-13 (26.97 ng/ml; 95% CI: 22.81-28.74 ng/ml;
P<0.001; n=65), POD 14-20 (25.92 ng/ml; 95% CI: 17.85-35.89
ng/ml; P=0.001; n=26) and POD 21-27 (22.63 ng/ml; 95% CI:
20.03-30.09 ng/ml; P<0.001; n=19). There was no significant
difference between the POD 27-34 and preoperative levels (Fig. 1). The ‘n’ for each time point
varied for PAI-1 and, therefore, the preoperative median protein
values are different at each time point. Because of this, as
regards the bar graph figures, at each time point, in addition to a
bar showing the postoperative result, there is an adjacent bar (on
the left) providing the median preoperative result. There was no
significant correlation between age and operative time for plasma
PAI-1 levels at all six postoperative time points. No significant
differences were found between the male and female groups at any
perioperative time point. The percentage increases from the median
baseline at each time point were as follows: 66.8% at POD 1, 9.0%
at POD 3, 60.6% at POD 7-13, 51.5% at POD 14-20 and 47.7% at POD
21-27. Of note, no direct association was observed between
advancing cancer stage and preoperative PAI-1 levels, except for
the significantly higher levels in the stage II subgroup compared
with the stage I subgroup (P=0.038).
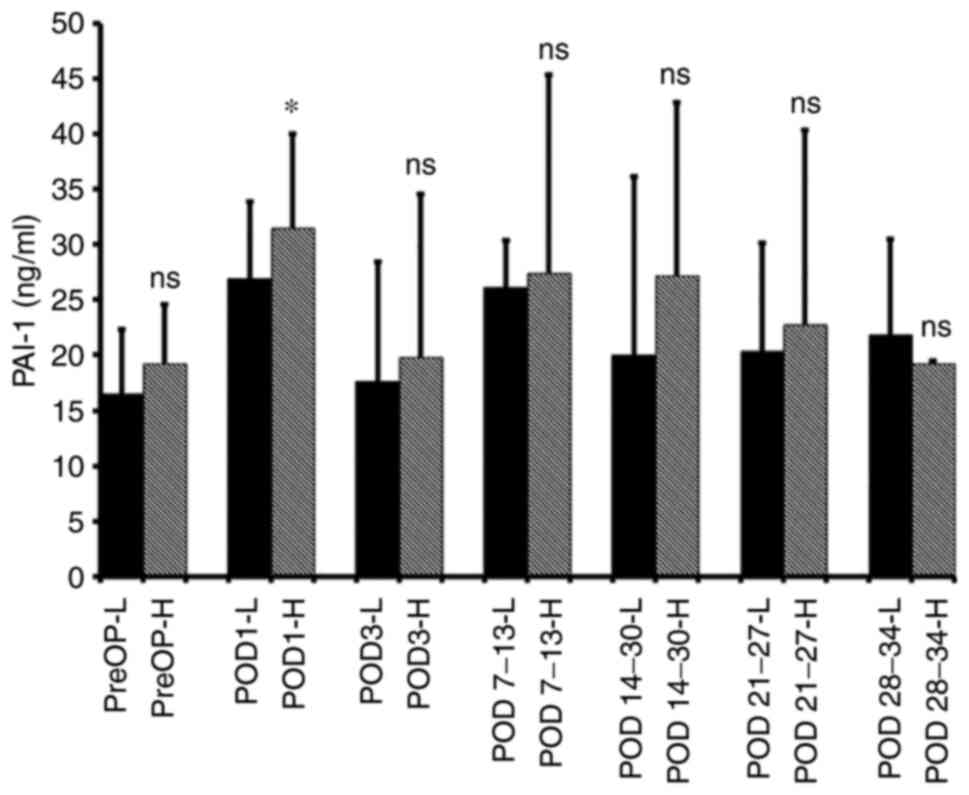
The influence of the incision size (i.e., the extent
of abdominal wall trauma) on postoperative PAI-1 levels was also
considered. The median PAI-1 levels in the hand-assisted surgery
group (mean incision length, 10.2±3.2 cm) were higher compared with
those in the laparotomy-assisted surgery group (mean incision
length, 6.6±3.7 cm) for 4 weeks after surgery; however, the
difference was significant only on POD 1 (P=0.02; Fig. 2).
Discussion
In patients with CRC undergoing MICR, the plasma
levels of PAI-1 were significantly elevated from POD 1 through to
POD 27. The percentage change from baseline during the 2nd through
to the 4th postoperative week ranged from 47.7 to 60.6%. No
significant correlation was observed between the abdominal incision
length or operative time and the height of the elevations (except
for the POD 1 results). If the plasma protein elevation was caused
by wound healing, as suggested below, then longer incisions would
have been expected to be associated with more pronounced
elevations. The failure to demonstrate a direct association between
incision length and postoperative blood PAI-1 levels may be
associated with the relatively small number of patients in the
present study. Another factor may have been the narrow range of
incision lengths found in this population of patients who underwent
only minimally invasive procedures. As regards the preoperative
PAI-1 levels and cancer stage, no significant correlation was found
between the pathological cancer stage and blood PAI-1
concentration, except for the significantly higher levels in the
stage II subgroup compared with the stage I subgroup (P=0.038).
The sustained elevation of its plasma levels after
surgery place PAI-1 among the growing group of proteins the plasma
concentrations of which remain significantly elevated for 2-5 weeks
after cancer resection. These proteins include VEGF, Ang-2, PIGF,
sVCAM-1, IL-8, MCP-1, CHI3L1, MMP-3 and CXCL-16 (3-5,13-17).
These long-duration sustained postoperative elevations are uniquely
observed after MICR.
Surgery and anesthesia are well known to transiently
alter the blood levels of several proteins, including IL-1, TNF,
IL-6, hepatocyte growth factor, fibroblast growth factor,
C-reactive protein, IL-4, IL-10 and granulocyte colony-stimulating
factor. The majority of these plasma protein elevations resolve in
a matter of hours or days. Most of these transient changes are
considered to be associated with either the acute inflammatory
response (principal mediators, IL-1β, TNF and IL-6) or the
hypothalamic/pituitary/adrenal axis-generated response to surgical
trauma, which results in the production/release of
adrenocorticotropic hormone, cortisone, norepinephrine and
vasopressin, as well cytokines and other proteins (34,35).
The origin and etiology of the persistent
postoperative plasma elevation of PAI-1 and the other
aforementioned long-duration proteins are unknown. Although several
types of cancer express PAI-1 and these other proteins, CRC is
unlikely to be the source of the postoperative plasma elevations
observed in this study, as these increases were observed after the
primary tumor had been resected. Evidence suggests (36) that wound healing contributes to the
elevated blood levels of eight of the proteins with long-duration
increases. The levels of these proteins in the fluid from surgical
wounds were found to be 3-40 times higher compared with their
plasma levels, which were significantly elevated over their
preoperative baselines (36). The
plasma proteins in question have been hypothesized to follow a
concentration gradient from the healing surgical wounds to the
bloodstream (31). Given the role
of PAI-1 in wound healing, this hypothesis may explain the
persistent plasma elevations of PAI-1 after surgery.
PAI-1 inhibits uPA and tPA, thereby regulating
plasmin-related fibrinolysis, which is critical to the normal wound
healing process (22). Plasmin is
a critical protease that breaks down fibrin clots that form soon
after wounding during the ‘inflammatory’ stage of wound healing.
Through removal of the fibrin clot, the second phase of wound
healing (the proliferative stage) is stimulated; in this phase,
fibroblasts generate collagen and tissue fibronectin, which replace
the fibrin (23). The modulation
of plasmin during wound healing has also been shown to be
necessary, as indicated by observations that PAI-1 is expressed in
keratinocytes, fibroblasts, and other cells found in healing
wounds, and its absence and overexpression affect the wound healing
process (37). The role of PAI-1
in wound healing is complex and incompletely understood, as it has
been demonstrated by experimental and human evidence that, under
different conditions, PAI-1 can both promote and inhibit wound
healing (38). Given the role of
PAI-1 in wound healing, it is reasonable to infer that the wounds
may be the source of the elevated plasma PAI-1 levels observed
after surgery.
In addition to regulating fibrinolysis and cell
migration, PAI-1 also affects angiogenesis (24). PAI-1 regulates angiogenesis via
several mechanisms, including the inhibition of proteolysis and the
breakdown of MMPs (39,40). PAI-1 has been shown to exert both
pro- and antiangiogenic effects (38,41).
PAI-1 in its active form binds vitronectin, a glycoprotein that is
found in both the plasma and the ECM, and plays a key role in cell
adhesion via integrin binding (41). When PAI-1 is bound to vitronectin,
the integrin adhesion site on vitronectin is blocked, thus
diminishing EC migration from the circulation to the periphery and
hindering angiogenesis (41). In
addition, PAI-1 directly inhibits proteinases that inhibit vessel
formation (41). By inhibiting EC
migration to vitronectin, PAI-1 promotes EC binding to fibronectin
(a critical cell adhesion glycoprotein) in the wound, thereby
facilitating angiogenesis. Thus, the composition of the ECM
(vitronectin vs. fibronectin balance) may determine whether the net
effect of PAI-1 is pro- or antiangiogenic. Of note, in response to
hypoxia, the transcription of PAI-1 increases via elevated PAI-1
promoter activity and genistein-sensitive tyrosine kinase-mediated
phosphorylation of transcription factors (42). As tumors have high fibronectin
levels and a hypoxic stroma, PAI-1 promotes the migration of ECs
away from the well-oxygenated, vitronectin-rich extravascular space
and into the hypoxic, fibronectin-rich tumor (24). Thus, PAI-1 may promote angiogenesis
in tumors while inhibiting new vessel formation in well-oxygenated
environments; high PAI-1 levels during the first month after MICR
may stimulate tumor angiogenesis in residual tumor deposits. The
results of an experimental study suggested that PAI-1 may also
affect VEGF levels in wounds. Chan et al (37) compared skin wound healing between
PAI-1 knockout (PAI-1-/-) and wild-type (WT) mice
(PAI-1+/+). Whereas VEGF expression in the connective
tissue of the wounds in WT mice was readily observed, VEGF was
poorly expressed in the wounds of PAI-1 knockout mice.
PAI-1 may also influence tumor cell growth. Cell
cycle progression, a hallmark of cancer, is regulated by cyclins
and cyclin-dependent kinases (CDKs) that control whether a cell can
progress through the cell cycle and ultimately replicate (43). PAI-1-deficient tumor cells cannot
progress through G1 phase into the S phase, and they exhibit
elevated levels of p53 and diminished levels of G1-phase transition
complexes (cyclin D3/CDK4/6 and cyclin E/CDK2), thus suggesting
that PAI-1 promotes cell cycle progression and tumor cell
proliferation (44). In addition,
PAI-1 modulates uPA and extracellular cell signaling molecules and
interactors. The effect of this modulated interaction is sustained
Ras/MAPK1 pathway activity, which promotes cell proliferation
(45).
In addition to promoting cell proliferation, PAI-1
inhibits spontaneous apoptosis (46). Knockout of PAI-1 results in
increased plasmin activity, which in turn facilitates spontaneous
apoptosis, as plasmin cleaves FasL and generates a pro-apoptotic
FasL fragment (40). PAI-1 thereby
protects ECs and potentially tumor cells from Fas/FasL-mediated
apoptosis (47). PAI-1 also
inhibits the activation and activity of caspase-3, thus promoting
apoptosis via the Fas/FasL pathway (48). By attenuating apoptotic signaling,
PAI-1 reduces the tumor response to chemotherapy (46,49).
Whether PAI-1 promotes tumor cell migration and
metastasis remains controversial. In vivo experiments have
demonstrated both pro- and anti-metastatic effects. Higher plasma
levels of PAI-1 were found to be correlated with larger tumors and
more frequent liver metastases in CRC, and its silencing reduces
the expression of MMPs, thus preventing the degradation of ECM and
cell mobility (33). By contrast,
overexpression of PAI-1 has been found to suppress cellular
invasion and liver metastasis in a pancreatic cancer cell line
(50). The different conclusions
may, in part, be attributed to differences in the experimental
protocols, tumor cell lines, inoculation methods, use of
immunocompetent vs. immunocompromised or knockout vs.
overexpressing mice, and examination of macro- vs. micrometastasis
(51).
Given the correlation between increased levels of
PAI-1 and worse oncological outcomes, researchers are striving to
formulate pharmacological inhibitors of PAI-1; the majority of
studies have been focused on forcing a conformational change of
PAI-1 into its inactive form (52,53).
PAI-039 (tiplaxtinin) is currently in clinical trials for
Alzheimer's disease and has been reported to suppress the growth of
bladder and cervical cancers (54). SK-216 has been shown to notably
reduce the number of intestinal polyps in a mouse model with a
defect in the adenomatous polyposis coli gene (55). Another small-molecule inhibitor
(TM5275) was shown to block ovarian cancer cell proliferation by
causing G2/M cell cycle arrest (56). However, to the best of our
knowledge, no PAI-1 inhibitor is currently being used in clinical
trials for cancer treatment.
There were several substantial limitations to the
present study. First, the number of post-hospital discharge time
points was considerably lower than the initial number of patients
and decreased at each successive time point. After the initial
postoperative office visit, the majority of the patients were not
seen again in the first month, and some patients refused additional
blood sampling. Since obtaining the late blood samples on the same
postoperative days was impossible for logistical reasons, it was
necessary to ‘bundle’ the specimens for postoperative weeks 2, 3, 4
and 5 and consider them as single time points. Furthermore, the
size of the study and the lack of long-term outcome data prevent a
meaningful correlation of PAI-1 levels and cancer outcomes. A more
extensive and comprehensive study would likely shed light on these
issues. Another shortcoming of this study is that the CRCs from the
study patients were not evaluated for PAI-1 expression.
PAI-1 belongs to the group of proteins the
postoperative blood levels of which remain elevated for 3-5 weeks
after MICR. The clinical ramifications of the surgery-associated
long-duration plasma protein changes are unknown. All nine proteins
on the list provided above plus PAI-1 serve key roles in the
complex process of angiogenesis, beyond their numerous other
effects. Moreover, as mentioned, several studies have provided
in vitro evidence that plasma collected from the 2nd and 3rd
weeks post-MICR increased EC invasion, migration and proliferation
beyond that observed in EC cultures into which preoperative plasma
from the same patients was added (28,49).
These findings suggest that the net effects of the surgery-related
changes to blood composition transiently render the plasma
proangiogenic during the first month after surgery. Despite being
unproven, a concern exists that tumor angiogenesis and, therefore,
tumor growth may be stimulated during the first month after surgery
in patients who have residual micro-metastases following resection
of the primary tumor. If this is the case, then the postoperative
period is a perilous time for patients with cancer who harbor
metastases. The development of anticancer treatments that could be
used perioperatively would be a logical next step.
In conclusion, the findings of the present study
demonstrated significant and persistent elevations of plasma PAI-1
levels over the preoperative baseline for 1 month after MICR for
CRC. Although the etiology of these postoperative changes was not
assessed in the present study, it was hypothesized that the acute
inflammatory response may account for the early PAI-1 elevations,
whereas the later postoperative elevations may be associated with
wound healing. Further studies are required to determine the cause
and possible ramifications of these changes.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a generous donation
(grant no. SL55002012 from the Thompson Family Foundation to the
Division of Colon and Rectal Surgery, Department of Surgery, Mount
Sinai West Hospital, New York.
Availability of data and materials
The datasets generated and/or analyzed during the
current study, other than those included in the article, are not
publicly available due to protection of patient privacy and
biosecurity reasons; all other data generated or analyzed during
this study are included in this published article.
Authors' contributions
HMCSK and RLW contributed to the design, conception
and statistical analysis; PA, DNG, EP, XY and VC contributed to the
study design, data collection, sample preparation, analysis and
interpretation; HMCSK and XY confirm the authenticity of all the
raw data. HMCSK, RLW and AS contributed to conducting of
experiments, data interpretation and critical revision of the
manuscript. All authors were involved in drafting the manuscript,
made revisions, and approved the article's final version.
Ethics approval and consent to
participate
The present study was conducted by using material
collected from patients who consented preoperatively to participate
in the Mount Sinai West Colorectal service's IRB-approved general
tissue and data banking protocol (reference no.: GCO1:
16-2619-Institutional Review Board of the Mount Sinai School of
Medicine, New York, USA; and reference no.: AAAA4473-Institutional
Review Board of the Columbia University Medical Center, New York,
USA).
Patient consent for publication
Written informed consent was obtained from all
participating patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Platell CF: Changing patterns of
recurrence after treatment for colorectal cancer. Int J Colorectal
Dis. 22:1223–1231. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Coffey JC, Wang JH, Smith MJ,
Bouchier-Hayes D, Cotter TG and Redmond HP: Excisional surgery for
cancer cure: Therapy at a cost. Lancet Oncol. 4:760–768.
2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kumara HM, Feingold D, Kalady M, Dujovny
N, Senagore A, Hyman N, Cekic V and Whelan RL: Colorectal resection
is associated with persistent proangiogenic plasma protein changes:
Postoperative plasma stimulates in vitro endothelial cell growth,
migration, and invasion. Ann Surg. 249:973–977. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shantha Kumara HM, Myers EA, Herath SA,
Jang JH, Njoh L, Yan X, Kirchoff D, Cekic V, Luchtefeld M and
Whelan RL: Plasma monocyte chemotactic protein-1 remains elevated
after minimally invasive colorectal cancer resection. World J
Gastrointest Oncol. 6:413–419. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shantha Kumara HM, Gaita D, Miyagaki H,
Yan X, Hearth SA, Njoh L, Cekic V and Whelan RL: Plasma chitinase
3-like 1 is persistently elevated during first month after
minimally invasive colorectal cancer resection. World J
Gastrointest Oncol. 8:607–614. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kumara HMCS, Bellini GA, Caballero OL,
Herath SAC, Su T, Ahmed A, Njoh L, Cekic V and Whelan RL:
P-Cadherin (CDH3) is overexpressed in colorectal tumors and has
potential as a serum marker for colorectal cancer monitoring.
Oncoscience. 4:139–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shantha Kumara HM, Kirman I, Feingold D,
Cekic V, Nasar A, Arnell T, Balik E, Hoffman A, Baxter R, Conte S
and Whelan RL: Perioperative GMCSF limits the proangiogenic plasma
protein changes associated with colorectal cancer resection. Eur J
Surg Oncol. 35:295–301. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Carter JJ, Feingold DL, Oh A, Kirman I,
Wildbrett P, Stapleton G, Asi Z, Fowler R, Bhagat G, Huang EH, et
al: Perioperative immunomodulation with Flt3 kinase ligand or a
whole tumor cell vaccine is associated with a reduction in lung
metastasis formation after laparotomy in mice. Surg Innov.
13:41–47. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Smith I, Robertson J, Kilburn L, Wilcox M,
Evans A, Holcombe C, Horgan K, Kirwan C, Mallon E, Sibbering M, et
al: Long-term outcome and prognostic value of Ki67 after
perioperative endocrine therapy in postmenopausal women with
hormone-sensitive early breast cancer (POETIC): An open-label,
multicentre, parallel-group, randomised, phase 3 trial. Lancet
Oncol. 21:1443–1454. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
O'Reilly MS, Holmgren L, Shing Y, Chen C,
Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH and Folkman J:
Angiostatin: A novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kirman I, Cekic V, Poltaratskaia N, Asi Z,
Bessler M, Huang EH, Forde KA and Whelan RL: Plasma from patients
undergoing major open surgery stimulates in vitro tumor growth:
Lower insulin-like growth factor binding protein 3 levels may, in
part, account for this change. Surgery. 132:186–192.
2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Allendorf JD, Bessler M, Horvath KD,
Marvin MR, Laird DA and Whelan RL: Increased tumor establishment
and growth after open vs. laparoscopic surgery in mice may be
related to differences in postoperative T-cell function. Surg
Endosc. 13:233–235. 1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shantha Kumara HM, Cabot JC, Yan X, Herath
SA, Luchtefeld M, Kalady MF, Feingold DL, Baxter R and Whelan RL:
Minimally invasive colon resection is associated with a persistent
increase in plasma PlGF levels following cancer resection. Surg
Endosc. 25:2153–2158. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shantha Kumara HM, Tohme ST, Herath SA,
Yan X, Senagore AJ, Nasar A, Kalady MF, Baxter R and Whelan RL:
Plasma soluble vascular adhesion molecule-1 levels are persistently
elevated during the first month after colorectal cancer resection.
Surg Endosc. 26:1759–1764. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shantha Kumara HM, Gaita DJ, Miyagaki H,
Yan X, Herath SA, Cekic V and Whelan RL: Minimally invasive
colorectal resection is associated with significantly elevated
levels of plasma matrix metalloproteinase 3 (MMP-3) during the
first month after surgery which may promote the growth of residual
metastases. Surg Endosc. 28:3322–3328. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shantha Kumara HMC, Pettke E, Shah A, Yan
X, Cekic V, Downing MA, Gandhi ND and Whelan RL: Plasma levels of
the proangiogenic protein CXCL16 remains elevated for 1 month after
minimally invasive colorectal cancer resection. World J Surg Oncol.
16(132)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shantha Kumara HMC, Sutton E, Bellini GA,
Yan X, Cekic V, Gandhi ND and Whelan RL: Plasma interleukin-8
levels are persistently elevated for 1 month after minimally
invasive colorectal resection for colorectal cancer. Mol Clin
Oncol. 8:471–476. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Belizon A, Balik E, Horst P, Feingold D,
Arnell T, Azarani T, Cekic V, Skitt R, Kumara S and Whelan RL:
Persistent elevation of plasma vascular endothelial growth factor
levels during the first month after minimally invasive colorectal
resection. Surg Endosc. 22:287–297. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shantha Kumara HM, Kirchoff D, Naffouje S,
Grieco M, Herath SA, Dujovny N, Kalady MF, Hyman N, Njoh L and
Whelan RL: Plasma from the second and third weeks after open
colorectal resection for cancer stimulates in vitro endothelial
cell growth, migration, and invasion. Surg Endosc. 26:790–795.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shantha Kumara HM, Grieco MJ, Caballero
OL, Su T, Ahmed A, Ritter E, Gnjatic S, Cekic V, Old LJ, Simpson
AJ, et al: MAGE-A3 is highly expressed in a subset of colorectal
cancer patients. Cancer Immun. 12(16)2012.PubMed/NCBI
|
|
21
|
Shantha Kumara H, Kirchoff D, Caballero
OL, Su T, Ahmed A, Herath SA, Njoh L, Cekic V, Simpson AJ,
Cordon-Cardo C and Whelan RL: Expression of the cancer testis
antigen IGF2BP3 in colorectal cancers; IGF2BP3 holds promise as a
specific immunotherapy target. Oncoscience. 2:607–614.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shetty S and Idell S: FIBRINOLYSIS |
Plasminogen Activator and Plasmin. In: Encyclopedia of Respiratory
Medicine. Laurent GJ and Shapiro SD (eds). Academic Press,
pp205-210, 2006.
|
|
23
|
Wilkinson HN and Hardman MJ: . Wound
healing: Cellular mechanisms and pathological outcomes. Open Biol.
10(200223)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Isogai C, Laug WE, Shimada H, Declerck PJ,
Stins MF, Durden DL, Erdreich-Epstein A and DeClerck YA:
Plasminogen activator inhibitor-1 promotes angiogenesis by
stimulating endothelial cell migration toward fibronectin. Cancer
Res. 61:5587–5594. 2001.PubMed/NCBI
|
|
25
|
Dellas C and Loskutoff DJ: Historical
analysis of PAI-1 from its discovery to its potential role in cell
motility and disease. Thromb Haemost. 93:631–640. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Angenete E, Langenskiöld M, Palmgren I,
Falk P, Oresland T and Ivarsson ML: uPA and PAI-1 in rectal
cancer-relationship to radiotherapy and clinical outcome. J Surg
Res. 153:46–53. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lu JJ, Guo H, Gao B, Zhang Y, Lin QL, Shi
J, Liu JJ and Liu J: Prognostic value of urokinase plasminogen
activator system in non-small cell lung cancer: A systematic review
and meta-analysis. Mol Clin Oncol. 8:127–132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Harbeck N, Thomssen C, Berger U, Ulm K,
Kates RE, Höfler H, Jänicke F, Graeff H and Schmitt M: Invasion
marker PAI-1 remains a strong prognostic factor after long-term
follow-up both for primary breast cancer and following first
relapse. Breast Cancer Res Treat. 54:147–157. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Harbeck N, Schmitt M, Meisner C, Friedel
C, Untch M, Schmidt M, Sweep CG, Lisboa BW, Lux MP, Beck T, et al:
Ten-year analysis of the prospective multicentre Chemo-N0 trial
validates American Society of Clinical Oncology (ASCO)-recommended
biomarkers uPA and PAI-1 for therapy decision making in
node-negative breast cancer patients. Eur J Cancer. 49:1825–1835.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Suh YS, Yu J, Kim BC, Choi B, Han TS, Ahn
HS, Kong SH, Lee HJ, Kim WH and Yang HK: Overexpression of
plasminogen activator inhibitor-1 in advanced gastric cancer with
aggressive lymph node metastasis. Cancer Res Treat. 47:718–726.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Berger DH: Plasmin/plasminogen system in
colorectal cancer. World J Surg. 26:767–771. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nielsen HJ, Christensen IJ, Sørensen S,
Moesgaard F and Brünner N: Preoperative plasma plasminogen
activator inhibitor type-1 and serum C-reactive protein levels in
patients with colorectal cancer. The RANX05 colorectal cancer study
group. Ann Surg Oncol. 7:617–623. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen H, Peng H, Liu W, Sun Y, Su N, Tang
W, Zhang X, Wang J, Cui L, Hu P and Liu S: Silencing of plasminogen
activator inhibitor-1 suppresses colorectal cancer progression and
liver metastasis. Surgery. 158:1704–1713. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Desborough JP: The stress response to
trauma and surgery. Br J Anaesth. 85:109–117. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kany S, Vollrath JT and Relja B: Cytokines
in inflammatory disease. Int J Mol Sci. 20(6008)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shantha Kumara H, Yan XH, Pettke E, Cekic
V, Gandhi ND, Bellini GA and Whelan RL: Plasma and wound fluid
levels of eight proangiogenic proteins are elevated after
colorectal resection. World J Gastrointest Oncol. 11:470–488.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chan JC, Duszczyszyn DA, Castellino FJ and
Ploplis VA: Accelerated skin wound healing in plasminogen activator
inhibitor-1-deficient mice. Am J Pathol. 159:1681–1688.
2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Maquerlot F, Galiacy S, Malo M, Guignabert
C, Lawrence DA, d'Ortho MP and Barlovatz-Meimon G: Dual role for
plasminogen activator inhibitor type 1 as soluble and as
matricellular regulator of epithelial alveolar cell wound healing.
Am J Pathol. 169:1624–1632. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Devy L, Blacher S, Grignet-Debrus C, Bajou
K, Masson V, Gerard R.D, Gils A, Carmeliet G, Carmeliet P, Declerck
PJ, et al: The pro- or antiangiogenic effect of plasminogen
activator inhibitor 1 is dose dependent. FASEB J. 16:147–154.
2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bajou K, Peng H, Laug WE, Maillard C, Noel
A, Foidart JM, Martial JA and DeClerck YA: Plasminogen activator
inhibitor-1 protects endothelial cells from FasL-mediated
apoptosis. Cancer Cell. 14:324–334. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Stefansson S, Petitclerc E, Wong MK,
McMahon GA, Brooks PC and Lawrence DA: Inhibition of angiogenesis
in vivo by plasminogen activator inhibitor-1. J Biol Chem.
276:8135–8141. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Uchiyama T, Kurabayashi M, Ohyama Y,
Utsugi T, Akuzawa N, Sato M, Tomono S, Kawazu S and Nagai R:
Hypoxia induces transcription of the plasminogen activator
inhibitor-1 gene through genistein-sensitive tyrosine kinase
pathways in vascular endothelial cells. Arterioscler Thromb Vasc
Biol. 20:1155–1161. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Casimiro MC, Crosariol M, Loro E, Li Z and
Pestell RG: Cyclins and cell cycle control in cancer and disease.
Genes Cancer. 3:649–657. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Giacoia EG, Miyake M, Lawton A, Goodison S
and Rosser CJ: PAI-1 leads to G1-phase cell-cycle progression
through cyclin D3/cdk4/6 upregulation. Mol Cancer Res. 12:322–334.
2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Webb DJ, Thomas KS and Gonias SL:
Plasminogen activator inhibitor 1 functions as a urokinase response
modifier at the level of cell signaling and thereby promotes MCF-7
cell growth. J Cell Bio. 152:741–752. 2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kwaan HC, Wang J, Svoboda K and Declerck
PJ: Plasminogen activator inhibitor 1 may promote tumour growth
through inhibition of apoptosis. Br J Cancer. 82:1702–1708.
2000.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fang H, Placencio VR and DeClerck YA:
Protumorigenic activity of plasminogen activator inhibitor-1
through an antiapoptotic function. J Natl Cancer Inst.
104:1470–1484. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen Y, Kelm RJ Jr, Budd RC, Sobel BE and
Schneider DJ: Inhibition of apoptosis and caspase-3 in vascular
smooth muscle cells by plasminogen activator inhibitor type-1. J
Cell Biochem. 92:178–188. 2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rømer MU, Larsen L, Offenberg H, Brünner N
and Lademann UA: Plasminogen activator inhibitor 1 protects
fibrosarcoma cells from etoposide-induced apoptosis through
activation of the PI3K/Akt cell survival pathway. Neoplasia.
10:1083–1091. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Inoue M, Sawada T, Uchima Y, Kimura K,
Nishihara T, Tanaka H, Yashiro M, Yamada N, Ohira M and Hirakawa K:
Plasminogen activator inhibitor-1 (PAI-1) gene transfection
inhibits the liver metastasis of pancreatic cancer by preventing
angiogenesis. Oncol Rep. 14:1445–1451. 2005.PubMed/NCBI
|
|
51
|
Kubala MH and DeClerck YA: The plasminogen
activator inhibitor-1 paradox in cancer: A mechanistic
understanding. Cancer Metastasis Rev. 38:483–492. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Placencio VR and DeClerck YA: Plasminogen
activator inhibitor-1 in cancer: Rationale and insight for future
therapeutic testing. Cancer Res. 75:2969–2974. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fortenberry YM: Plasminogen activator
inhibitor-1 inhibitors: A patent review (2006-present). Expert Opin
Ther Pat. 23:801–815. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gomes-Giacoia E, Miyake M, Goodison S and
Rosser CJ: Targeting plasminogen activator inhibitor-1 inhibits
angiogenesis and tumor growth in a human cancer xenograft model.
Mol Cancer Ther. 12:2697–2708. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Mutoh M, Niho N, Komiya M, Takahashi M,
Ohtsubo R, Nakatogawa K, Ueda K, Sugimura T and Wakabayashi K:
Plasminogen activator inhibitor-1 (Pai-1) blockers suppress
intestinal polyp formation in Min mice. Carcinogenesis. 29:824–829.
2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Mashiko S, Kitatani K, Toyoshima M,
Ichimura A, Dan T, Usui T, Ishibashi M, Shigeta S, Nagase S, Miyata
T and Yaegashi N: Inhibition of plasminogen activator inhibitor-1
is a potential therapeutic strategy in ovarian cancer. Cancer Biol
Ther. 16:253–260. 2015.PubMed/NCBI View Article : Google Scholar
|