Introduction
Immune checkpoints constitute one of the most
important mechanisms for regulating the activation of the immune
system (1). In cancer, the
overexpression of programmed cell death-ligand 1 (PD-L1) on cancer
cells and/or in the cancer microenvironment leads to the
neutralization of activated programmed cell death-1 (PD-1)-positive
cancer-specific T cells (2).
Therapies with antibodies targeting PD-1 and its ligands restore
T-cell functions. The effectiveness of these therapies has been
widely demonstrated in clinical trials, and immune checkpoint
inhibitors (ICIs) are becoming widely used for the treatment of
various cancers (3). However,
characteristic side effects, which are referred to as
immune-related adverse events, may appear (4-6),
and they have important clinical implications for the management of
patients treated with ICIs. Furthermore, complications from
infections have recently been reported to be an important issue in
patients receiving treatment with ICIs (7). The development of mycobacterial
infections has also been reported, but they have mostly been seen
in cases with tuberculosis (1,8-10),
and only a few cases involved non-tuberculous mycobacteriosis
(11).
We herein report a case of Mycobacterium
avium complex lung disease (MAC-LD) that developed rapidly in a
patient who was being treated with nivolumab for gastric
cancer.
Case report
An 82-year-old man visited the Nagoya City
University West Medical Center (Nagoya, Japan) in March 2017 with a
chief complaint of epigastric pain that started 3 months earlier,
and he was diagnosed with stage IV gastric cancer.
Tegafur/gimeracil/oteracil (TS-1) was administered as first-line
chemotherapy (40 mg twice/day, consisting of 42-day cycles of
28-day consecutive administration followed by 14-day drug holiday,
for a total of 9 cycles), and paclitaxel was administered as the
second-line chemotherapy (65 mg/m2 on days 1, 8 and 15
every 4 weeks for a total of 2 cycles), but neither treatment was
effective. Due to increased bleeding from the tumor, the patient
received radiation therapy (45 Gy) to control the bleeding. In
October 2018, the patient was initiated on nivolumab (240 mg every
4 weeks) as the third-line chemotherapy; the nivolumab treatment
induced a partial response, and was thus continued. After the 22nd
course of the treatment, the patient experienced loss of appetite
for 1 week, and was then referred to the Department of Respiratory
Medicine of the Nagoya City University West Medical Center due to
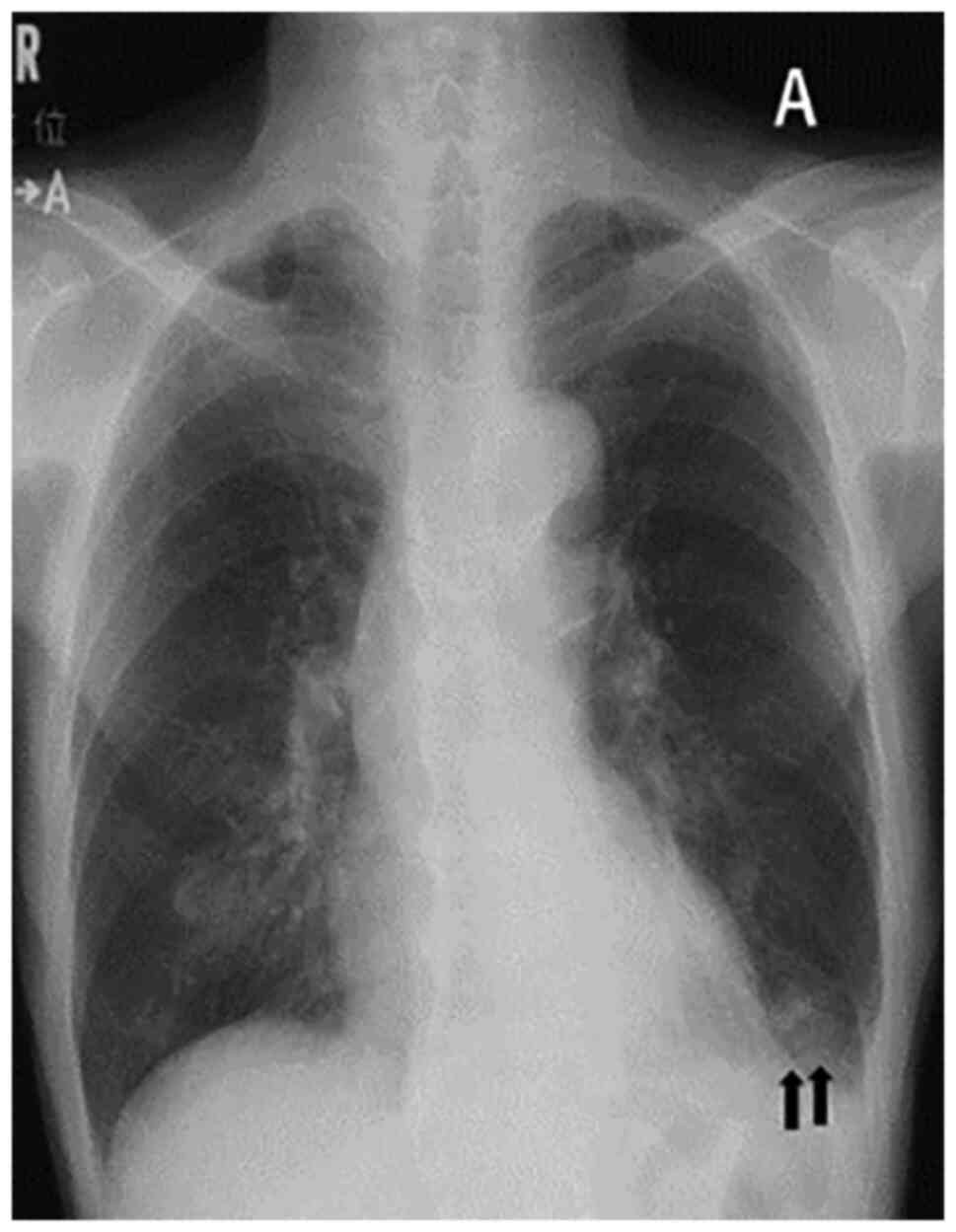
the appearance of infiltration shadows in the lower lobe of the
left lung on chest X-ray (Fig. 1A)
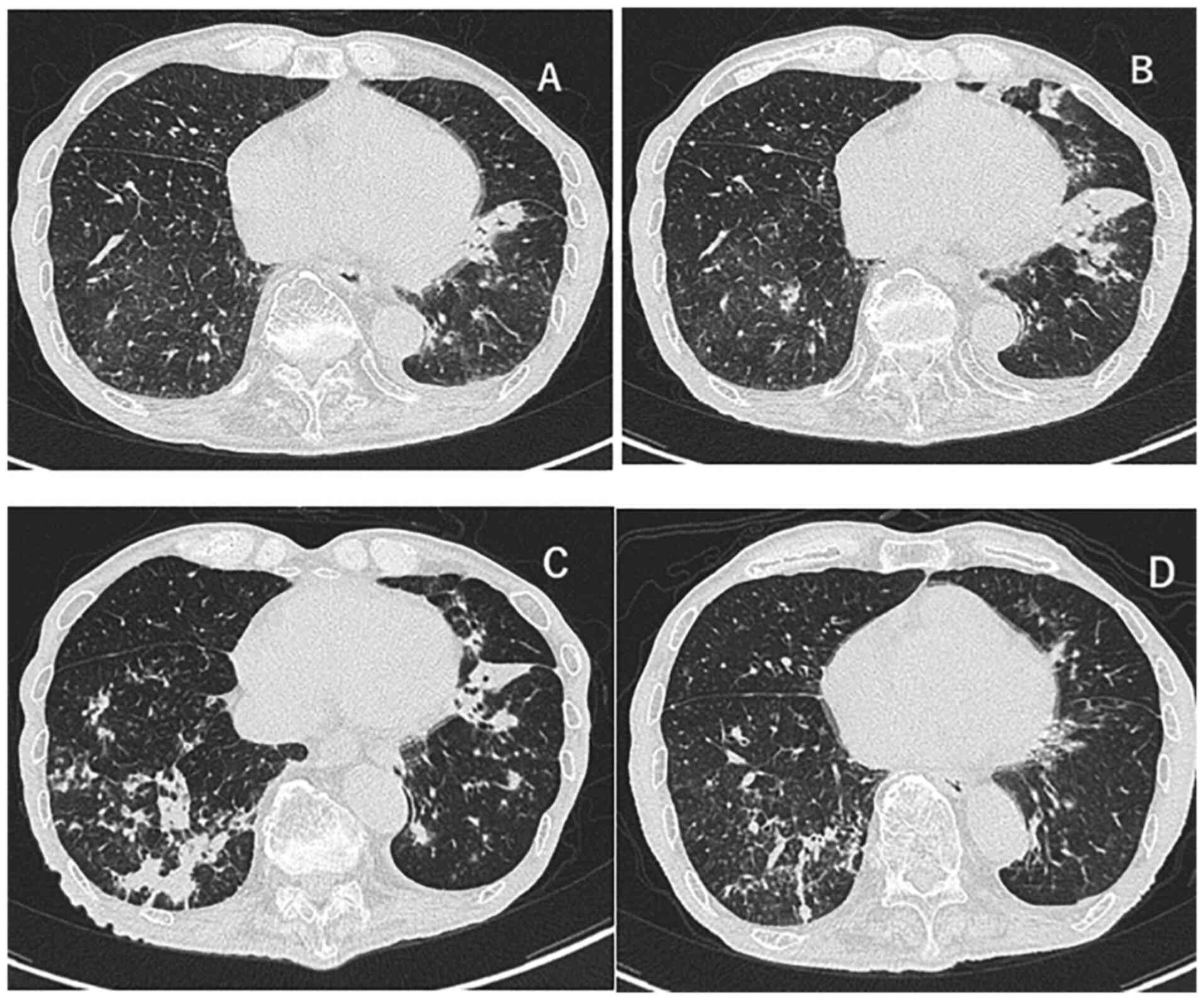
and chest CT (Fig. 2A) images. On
the chest CT images taken 3 months ago, no abnormality was found
bilaterally in the lung fields. The patient had no fever, and the
blood test results revealed a white blood cell count of 5,200/µl
(normal range, 3,300-8,600/µl) and a C-reactive protein level of
0.6 mg/dl (normal range, 0.0-0.3 mg/dl). The patient received
moxifloxacin per os 400 mg/day for 7 days on suspicion of
bacterial pneumonia, but no treatment was administered subsequently
as his condition and inflammatory response on blood tests were
stable. One month later, the CT findings revealed an exacerbation,
the infiltrative shadow in the lower left lobe had expanded, and
other inflammatory shadows appeared in the left lingula and the
lower lobe of the right lung (Fig.
2B); however, no fever or oxygen desaturation were observed.
The results of the blood examination that was performed at that
time are listed in Table I. No
obvious abnormalities were observed, except for a slight increase
in the C-reactive protein level to 3.2 mg/dl. Mycobacterium
intracellulare was detected twice in sputum cultures performed
at the time, and no other causative bacteria were detected. The
shadows were found mainly in the lower lobe of the left lung, and
not in the middle lobe, lingula or lung apex, where lesions are
usual observed in MAC-LD, so treatment for bacterial pneumonia was
again administered. Meropenem at a dose of 1,000 mg/day was
selected as an antibiotic and the nivolumab treatment was
discontinued; however, the lung shadows were further exacerbated
after 4 weeks (Fig. 2C) and the
case was diagnosed as MAC-LD. The standard treatment for MAC-LD is
clarithromycin 500 mg twice per day, rifampicin 10 mg/kg per day
and ethambutol 15 mg/kg per day (12). Since the patient weighed 45 kg, the
standard doses for rifampicin and ethambutol were 450 and 675
mg/day, respectively. However, as the patient was at an advanced
age and suffered from gastric cancer, a decision was made to start
with a dose lower than the standard dose of each drug, and
treatment with all three drugs was initiated: Clarithromycin 600
mg/day, rifampicin 300 mg/day and ethambutol 500 mg/day. Since it
became difficult to continue treatment due to severe nausea as a
side effect of the treatment, the medication schedule was changed
from daily to 3 times per week, after which time the nausea
markedly improved and the patient was able to continue receiving
treatment. At 3 months after the start of medication, the lung
shadows improved (Fig. 2D), and at
7 months after the start of treatment the sputum cultures became
negative for acid-fast bacilli. Although resumption of nivolumab
was considered, the patient declined treatment. He is currently
being followed up and is on vonoprazan fumarate (20 mg/day), but
receives no chemotherapy for gastric cancer. An abdominal CT
examination in August 2021 revealed thickening of the gastric wall,
raising the suspicion of disease recurrence. The date of the last
follow-up visit was 19 October 2021, at which time no infiltrative
shadow in the lung was detected.
 | Table ILaboratory data on admission. |
Table I
Laboratory data on admission.
| Laboratory
parameters | Values (normal
range) |
|---|
| Hematology | |
|
White blood
cell count, /µl | 5,040
(3,300-8,600) |
|
Neutrophils,
% | 72.8 (37-73) |
|
Lymphocytes,
% | 19.2 (20-55) |
|
Monocytes,
% | 5.0 (3-10) |
|
Eosinophils,
% | 2.8 (0-11) |
|
Basophils,
% | 0.2 (0-2) |
|
Red blood
cell count, x104/µl | 321 (435-555) |
|
Hemoglobin,
g/dl | 11.2 (13.7-16.8) |
|
Platelet
count, x104/µl | 22.5 (15.8-34.8) |
| Biochemistry | |
|
Total
protein, g/dl | 7.3 (6.6-8.1) |
|
Albumin,
g/dl | 3.1 (4.1-5.1) |
|
Total
bilirubin, mg/dl | 0.4 (0.4-1.5) |
|
Alkaline
phosphatase, IU/l | 447 (106-322) |
|
Aspartate
amino transferase, IU/l | 38 (13-30) |
|
Alanine
aminotransferase, IU/l | 25 (10-42) |
|
Lactate
dehydrogenase, IU/l | 147 (124-222) |
|
Creatinine
kinase, IU/l | 50 (59-248) |
|
Blood urine
nitrogen, mg/dl | 14.3 (8-20) |
|
Creatinine,
mg/dl | 0.79 (0.65-1.07) |
|
Na,
mEq/l | 134 (138-145) |
|
K,
mEq/l | 3.7 (3.6-4.8) |
|
Cl,
mEq/l | 101 (101-108) |
|
Ca,
mg/dl | 8.2 (8.8-10.1) |
|
Glucose,
mg/dl | 135 (73-109) |
| Serology | |
|
C-reactive
protein, mg/dl | 3.2 (0.0-0.3) |
| Tumor markers | |
|
Carcinoembryonic
antigen, ng/ml | 1.9 (0.0-5.0) |
|
Carbohydrate
antigen 19-9, U/ml | 11.0 (0.0-37.0) |
Discussion
We herein resent a case of MAC-LD that occurred
during ICI immunotherapy. In a previous report, the underlying
disease of the patients who developed MAC-LD during ICI
immunotherapy was lung cancer (11). In the present case, the underlying
malignant disease was gastric cancer, and no underlying lung
disease was found. This indicated that MAC-LD may develop during
ICI immunotherapy for various types of cancer, and is not limited
to only lung cancer.
In this case, the clinical course was more acute
than the usual course experienced in MAC-LD (13). The exact mechanism of MAC-LD is
unknown, but anti-PD-1/PD-L1 antibodies are known to have potential
antibacterial effects mediated by the upregulation of
T-cell-mediated immunity (14). In
fact, there is a case report in which the coexisting
Mycobacterium abscessus lung disease improved when nivolumab
was used to treat lung cancer (15). On the other hand, some cases of
rapid-onset Mycobacterium tuberculosis (MTB) infection
occurring during ICI immunotherapy have been reported (1,8-10).
In MTB, inhibition of the PD-1/PD-L1 pathway causes the
upregulation of interferon-g production (16). While this mechanism could be
expected to suppress MTB, it may also potentially lead to a
hypersensitivity reaction to microorganisms that is similar to
immune reconstitution inflammatory syndrome due to the inhibition
of the PD-1/PD-L1 pathway (8).
Indeed, transbronchial pulmonary biopsy specimens from patients who
developed MTB during ICI immunotherapy have been reported to
exhibit diffuse lymphocyte infiltration within the specimens
(8). Another hypothesis attributes
MTB infection to immune checkpoint-related lymphopenia (9); however, our patient did not develop
lymphopenia during the clinical course. Therefore, it was inferred
that MAC-LD may have developed rapidly due to a hypersensitivity
reaction. In addition, the time period from the start of ICI
treatment to the onset of MAC-LD was 20 months. In three previously
reported cases (11), this time
period was also between 17 and 19 months. This indicates that
attention should be paid to long-term MAC-LD complications during
ICI immunotherapy. Interestingly, all three previously reported
cases (11) had a history of
radiation therapy. In the present case, part of the lower left lobe
was included in the radiation field of the radiation therapy for
gastric cancer. It is unclear to what extent a medical history of
radiation is implicated in the development of MAC-LD during ICI
treatment, and further case reports are needed to compare findings
and outcomes.
In patients with non-cavitary nodular/bronchiectatic
macrolide-susceptible MAC-LD, a thrice-weekly macrolide-based
regimen is recommended rather than a daily macrolide-based regimen
(12). In the present case, daily
therapy was first selected due to the acute onset during gastric
cancer treatment. Owing to severe treatment-related nausea, the
patient was switched to a thrice-weekly regimen, without
compromised effectiveness. MAC treatment may be difficult to
continue due to various factors, such as old age and the presence
of cancer or other comorbidities. Intermittent therapy may also be
effective, even in cases such as the present, in which MAC-LD
occurred during ICI immunotherapy.
In conclusion, we herein present a case of MAC-LD
that occurred in a patient with gastric cancer receiving ICI
immunotherapy. It is important for clinicians to include MAC-LD as
one of the differential diseases for pneumonia during ICI
immunotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY was involved in manuscript writing and
acquisition of data. OT wrote the manuscript. YT and KS treated the
patient for gastric cancer. SO, KY, EK and YI were involved in the
treatment of the patient and interpreted the CT imaging and the
laboratory test results. YY, OT and KA confirm the authenticity of
all the raw data. All the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the clinical data and any associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Picchi H, Mateus C, Chouaid C, Besse B,
Marabelle A, Michot JM, Champiat S, Voisin AL and Lambotte O:
Infectious complications associated with the use of immune
checkpoint inhibitors in oncology: Reactivation of tuberculosis
after anti PD-1 treatment. Clin Microbiol Infect. 24:216–218.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sunshine J and Taube JM: PD-1/PD-L1
inhibitors. Curr Opin Pharmacol. 23:32–38. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Michot JM, Bigenwald C, Champiat S,
Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A,
Bahleda R, Hollebecque A, et al: Immune-related adverse events with
immune checkpoint blockade: A comprehensive review. Eur J Cancer.
54:139–148. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fujita K, Kim YH, Kanai O, Yoshida H, Mio
T and Hirai T: Emerging concerns of infectious diseases in lung
cancer patients receiving immune checkpoint inhibitor therapy.
Respir Med. 146:66–70. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fujita K, Terashima T and Mio T: Anti-PD1
antibody treatment and the development of acute pulmonary
tuberculosis. J Thorac Oncol. 11:2238–2240. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chu YC, Fang KC, Chen HC, Yeh YC, Tseng
CE, Chou TY and Lai CL: Pericardial tamponade caused by a
hypersensitivity response to tuberculosis reactivation after
anti-PD-1 treatment in a patient with advanced pulmonary
adenocarcinoma. J Thorac Oncol. 12:e111–e114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Takata S, Koh G, Han Y, Yoshida H,
Shiroyama T, Takada H, Masuhiro K, Nasu S, Morita S, Tanaka A, et
al: Paradoxical response in a patient with non-small cell lung
cancer who received nivolumab followed by anti-Mycobacterium
tuberculosis agents. J Infect Chemother. 25:54–58.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fujita K, Yamamoto Y, Kanai O, Okamura M,
Nakatani K and Mio T: Development of Mycobacterium avium
complex lung disease in patients with lung cancer on immune
checkpoint inhibitors. Open Forum Infect Dis.
7(ofaa067)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Daley CL, Iaccarino JM, Lange C, Cambau E,
Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE,
Guglielmetti L, et al: Treatment of nontuberculous mycobacterial
pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical
practice guideline: Executive Summary. Clin Infect Dis. 71:e1–e36.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Daley CL: Mycobacterium avium
complex disease. Microbiol Spectr 5, 2017.
|
|
14
|
Rao M, Valentini D, Dodoo E, Zumla A and
Maeurer M: Anti-PD-1/PD-L1 therapy for infectious diseases:
Learning from the cancer paradigm. Int J Infect Dis. 56:221–228.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ishii S, Tamiya A, Taniguchi Y, Tanaka T,
Abe Y, Isa SI, Tsuyuguchi K, Suzuki K and Atagi S: Improvement of
Mycobacterium abscessus pulmonary disease after nivolumab
administration in a patient with advanced non-small cell lung
cancer. Intern Med. 57:3625–3629. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jurado JO, Alvarez IB, Pasquinelli V,
Martínez GJ, Quiroga MF, Abbate E, Musella RM, Chuluyan HE and
García VE: Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway
inhibits T cell effector functions during human tuberculosis. J
Immunol. 181:116–125. 2008.PubMed/NCBI View Article : Google Scholar
|