Introduction
Neoadjuvant chemotherapy (NAC) is a well-established
treatment for early breast cancer. NAC allows for breast conserving
surgery by reducing the size of the tumor and provides an
evaluation of real time sensitivity to therapy. This is very
essential in determining the effectiveness of treatment.
Preoperative assessment via imaging is important for surgical
planning. It is also necessary to understand the characteristics of
each imaging modality for diagnosis.
Dynamic contrast enhanced magnetic resonance imaging
(MRI) has been widely used in breast cancer screening, determining
the extent of disease, monitoring response to NAC, evaluating for
rupture or cancer detection in patients with implants because of
its high spatial resolution. 18F-fluorodeoxyglucose
positron-emission tomography/computed tomography (FDG PET/CT) has
also been used in whole-body examination, assessing distant
metastasis during initial staging and later surveillance. However,
MRI with the breast extended in the supine position are more useful
for diagnosing the condition inside the breast in detail.
In predicting pathological complete response (pCR)
to NAC, MRI of the breast is more accurate compared with other
imaging modalities, such as mammography (MG) and ultrasound (US)
(1-5).
FDG PET/CT is known to be an accurate imaging modality for response
evaluation of NAC in breast cancer (6-8).
Recently, PET/MRI, which can obtain images by combining metabolic
analysis with PET and morphologic and vascularity analysis with
contrast enhanced MRI simultaneously, has attracted attention as a
new imaging modality. While several studies have reported the use
of PET/MRI in breast cancer (9-11),
the present study aimed to evaluate the efficacy of PET/MRI in the
assessment of NAC.
Materials and methods
Patient selection and NAC regimen
This study protocol was approved by the local
institutional review board, and written informed consent was
obtained from all patients. Patients were not required to give
informed consent to this study because the analysis used anonymous
clinical data and images obtained after each patient agreed to
treatment by written consent. We also applied the opt-out method to
obtain consent for this study.
A total of 74 patients with stage II-III invasive
breast cancer treated with NAC and surgery from September 2016 to
March 2019 were enrolled, and the data were analyzed
retrospectively. All patients underwent preoperative imaging
evaluation with PET/MRI, MG, and US. Prior to NAC, 59 patients also
underwent the same examinations before NAC while 15 patients
underwent MRI, MG, and US. All the patients received anthracycline
followed by a taxane regimen, except for two patients who received
a platinum regimen followed by a taxane regimen. In addition, all
patients with human epidermal growth factor receptor 2 (HER2)
positive disease were treated with trastuzumab, and one of them was
also treated with pertuzumab.
Imaging assessment through mammography
and digital breast tomosynthesis
Clinical image data were acquired in the
mediolateral-oblique and cranio-caudal positions using an a-Se
full-field digital mammography (FFDM) system with a spatial
resolution of 85 µm (MAMMOMAT Inspiration, Siemens). In 63 patients
(85.1%), two-view digital breast tomosynthesis (DBT) was performed
with the same compression angle and compression pressure as the
FFDM. With one-view DBT, the radiation dose was 1.5 times of that
with one-view FFDM. The radiation dose with ACR 156 was 1.2 mGy
with FFDM. FFDM and reconstructed 1mm slice images from DBT were
reviewed at a dedicated workstation. Complete response (CR) was
defined as undetectable lesions or disappearance of density after
NAC. Cases with only residual calcification were also defined as CR
in this study.
Imaging assessment through
ultrasound
Whole-breast US was performed with an 8 MHz
wide-band high-resolution transducer (aplio™ XV, Toshiba Medical
Systems). The longest diameter of the hypoechoic part of the lesion
was measured. Undetectable lesions were defined as CR.
Imaging assessment through
PET/MRI
The images of PET/MRI that we evaluated in this
study were organized from whole body PET/MRI images and breast
PET/MRI images. Patients were instructed to fast for at least 4 h
before the scan. Blood glucose levels were required to be <350
mg/dl.
Whole body PET/MRI
Whole body PET/MRI images were obtained using a PET
scanner with 3T MRI (SIGNA; GE Healthcare). All PET images were
acquired after intravenous injection of body weight-adapted
18F-FDG doses (4 MBq/kg) followed by a resting period of
55-65 min in a supine position as the early phase. The data were
recorded in 5-6 bed positions, for 2 min per bed position, and 2.8
mm imaging slices were obtained. The display field of view (DFOV)
and matrix size were 50x35 cm and 256x256, respectively. Images
were reconstructed using the time-of-flight method (VUE Point) with
four iterations and 32 subsets.
MR-based sequence for attenuation correction of PET
images was performed using T1 weighted image (T1WI) with axial
3D-spoiled gradient echo (SPGR) sequence (LAVA-FLEX: FA/TR/TE: 5
degree/4 ms/1.7 ms, FOV: 50x50 cm, matrix size: 256x128, slice
thickness: 5.2 mm). Regarding whole body MRI, T1WI with axial
3D-SPGR sequence (LAVA: FA/TR/TE: 12 degree/5400 ms/2.6 ms, FOV:
50x37.5 cm, matrix size: 512x512, slice thickness: 2 mm) and T2
weighted image (T2WI) with single shot fast spin echo (SSFSE:
FA/TR/TE: 90 degree/1600 ms/80 ms, FOV: 50x37.5 cm, matrix size:
512x512, slice thickness: 6 mm) without fat suppression were
obtained.
Breast PET/MRI
Late phase breast PET images were obtained 75-95 min
after patients were given injections in a prone position. The DFOV
and matrix size were 30 cm and 256x256. The images were
reconstructed using the time-of-flight method (VUE Point) with four
iterations and 32 subsets. The data were acquired for 10 min in a
single bed position (89 slices, 249 mm). Breast MRI was performed
utilizing the 8-channel breast coil with fat suppression methods.
The sequences consisted of axial T2WI (FSE-XL and IDEAL; FOV:
320x320, FA/TR/TE: 111 degree/5400 ms/80 ms, matrix size: 224x320,
bandwidth: 90 kHz, slice thickness: 4 mm), axial DWI (EPI and
CHESS; FOV: 320x320, FA/TR/TE: 249 degree/6300 ms/67 ms, matrix
size: 96x128, bandwidth: 250 kHz, slice thickness: 4 mm). Dynamic
contrast-enhanced axial T1WI consisted of pre-contrast and three
post-contrast enhanced phases (90, 180, and 270 sec) of T1WI
(VIBRANT and CHESS; FOV: 320x320, FA/TR/TE: 12 degree/5.3 ms/2.4
ms, matrix size: 400x400, bandwidth: 83 kHz, slice thickness: 3
mm). Moreover, early contrast at 30 sec after contrasted images
(VIBRANT and CHESS; FOV: 320x320, FA/TR/TE: 12 degree/5.3 ms/2.1
ms, matrix size: 224x320, bandwidth: 90 kHz, slice thickness: 3 mm)
were obtained. Meglumine gadoterate (Magnescope, Guerbet) was used
as contrast agent, and it was automatically injected in the
antecubital vein at 0.2 ml/kg bodyweight.
The breast PET and MR images were evaluated
independently; the fusion images of early phase (90 sec) dynamic
contrast-enhanced T1WI and late phase PET were also assessed. The
frequency of fusion of the images was organized from PET by 30% and
MRI by 70%. The data were analyzed by experienced radiologists.
Undetectable MRI enhancements without meaningful
maximum standardized uptake values (SUVmax) of the tumor lesion of
PET were defined as CR. Significant FDG uptake (SUV values) was
defined as those values that were higher than that for whole body
average FDG uptake. The tumor shapes on early phase (90 sec)
dynamic contrast-enhanced MRI were classified into the ‘mass type’
and ‘non-mass type’. The relationships between tumor shapes or
molecular subtypes and pCR were also evaluated. The imaging data
and diagnoses were analyzed by two experienced radiological
specialists and breast surgeons with consensus.
Pathological assessment
Pathologic examination and immunohistochemistry were
performed by dedicated breast pathologists. The histologic type of
the tumor, tumor size, and histologic grade were determined from
formalin-fixed paraffin-embedded tumor sections cut at a thickness
of 4 µm and stained with hematoxylin and eosin. The status of
estrogen receptor (ER), progesterone receptor (PgR), and HER2 were
analyzed with specimens of core needle biopsy before starting NAC.
ER and PgR expressions were scored as positive or negative with a
nuclear immunostaining cut-off of 1%. ER and/or PgR positive tumors
were defined as hormone receptor (HR) positive. HER2 was considered
as positive if expression was scored at 3+ in immunohistochemistry
or if expression was scored at 2+ with HER2 gene
amplification by fluorescent in situ hybridization. pCR was
defined as the absence of residual invasive cancer cells in the
breast.
Statistical analysis
Cases with missing data were excluded. The
characteristics among patients with invasive breast cancer were
compared using Fisher's exact test. The sensitivity and specificity
for the prediction of NAC effect were calculated for each modality.
All data analyses were performed using Stata version 14 (Stata
Corp.). All tests were two-sided, and P-values <0.05 were
considered statistically significant.
Results
Patient and tumor characteristics
All patient and tumor characteristics are listed in
Table I. A total of 74 women with
stage II or III breast cancer were included in the study. Their
mean age was 48 years (range, 30-78 years). Most of the tumors
(95.9%) were invasive ductal carcinomas. There was one case of
invasive lobular carcinoma and two cases of metaplastic carcinomas.
Four tumors were classified as histological grade 1 and 69 tumors
as histological grade 2 or 3. Immunohistochemical examination
revealed that 30 patients had HR+/HER2-
disease, 16 had HR+/HER2+ disease, 10 had
HR-/HER2+ disease, and 18 had
HR-/HER2- disease. According to the MRIs
obtained before NAC, 27 (36.5%) of the tumors were ‘mass’ type
lesions and 40 (54.1%) were ‘non-mass’ type lesions.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient
characteristics | Total (n=74) |
HR+/HER2-
(n=30) |
HR+/HER2+
(n=16) |
HR-/HER2+
(n=10) |
HR-/HER2-
(n=18) | P-value |
|---|
| Age, years, median
(range) | 48 (30-78) | 44 (30-78) | 50 (37-69) | 58 (45-71) | 50 (33-78) | 0.14 |
| Stage prior to NAC, n
(%) | | | | | | 0.11 |
|
2 | 53 (71.6) | 21 (70.0) | 15 (93.8) | 6 (60.0) | 11 (61.1) | |
|
3 | 21 (28.4) | 9 (30.0) | 1 (6.3) | 4 (40.0) | 7 (38.9) | |
| Tumor-stage prior
to NAC, n (%) | | | | | | 0.58 |
|
T1 | 13 (17.6) | 5 (16.7) | 5 (31.3) | 1 (10.0) | 2 (11.1) | |
|
T2 | 48 (64.9) | 20 (66.7) | 10 (62.5) | 7 (70.0) | 11 (61.1) | |
|
T3 | 11 (14.9) | 5 (16.7) | 1 (6.3) | 1 (10.0) | 4 (22.2) | |
|
T4 | 2 (2.7) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 1 (5.6) | |
| Node-stage prior to
NAC, n (%) | | | | | | 0.61 |
|
N0 | 12 (16.2) | 3 (10.0) | 3 (18.8) | 2 (20.0) | 4 (22.2) | |
|
N1 | 51 (68.9) | 23 (76.7) | 12 (75.0) | 6 (60.0) | 10 (55.6) | |
|
N2 | 2 (2.7) | 1 (3.3) | 0 (0.0) | 1 (10.0) | 0 (0.0) | |
|
N3 | 9 (12.2) | 3 (10.0) | 1 (6.3) | 1 (10.0) | 4 (22.2) | |
| Type of lesion on
MRI, n (%) | | | | | | 0.58 |
|
Mass | 27 (36.5) | 9 (30.0) | 7 (43.8) | 3 (30.0) | 8 (44.4) | |
|
Non-mass | 40 (54.1) | 19 (63.3) | 9 (56.3) | 5 (50.0) | 7 (38.9) | |
|
NA | 7 (9.5) | 2 (6.7) | 0 (0.0) | 2 (20.0) | 3 (16.7) | |
| Histology, n
(%) | | | | | | 0.74 |
|
IDC | 71 (95.9) | 28 (93.3) | 16 (100.0) | 9 (90.0) | 18 (100.0) | |
|
ILC | 1 (1.4) | 1 (3.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
|
Metaplastic
carcinoma | 2 (2.7) | 1 (3.3) | 0 (0.0) | 1 (10.0) | 0 (0.0) | |
Identification by each modality
All the lesions were visually detectable using
PET/MRI and US. Two lesions were difficult to identify using MG;
these patients received only FFDM, and their data were excluded
from the assessment of CR using MG.
pCR rate and tumor
characteristics
Eighteen patients (24.3%) achieved pCR. HR negative
and HER2 positive cases demonstrated a significant correlation with
pCR compared with HR positive cases and triple negative cases
(P=0.017). Furthermore, patients with ‘mass’ type lesions who
underwent MRI before NAC experienced pCR at a higher frequency than
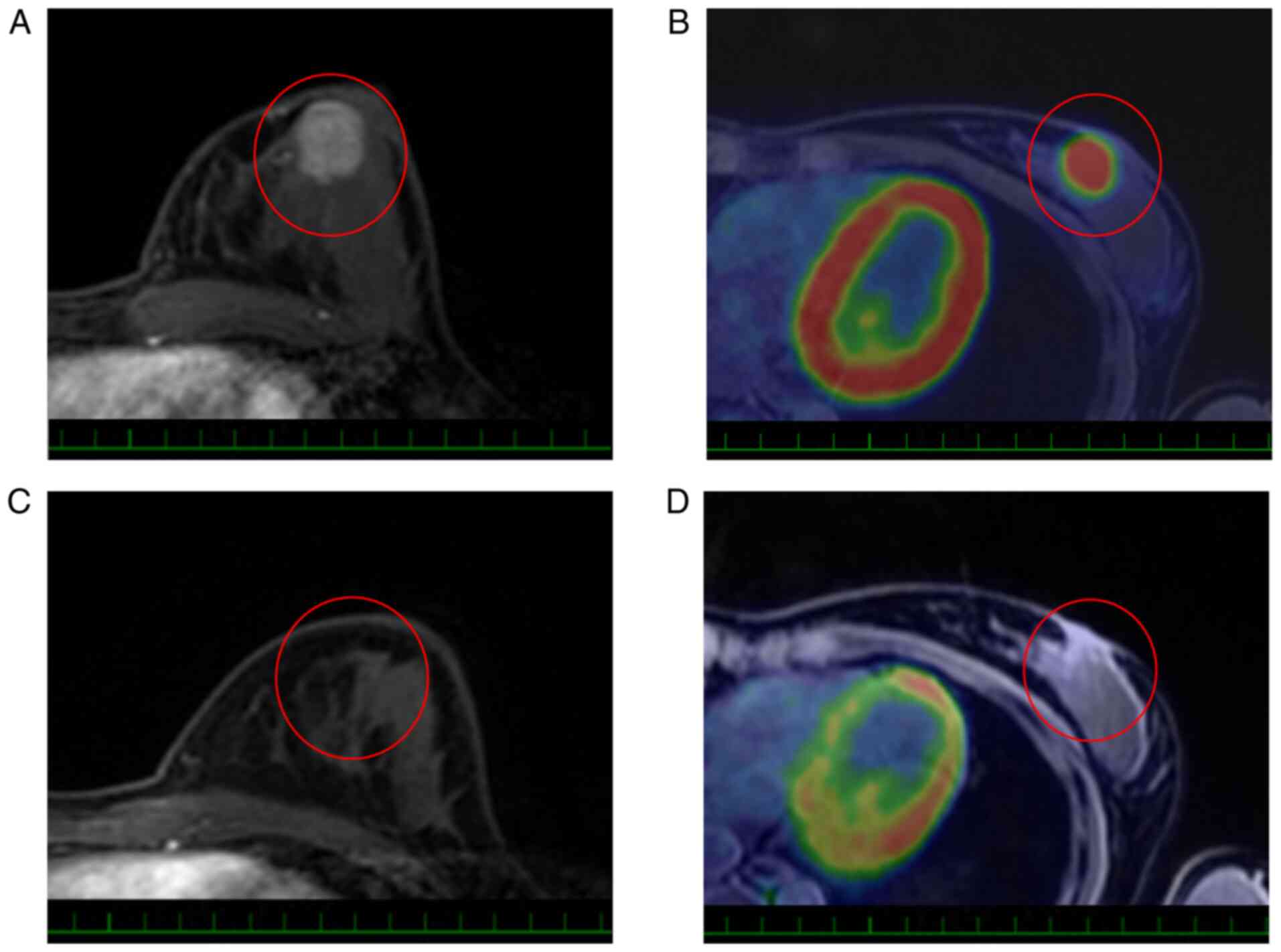
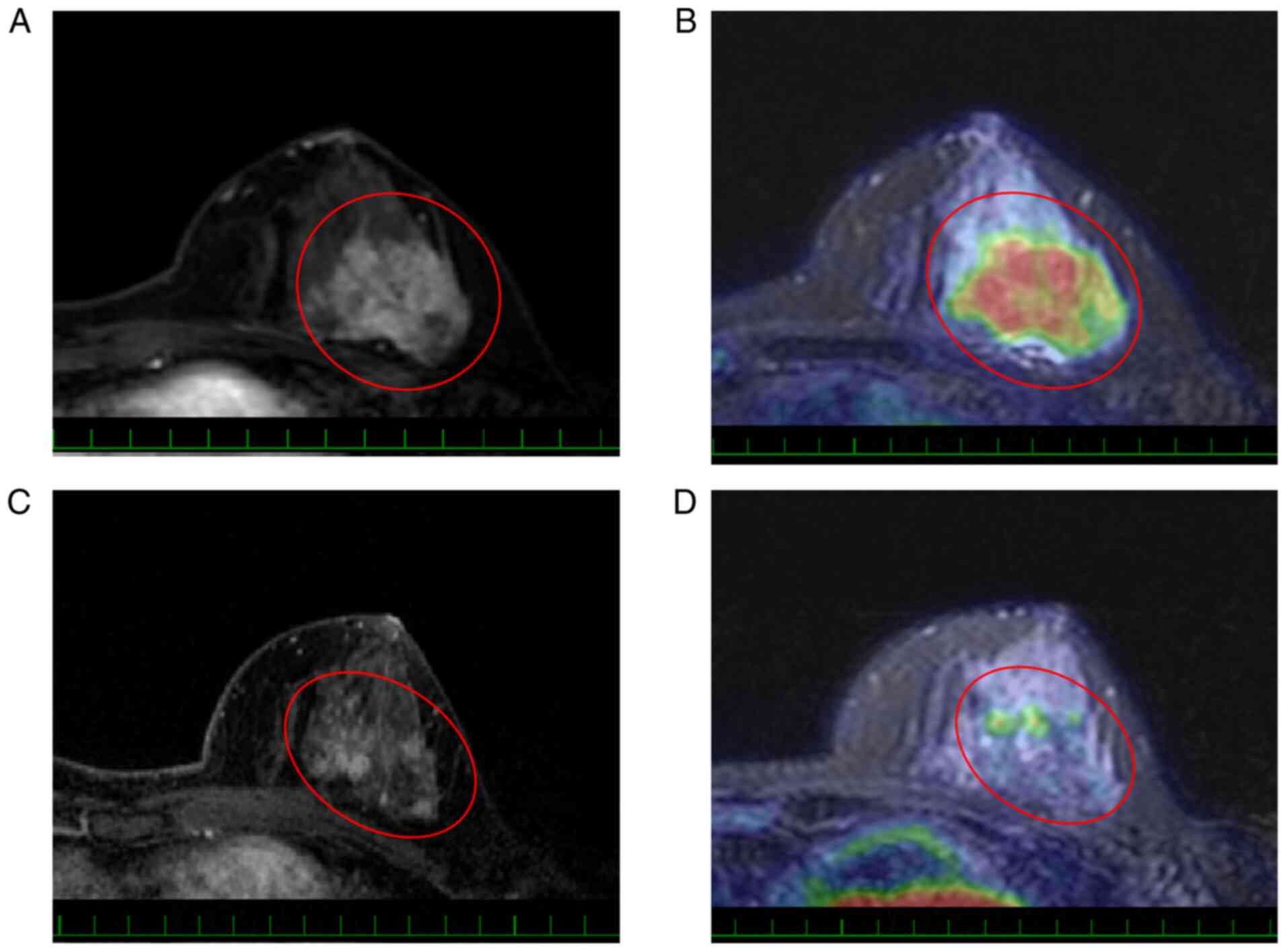
those with ‘non-mass’ type lesions (Table II). Typical responses to NAC are
shown in Figs. 1 and 2.
 | Table IIComparison of patients who achieved
pCR and non-pCR. |
Table II
Comparison of patients who achieved
pCR and non-pCR.
| Patient
characteristics | pCR (n=18) | Non-pCR (n=56) | P-value |
|---|
| Age, years, median
(range) | 49 (32-78) | 48 (30-78) | 0.79 |
| Stage, n (%) | | | 0.76 |
|
2 | 12 (66.7) | 41 (73.2) | |
|
3 | 6 (33.3) | 15 (26.8) | |
| Type of lesion, n
(%) | | | 0.02 |
|
Mass | 11 (61.1) | 16 (28.6) | |
|
Non-mass | 5 (27.8) | 35 (62.5) | |
|
NA | 2 (11.1) | 5 (8.9) | |
| Histology, n
(%) | | | 0.57 |
|
IDC | 17 (94.4) | 54 (96.4) | |
|
ILC | 0 (0.0) | 1 (1.8) | |
|
Metaplastic
carcinoma | 1 (5.6) | 1 (1.8) | |
| Subtype, n (%) | | | 0.02 |
|
HR+/HER2- | 3 (16.7) | 27 (48.2) | |
|
HR+/HER2+ | 4 (22.2) | 12 (21.4) | |
|
HR-/HER2+ | 6 (33.3) | 4 (7.1) | |
|
HR-/HER2- | 5 (27.8) | 13 (23.2) | |
pCR prediction with PET/MRI
The overall sensitivity and specificity of pCR
prediction with PET/MRI were 72.2 and 78.6%, respectively. Among
the 74 cases, 20 had undetectable enhancement on MRI and 51 cases
did not demonstrate substantial SUV uptake on PET after NAC.
Therefore, the accuracy of pCR prediction with PET/MRI depended
more on the MRI than on the PET. Among pCR patients, 13 (72.2%)
showed undetectable enhancement of MRI and 17 patients (94.4%)
showed lack of meaningful SUVmax.
Sensitivity and specificity of pCR
prediction with each modality based on receptor status
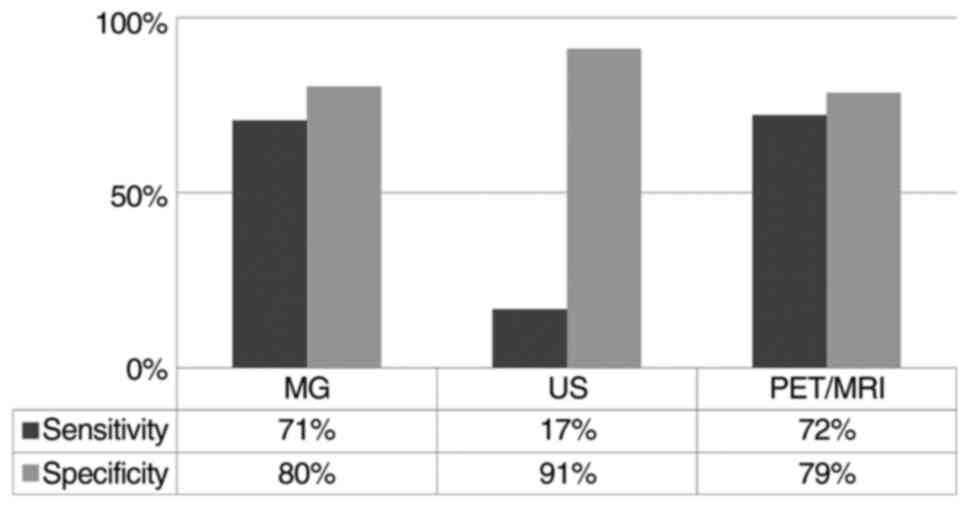
The overall sensitivity and specificity of MG and US
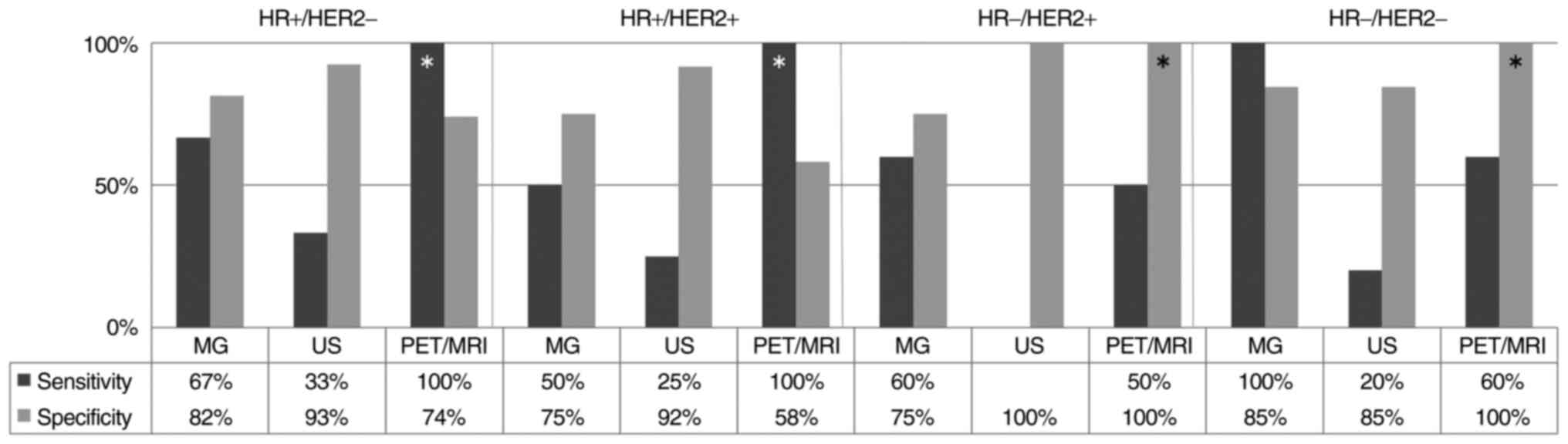
were 70.6 and 80.4%, and 16.7 and 91.1%, respectively (Fig. 3). In HR positive tumors, the
sensitivity of PET/MRI was 100%. In addition, in HR negative
tumors, the specificity of PET/MRI was 100% (Fig. 4). None of the HR negative and HER2
positive patients were predicted to have achieved pCR with US.
Discussion
NAC is a standard therapy for locally advanced
breast cancer as it allows tumor downstaging and facilitates breast
conserving surgery. In addition, some trials have been reported
wherein surgery was avoided in patients who achieved CR after NAC
(12-14).
Furthermore, the efficacy of six months of capecitabine
administration after surgery for non-pCR patients has also been
reported (15). At present, the
accurate assessment of the effect of NAC is more important for the
management of successful therapy than it was before.
MRI and PET/CT have been reported to be more
accurate modalities for predicting pCR than both MG and US
(16-19).
The sensitivity and specificity of the pCR prediction were reported
to be 65 and 88%, respectively, for MRI, and 86 and 72%,
respectively, for PET/CT (20).
PET/MRI is considered as a new modality that can make use of the
advantages of both PET and MRI. In this study, the utility of
PET/MRI for predicting pCR was assessed.
The overall sensitivity and specificity of PET/MRI
were found to be acceptable. In particular, this study found that
the sensitivity of PET/MRI in HR-positive tumors and the
specificity in HR negative tumors were excellent; HER2 status did
not affect the results. This meant that tumor disappearance was
easily identified in HR positive tumors while the residual tumor
was easily detected in HR negative tumors. This finding could be
attributed to the association between the HR status and the
morphological tumor features after NAC. HR positive tumors have an
infiltrative shrinkage pattern and lower cellularity, whereas HR
negative tumors are more likely to have a homogeneous tumor
composition with centripetal shrinkage pattern and have
significantly higher cancer cellularity than HR positive tumors
(21).
Previous studies showed that the pCR prediction
accuracy of MRI was higher in those with HR negative tumors than in
those with HR positive tumors (22,23).
Lee et al (21) explained
this distinction as the difference in sensitivity to NAC. We also
demonstrated that pCR was significantly more often observed among
HR negative patients. Hence, the difference in prediction accuracy
between this study and previous studies cannot be explained by the
therapy effect alone. This difference may be attributed to
improvements in PET and MRI accuracy or the technical difference
between PET/MRI and PET/CT. Although former studies operated with a
1.5T MRI systems, we used a 3.0T MRI system which has higher
spatial resolution. While CT attenuation correction was based on
the tissue density information provided by plain CT, MRI does not
rely on tissue density. MRI contributes to soft-tissue contrast and
vascularity information in detail. Dynamic contrast enhanced MRI
allows measurement of the kinetic parameters related to
permeability and perfusion. Moreover, advanced sequences, such as
diffusion or apparent diffusion coefficient, which can provide
helpful information, are available from MRI data. In addition,
compared to PET/CT, PET/MRI in the prone position can be
advantageous since clinicians can collect morphologic and metabolic
imaging information simultaneously, and this might contribute to
precision improvement.
When the response was analyzed according to the
tumor shape on MRI, mass-type lesion was significantly related to
pCR. Although HR-/HER2-tumors showed unifocal masses at the
baseline more often (24), this
cohort did not demonstrate obvious relationships between shapes on
MRI and tumor subtypes. One explanation for this is provided by Loo
et al (22), who showed
that the earlier reported correlation between subtype and
morphology of the tumor on MRI was valid for relatively large
tumors selected for NAC (22).
During NAC, metabolic reduction within a tumor
occurs much earlier than reduction in vascularity and shrinkage of
tumor volume (25). Similarly, in
our study, metabolic CR cases of PET were observed more frequently
than cases with disappearance of enhancement on MRI. Metabolic
analysis might investigate only the initial effect of NAC;
therefore, by integrating it with morphology and vascularity
analysis, a more accurate prediction may be possible.
In a previous assessment of each modality in pCR
prediction, the sensitivity and specificity of MG were reported to
be 48 and 81%, respectively (26).
The sensitivity of MG in the cohort of the current study was
improved compared with that in this previous report. Although there
are few studies on the efficacy of DBT in determining NAC effect,
previous studies have demonstrated that DBT was also a useful
modality like MRI (3,27). Consistent with these findings, our
result of pCR prediction with MG was appropriate because most of
the tumors were evaluated via DBT. In addition, the pCR criteria
for MG included residual microcalcification which explains the
optimum results obtained. We considered that microcalcification
without density on MG was from cancer treated previously, although
residual calcification could be due to both treated cancer and a
residual tumor. Therefore, DBT was more useful in detecting the
density around areas of calcification compared to conventional
MG.
In contrast, the sensitivity of US was insufficient.
Croshaw et al (26) also
reported low sensitivity (33%) and high specificity (90%) for US.
We found that US could detect fibrosis and edema that was
associated with complete response to NAC. When the US images of pCR
patients diagnosed as non-pCR were reviewed, all of the detected
lesions were described as hypoechoic areas and did not form mass
shapes. With careful observation using US, the specificity was
extremely good.
Although our study identified the usefulness of
PET/MRI in the prediction of response to NAC, it has several
limitations. First, we defined pCR as having no residual invasive
disease. Consequently, our results might not be relevant to cases
that do not include surgery as an option for patients with expected
pCR. Despite this, there is no evidence that residual in
situ carcinoma increases future distant relapse risk (28-31).
Second, this was a retrospective study with a small sample size.
Third, most of patients were treated with anthracycline-based
regimens. Therefore, our findings may not be applicable to patients
who received other regimens. Further large trials should be
performed to confirm the results of our study.
In conclusion, PET/MRI provides a different
diagnostic approach consisting of a multi-modality system. Although
the diagnostic accuracy of the responses to NAC was similar to
previous imaging modalities, under specific conditions, the
usefulness of PET/MRI was confirmed.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CS and NU conceived and designed the present study.
CS wrote the manuscript with support from NU, HK, TK and AS. NU,
HK, TK, CW, TM, SS, KJ, EI, ST and AS acquired imaging data. CW,
TM, SS, KJ, EI, ST, TK and AS analyzed and interpreted the patient
data regarding breast cancer and imaging features. KS and MY
performed the histological examination of the breast cancer
tissues. CS and NU confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study protocol was approved (approval no.
2017-278) by the local institutional review board of the National
Cancer Center (Tokyo, Japan). Written informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Balu-Maestro C, Chapellier C, Bleuse A,
Chanalet I, Chauvel C and Largillier R: Imaging in evaluation of
response to neoadjuvant breast cancer treatment benefits of MRI.
Breast Cancer Res Treat. 72:145–152. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Drew PJ, Kerin MJ, Mahapatra T, Malone C,
Monson JR, Turnbull LW and Fox JN: Evaluation of response to
neoadjuvant chemoradiotherapy for locally advanced breast cancer
with dynamic contrast-enhanced MRI of the breast. Eur J Surg Oncol.
27:617–620. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shin HJ, Kim HH, Ahn JH, Kim SB, Jung KH,
Gong G, Son BH and Ahn SH: Comparison of mammography, sonography,
MRI and clinical examination in patients with locally advanced or
inflammatory breast cancer who underwent neoadjuvant chemotherapy.
Br J Radiol. 84:612–620. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Atkins JJ, Appleton CM, Fisher CS, Gao F
and Margenthaler JA: Which imaging modality is superior for
prediction of response to neoadjuvant chemotherapy in patients with
triple negative breast cancer? J Oncol. 2013(964863)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dialani D, Chadashxili T and Slanetz P:
Role of imaging in neoadjuvant therapy for breast cancer. Ann Surg
Oncol. 22:1416–1424. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pengel KE, Koolen BB, Loo CE, Vogel WV,
Wesseling J, Lips EH, Rutgers EJ, Valdés Olmos RA, Vrancken Peeters
MJ, Rodenhuis S and Gilhuijs KG: Combined use of 18F-FDG PET/CT and
MRI for response monitoring of breast cancer during neoadjuvant
chemotherapy. Eur J Nucl Med Mol Imaging. 41:1515–524.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li H, Yao L, Jin P, Hu L, Li X, Guo T and
Yang K: MRI and PET/CT for evaluation of the pathological response
to neoadjuvant chemotherapy in breast cancer: A systematic review
and meta-analysis. Breast. 40:106–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pahk K, Kim S and Choe KG: Early
prediction of pathological complete response in luminal B type
neoadjuvant chemotherapy-treated breast cancer patients: Comparison
between interim 18F-FDG PET/CT and MRI. Nucl Med Commun.
36:887–891. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Melsaether A and Moy L: Breast PET/MR
imaging. Radiol Clin North Am. 55:579–589. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rice SL and Friedman KP: Clinical PET-MR
imaging in breast cancer and lung cancer. PET Clin. 11:387–402.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Catalano OA, Horn GL, Signore A, Iannace
C, Lepore M, Vangel M, Luongo A, Catalano M, Lehman C, Salvatore M,
et al: PET/MR in invasive ductal breast cancer: Correlation between
imaging markers and histological phenotype. Br J Cancer.
116:893–902. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Heys SD and Chaturvedi S: Primary
chemotherapy in breast cancer: The beginning of the end or the end
of the beginning for the surgical oncologist? World J Surg Oncol.
1(14)2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rea D, Tomlins A and Francis A: Time to
stop operating on breast cancer patients with pathological complete
response? Eur J Surg Oncol. 39:924–930. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mamounas EP: Impact of neoadjuvant
chemotherapy on locoregional surgical treatment of breast cancer.
Ann Surg Oncol. 22:1425–1433. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES,
Yokota I, Kuroi K, Im SA, Park BW, Kim SB, et al: Adjuvant
capecitabine for breast cancer after preoperative chemotherapy. N
Engl J Med. 376:2147–2159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
De Los Santos J, Bernreuter W, Keene K,
Krontiras H, Carpenter J, Bland K, Cantor A and Forero A: Accuracy
of breast magnetic resonance imaging in predicting pathologic
response in patients treated with neoadjuvant chemotherapy. Clin
Breast Cancer. 11:312–319. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Marinovich ML, Houssami N, Macaskill P,
Sardanelli F, Irwig L, Mamounas EP, von Minckwitz G, Brennan ME and
Ciatto S: Meta-analysis of magnetic resonance imaging in detecting
residual breast cancer after neoadjuvant therapy. J Natl Cancer
Inst. 105:321–333. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gu YL, Pan SM, Ren J, Yang ZX and Jiang
GQ: Role of magnetic resonance imaging in detection of pathologic
complete remission in breast cancer patients treated with
neoadjuvant chemotherapy: A meta-analysis. Clin Breast Cancer.
17:245–255. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Akimoto E, Kadoya T, Kajitani K, Emi A,
Shigematsu H, Ohara M, Masumoto N and Okada M: Role of
18F-PET/CT in predicting prognosis of patients with
breast cancer after neoadjuvant chemotherapy. Clin Breast Cancer.
1:45–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu Q, Wang C, Li P, Liu J, Huang G and
Song S: The role of (18)F-FDG PET/CT and MRI in assessing
pathological complete response to neoadjuvant chemotherapy in
patients with breast cancer: A systematic review and meta-analysis.
Biomed Res Int. 2016(3746232)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee HJ, Song IH, Seo AN, Lim B, Kim JY,
Lee JJ, Park IA, Shin J, Yu JH, Ahn JH and Gong G: Correlations
between molecular subtypes and pathologic response patterns of
breast cancers after neoadjuvant chemotherapy. Ann Sug Oncol.
22:392–400. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Loo CE, Straver ME, Rodenhuis S, Muller
SH, Wesseling J, Vrancken Peeters MJ and Gilhuijs KG: Magnetic
resonance imaging response monitoring of breast cancer during
neoadjuvant chemotherapy: Relevance of breast cancer subtype. J
Clin Oncol. 29:660–666. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Koolen BB, Pengel KE, Wesseling J, Vogel
WV, Vrancken Peeters MJ, Vincent AD, Gilhuijs KG, Rodenhuis S,
Rutgers EJ and Valdés Olmos RA: FDG PET/CT during neoadjuvant
chemotherapy may predict response in ER-positive/HER2-negative and
triple negative, but not in HER2-positive breast cancer. Breast.
22:691–697. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tsunoda-Shimizu H, Hayashi N, Hamaoka T,
Kawasaki T, Tsugawa K, Yagata H, Kikuchi M, Suzuki K and Nakamura
S: Determining the morphological features of breast cancer and
predicting the effects of neoadjuvant chemotherapy via diagnostic
breast imaging. Breast Cancer. 15:133–140. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim TH, Yoon JK, Kang DK, Kang SY, Jung
YS, Han S, Kim JY, Yim H and An YS: Value of volume-based metabolic
parameters for predicting survival in breast cancer patients
treated with neoadjuvant chemotherapy. Medicine (Baltimore).
95(e4605)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Croshaw R, Shapira-Wright H, Svensson E,
Erb K and Julian T: Accuracy of clinical examination, digital
mammogram, ultrasound, and MRI in determining postneoadjuvant
pathologic tumor response in operable breast cancer patients. Ann
Surg Oncol. 18:3160–3163. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Uchiyama N, Kinoshita T, Hojo T, Asaga S,
Suzuki J, Kawawa Y and Otsuka K: Usefulness of adjunction of
digital breast tomosynthesis (DBT) to full-field digital
mammography (FFDM) in evaluation of pathological response after
neoadjuvant chemotherapy (NAC) for breast cancer. Breast Imaging.
7361:354–361. 2012.
|
|
28
|
Jones RL, Lakhani SR, Ring AE, Ashley S,
Walsh G and Smith IE: Pathological complete response and residual
DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J
Cancer. 94:358–362. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kaufmann M, Hortobagyi GN, Goldhirsch A,
Scholl S, Makris A, Valagussa P, Blohmer JU, Eiermann W, Jackesz R,
Jonat W, et al: Recommendations from an international expert panel
on the use of neoadjuvant (primary) systemic treatment of operable
breast cancer: An update. J Clin Oncol. 24:1940–1949.
2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mazouni C, Peintinger F, Wan-Kau S, Andre
F, Gonzalez-Angulo AM, Symmans WF, Meric-Bernstam F, Valero V,
Hortobagyi GN and Pusztai L: Residual ductal carcinoma in situ in
patients with complete eradication of invasive breast cancer after
neoadjuvant chemotherapy does not adversely affect patient outcome.
J Clin Oncol. 25:2650–2655. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Symmans WF, Peintinger F, Hatzis C, Rajan
R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, et
al: Measurement of residual breast cancer burden to predict
survival after neoadjuvant chemotherapy. J Clin Oncol.
25:4414–4422. 2007.PubMed/NCBI View Article : Google Scholar
|