Introduction
Undifferentiated small round cell sarcoma is a newly
classified category of bone and soft tissue sarcoma according to
the 2020 World Health Organization classification (1). This newly defined category includes
Ewing sarcoma and small round cell sarcoma, previously known as
Ewing-like sarcoma. Ewing sarcoma is the most well-known, with the
characteristic chromosomal translocation abnormality t(11;22)
(q24;q12) causing fusion of the EWS RNA-binding protein 1
(EWSR1) and Friend leukemia virus integration site 1
(FLI1) genes (2). So-called
‘Ewing-like sarcoma’ is also an aggressive sarcoma comprising small
round tumor cells arising from bone and soft tissue. In terms of
histopathological features, Ewing-like sarcoma is morphologically
similar to Ewing sarcoma, but lacks the classical fusion of
EWSR1 and erythroblast transformation-specific (ETS)
family genes, such as FLI1 (1). According to molecular analyses over
the last two decades, undifferentiated small round cell sarcoma
(Ewing-like sarcoma) has been recognized to exhibit three different
genetic features: capicua transcriptional repressor
(CIC)-rearranged sarcoma (3); BCL6 corepressor
(BCOR)-rearranged sarcoma (4); and round cell sarcoma with
EWSR1-non-ETS fusion (5).
Of the undifferentiated small round cell sarcomas without
EWSR1-ETS gene fusion, CIC-rearrangement sarcomas
account for the majority, at 60-70% (6). Molecular findings support these
sarcoma subtypes being biologically distinct from Ewing
sarcoma.
BCOR-rearrangement sarcoma was first
identified by Pierron et al in 2012 among 594 cases of
undifferentiated round cell sarcoma that morphologically resembled
Ewing sarcoma, but lacked the canonical EWSR1-ETS
translocation (4). RNA sequencing
and subsequent reverse transcription-polymerase chain reaction
(RT-PCR) demonstrated a novel BCOR-cyclin B3 (CCNB3)
fusion gene in 24 tumors (4%), resulting from a chromosome-X
paracentric inversion (4). This
inversion causes an in-frame fusion between exon 15 of BCOR
and exon 5 of CCNB3. BCOR-CCNB3 fusion is the most
frequent fusion seen among BCOR-rearrangement sarcomas,
accounting for ~60% (7).
BCOR-rearrangement sarcoma occurs slightly more often in
bone than in soft tissue, at a ratio of 1.5:1(4). Other fusion partners of BCOR
have recently been identified, namely mastermind-like
transcriptional coactivator 3 (MAML3), zinc finger CCCH
domain-containing protein 7B (ZC3H7B), and internal tandem
duplications (ITD) (8,9).
BCOR-MAML3 and ZC3H7B-BCOR fusion sarcomas have been
reported in a small number of tumors arising in soft tissue
(8). BCOR ITD has been
reported in a subgroup of soft-tissue undifferentiated round cell
sarcomas occurring in infants (9).
BCOR-rearrangement sarcoma of bone is gaining
widespread recognition among pathologists, but remains less
recognized by clinical orthopedic surgeons than osteosarcoma or
Ewing sarcoma. We present herein an additional case of
BCOR-CCNB3 sarcoma of the proximal tibia, and review the
relevant literature on BCOR-CCNB3 sarcoma of bone. Our
review of the literature focused on the clinical characteristics,
histopathology, and prognosis of BCOR-CCNB3 sarcoma arising
in bone.
Case presentation
This report was approved by the ethics committee at
Toyama University Hospital (Toyama, Japan), and the patient and his
parents provided written, informed consent for publication of this
report. A 12-year-old boy presented with a 6-month history of knee
pain on flexion of the knee joint and a slowly growing mass in the
anteromedial aspect of the proximal left tibia. There was no
special mention of medical history or his family history. Physical
examination revealed an elastic hard mass without tenderness or
redness, with mild warmth. Despite a lack of limitations to motion
of the knee joint, he experienced pain on full flexion of the knee
joint. Laboratory tests revealed regular leukocyte counts
(6,250/µl). The C-reactive protein level was not elevated at 0.02
mg/dl. Although the normal range of alkaline phosphatase (ALP) in
children is wide, the value of ALP was slightly high at 1,298 U/l.
Other biochemical blood test was normal. Plain radiographs of the
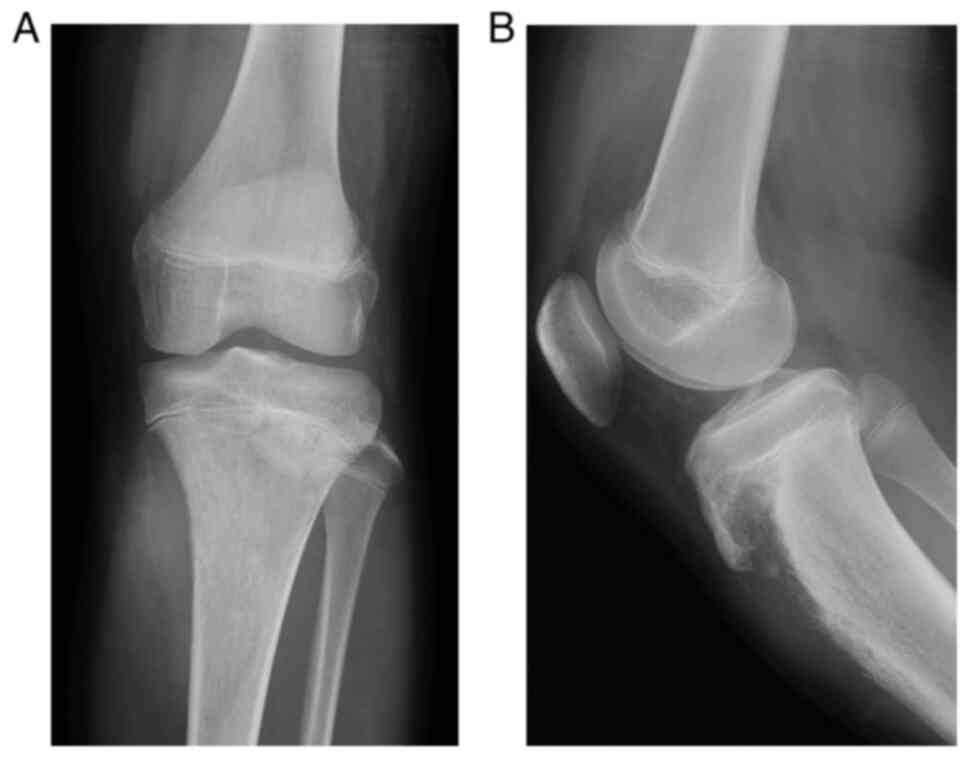
knee demonstrated an extraskeletal mass in the soft tissue at the
medial aspect of the proximal tibia on anteroposterior view and a
lytic lesion with cortical destruction of the proximal tibia on
lateral view (Fig. 1). Computed
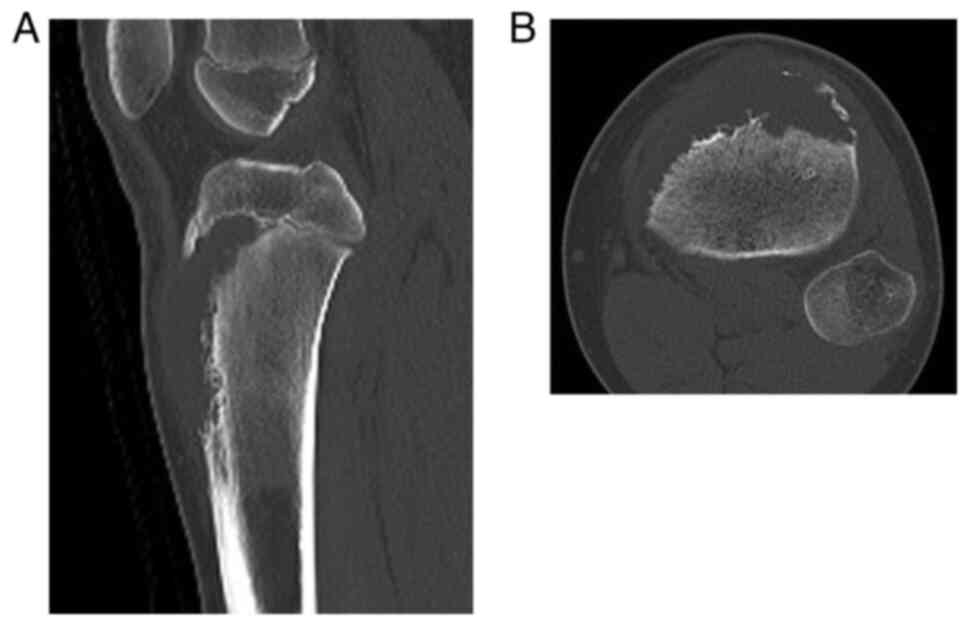
tomography (CT) confirmed a lytic bone tumor with periosteal
reaction in the proximal metaphysis of the tibia and a soft tissue
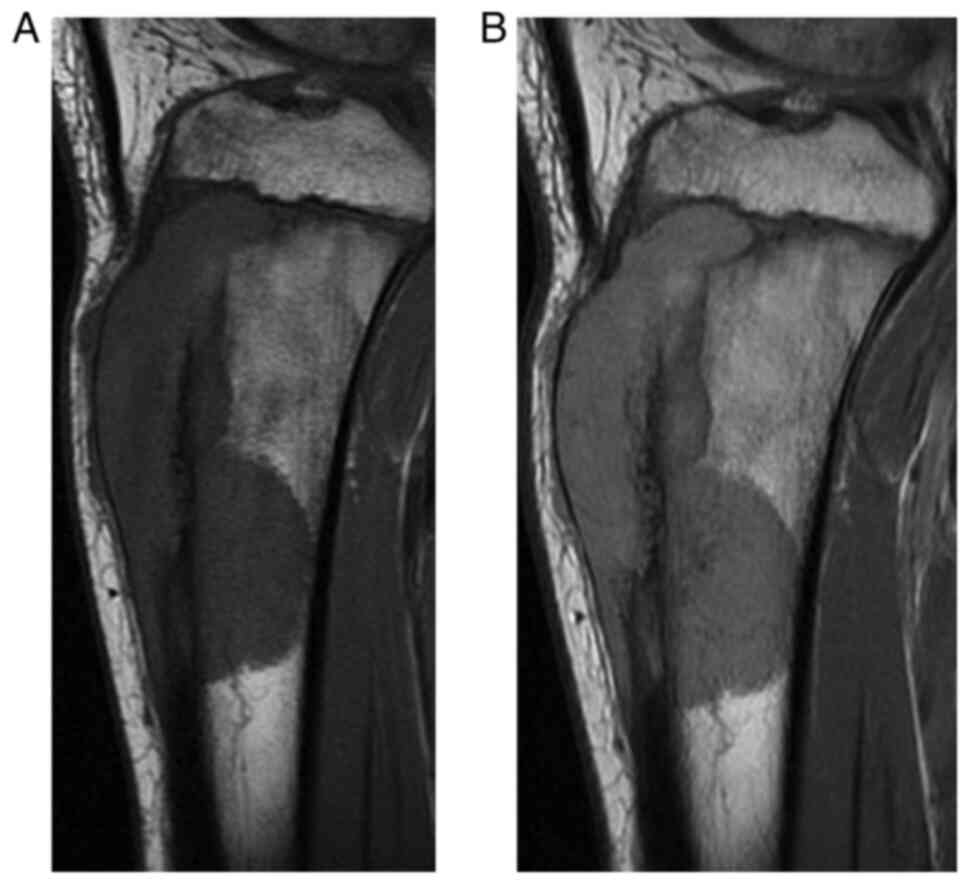
tumor with extraosseous soft tissue extension (Fig. 2). Magnetic resonance imaging (MRI)
showed the bone tumor expanding into soft tissue with almost
homogeneous hypointensity on T1-weighted imaging and slight
hyperintensity on T2-weighted imaging compared to muscle (Fig. 3). On
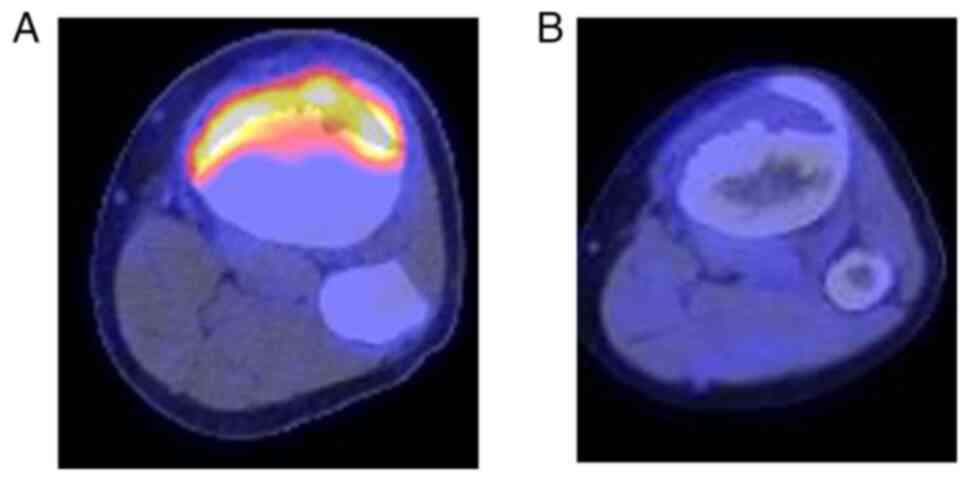
18F-fluoro-2-deoxyglucose positron emission tomography
(FDG-PET)/CT, accumulation of FDG was seen only in the bone tumor
of the proximal tibia, with a maximum standardized uptake value
(SUV) of 8.6 and no distant metastases. Based on these findings
from images, primary malignant bone tumor was highly suspected.
Differential diagnoses included osteosarcoma, Ewing sarcoma, and
malignant lymphoma. Open biopsy was performed to determine the
histopathological diagnosis.
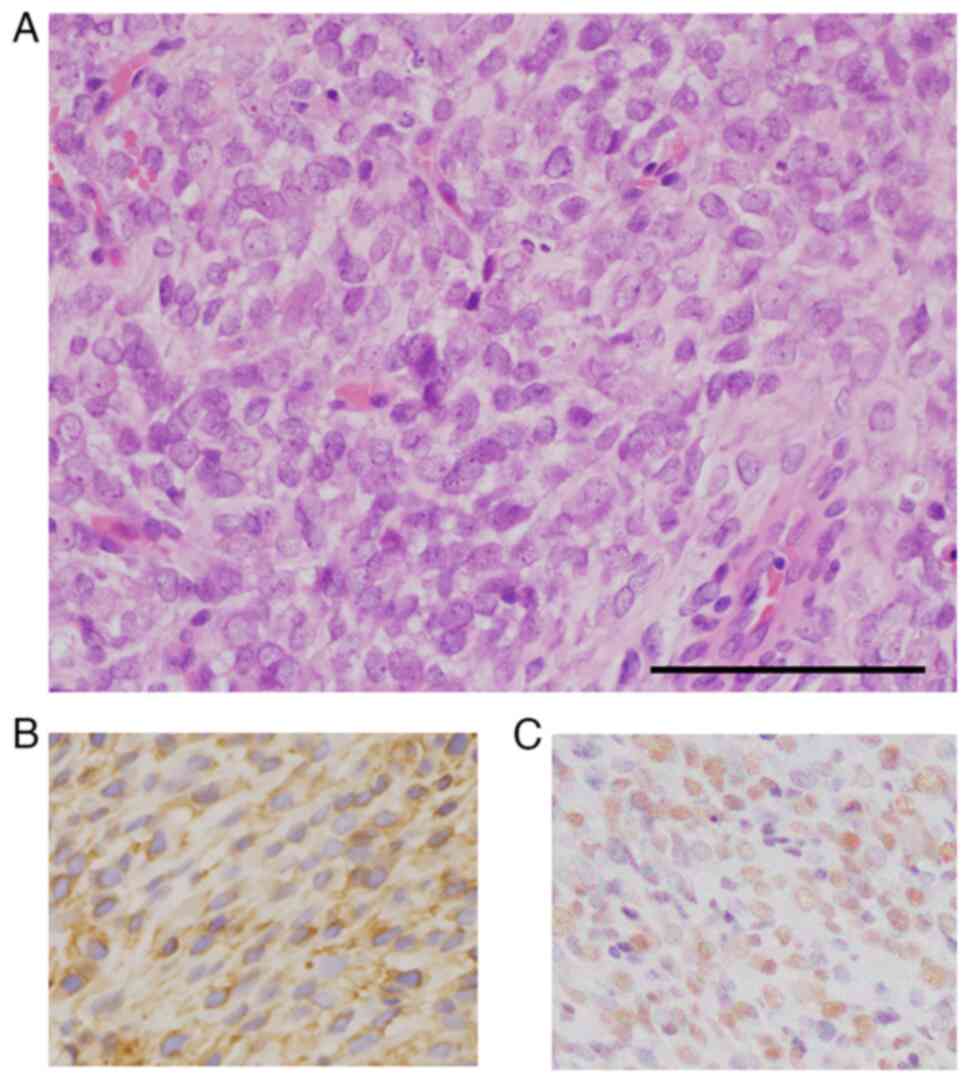
On histopathological evaluation, the tumor comprised
proliferation of the small, round to ovoid-shaped mesenchymal cells
without osteoid formation (Fig.
4A). The nuclei of some tumor cells appeared hyperchromatic
with finely dispersed chromatin. Immunohistochemically, tumor cells
were completely negative for AE1/AE3, S-100 protein, STAT6, CD34,
c-Myc, CD117 and alpha-smooth muscle actin. CD99 (clone 12E7, DAKO,
1:100) expression revealed weak membranous immunostaining (Fig. 4B), and CCNB3 (clone HPA000496;
Sigma-Aldrich, 1:200) expressed strong and diffuse nuclear
positivity (Fig. 4C). Considering
the histopathological findings, the pathological diagnosis favored
Ewing-like sarcoma rather than Ewing sarcoma. Additional molecular
examination by RT-PCR were performed using formalin-fixed
paraffin-embedded tissues. RT-PCR detected a BCOR-CCNB3
fusion transcript rather than CIC-double homeobox 4
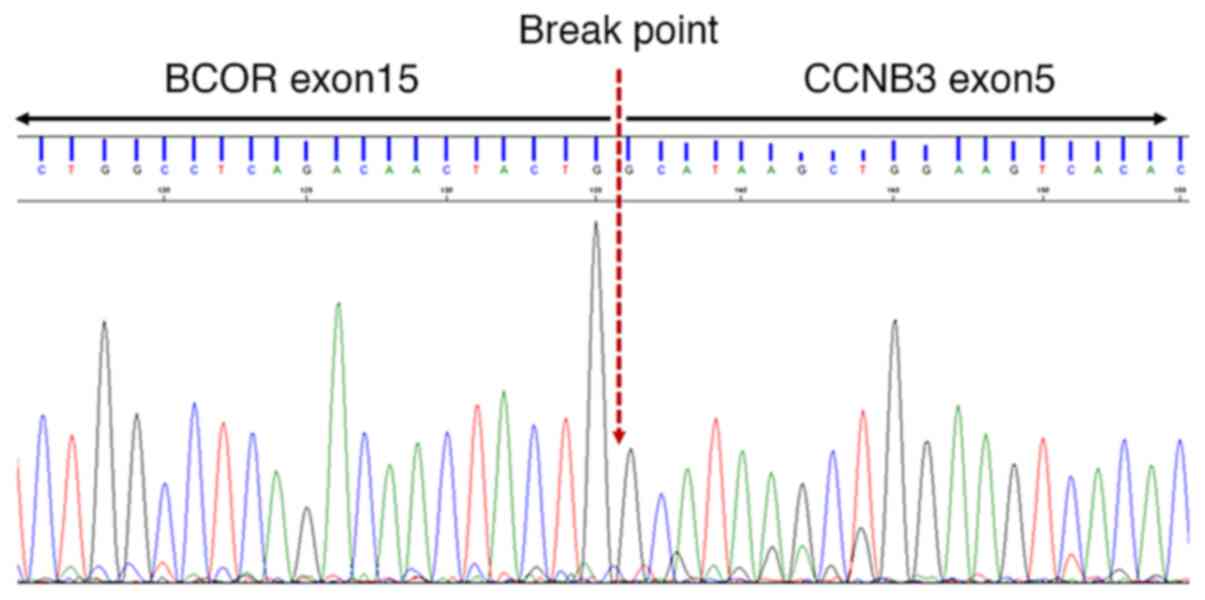
(DUX4). Additional direct Sanger sequencing using PCR
product revealed a BCOR-CCNB3 fusion (Fig. 5). The patient received 2.5 cycles
of neoadjuvant chemotherapy according to a Ewing sarcoma protocol
(10), using vincristine (1.5
mg/m2), doxorubicin (75 mg/m2), and
cyclophosphamide (1,200 mg/m2) alternating with
ifosfamide (1,800 mg/m2) and etoposide (100
mg/m2). On evaluation of the effect of neoadjuvant
chemotherapy, MRI showed a reduction in the size of the tumor,
which had extraskeletal extension into soft tissue, by ~20%.
FDG-PET/CT showed a decrease in maximum SUV from 8.6 to 2.5
(Fig. 6). Although there was
little reduction in tumor size, the activity of tumor cells was
judged to be attenuated. Following neoadjuvant chemotherapy, the
patient underwent surgery. The surgery comprised wide resection of
the bone tumor of the proximal tibia and endoprosthetic
reconstruction with a mega-prosthesis. The remaining patellar
tendon was sutured to the holes on the proximal tibia prosthesis
with a multi-strand sutures made by ultra-high molecular weight
polyethylene. Then, the extensor mechanism of the knee joint was
reconstructed by the gastrocnemius muscle transfer using the
quadriceps tendon and iliotibial band reported by Yoshida et
al (11). Specimens of
resected bone tumor contained less than 10% viable tumor cells and
were evaluated as showing good chemosensitivity to neoadjuvant
chemotherapy. Postoperatively, the patient received an additional
3.5 cycles of adjuvant chemotherapy with vincristine (1.5
mg/m2), doxorubicin (75 mg/m2) or
actinomycin-D (0.045 mg/kg), and cyclophosphamide (1,200
mg/m2) alternating with ifosfamide (1,800
mg/m2) and etoposide (100 mg/m2). Severe
adverse event during chemotherapy was white blood cell decreased
corresponding to Grade 4 by Common Terminology Criteria for Adverse
Events (Version 5.0). The patient developed a neutropenia rated
Grade 4 and received granulocyte-colony stimulating factor. Grade 3
anemia was treated with blood transfusion. The patient completed
the chemotherapy regimen as planned, with no sequelae. The patient
showed no evidence of local recurrence or distant metastasis at 12
months after completion of adjuvant chemotherapy. The range of
motion in his knee joint was 0-125 degrees without extension lag,
and he was able to walk without a cane. Functional outcome was
calculated with musculoskeletal society tumor score with the result
of 90%.
Discussion
BCOR-CCNB3 fusion sarcoma was first
identified by Pierron et al (4) in 2012 and was classified to the
emerging subgroup of undifferentiated small round cell sarcomas in
2020(1). However, due to the newly
established entity of sarcoma, a number of recent studies on
BCOR-CCNB3 have been re-diagnosed using molecular and
genetic techniques from tumors previously diagnosed as
undifferentiated sarcoma. This sarcoma remains a tumor that
orthopedic surgeons rarely encounter. To clarify the clinical
characteristics of the emerging subset of bone sarcomas with
BCOR-CCNB3 fusion, we evaluated a total of 72 cases of
BCOR-CCNB3 sarcoma of bone reported between 2012 and 2021
(including the present case), where at least the location of the
affected bone or outcome at final follow-up was described (Table I) (4,12-22).
The incidence of BCOR-CCNB3 fusion sarcoma is reportedly
1.5-14% among undifferentiated unclassified sarcomas (4,14,16).
BCOR-CCNB3 sarcoma of bone shows a striking predilection for
children, with over 90% of patients diagnosed at under 20 years
old. The mean age of cases for which age was described was 13.8
years (range, 2-25 years), similar to the cited peak incidence
between 5 and 20 years old for the Ewing sarcoma family of tumors
(23). Evaluating 70 cases with
descriptions of sex, males are affected more frequently, with a
male-to-female ratio of 6.7:1 (4,12-22).
The bone sites involved are most often a long bone of the limbs
(n=28, 38.9%), followed by the pelvis (n=27, 37.5%), calcaneus
(n=7, 9.7%), and spine (n=6, 8.3%). Among the long bone of limbs,
the most common location is the femur (n=13), followed by the tibia
(n=10) and fibula (n=4). BCOR-CCNB3 sarcoma of bone often
arises in the metaphyseal-diaphyseal portion of the femur or tibia
(12). These more common locations
of BCOR-CCNB3 sarcoma of bone are similar to those reported
for skeletal Ewing sarcoma (24).
 | Table IClinical features of
BCOR-CCNB3 fusion sarcoma of bone. |
Table I
Clinical features of
BCOR-CCNB3 fusion sarcoma of bone.
| Author | Cases | Mean age, years
(range) | Sex (n) | Location (n) | Treatment (n) | Outcome (n) | Medan F-U
(range) | 5-year OS rate | (Refs.) |
|---|
| Cohen-Gogo et
al [These 21 bone tumor cases included all cases in the article
reported by Pierron et al (4)] | 21 | 13.6 (6-22) | Male (14) | Pelvis (8) | EW (13) | CR/Al (12) | 86 months (alive in
sustained CR) | 76.5% | (12) |
| | | Female (5) | Femur (6) | IA*
(5) | DOD or De (9) | | | |
| | | NA (2) | Spine (2) | I*
(1) | | | | |
| | | | Tibia (1) | AML (1) | | | | |
| | | | Toe (1) | Local
treatment | | | | |
| | | | Clavicle (1) | only (1) | | | | |
| | | | Talus (1) | | | | | |
| | | | Rib (1) | | | | | |
| Puls et
al | 7 | 13.7 (11-16) | Male 6 | Fibula 2 | VIDE-VAI (5) | NED (5) | 78 months | 75% (including
BCOR-CCNB3 sarcoma arising in soft tissue) | (13) |
| | | | Female 1 | Pelvis (1) | EVIAD (1) | DOD (2) | (5-189) | | |
| | | | | Pubic ramus
(1) | I, D, MTX (1) | | | | |
| | | | | Femur (1) | | | | | |
| | | | | Tibia (1) | | | | | |
| | | | | Calcaneus (1) | | | | | |
| Peters et
al | 1 | 7 | Male | Calcaneus | VDC-IE | DOD | 157 months | NA | (14) |
| Shibayama et
al | 3 | 14.3 (11-17) | Male (3) | Pubis (2) | VDC-IE (2) | NED (3) | 80 months | NA | (15) |
| | | | | Calcaneus (1) | Osa (1) | | (7-102) | | |
| Ludwig et
al | 6 | 13.1 (5-18) | Male (6) | Sacro-iliac joint
(2) | NA (6) | NA | NA | NA | (16) |
| | | | | Ilium (1) | | | | | |
| | | | | Tibia (1) | | | | | |
| | | | | Acetabulum (1) | | | | | |
| | | | | Fibula (1) | | | | | |
| Yamada et
al | 1 | 12 | Female | Sacrum | NA | NA | NA | NA | (17) |
| Matsuyama et
al | 4 | | Male (4) | Sacrum (2) | NA 4 | NED (4) | 75.5 months | NA | (18) |
| | | | | Thoracic vertebra
(1) | | | (24-165) | | |
| | | | | Calcaneus (1) | | | | | |
| Krskova et
al | 1 | 15 | Male | Fibula | NA | DOD | 73 months | NA | (19) |
| Kao et
al | 20 | 14.3 (5-24) | Male (19) | Femur (5) | VDC-IE (3) | NED (7) | 45 months | 72% (including
BCOR-CCNB3 sarcoma arising in soft tissue) | (20) |
| | | | Female (1) | Tibia (4) | VD-IE (1) | AWD (4) | (10-113) | | |
| | | | | Calcaneus (3) | EW (3) | DOD (1) | | | |
| | | | | Sacrum (3) | Osa then EW
(1) | NA (8) | | | |
| | | | | Ilium (2) | Others (4) | | | | |
| | | | | Pubic ramus
(2) | NA (8) | | | | |
| | | | | Elbow (1) | | | | | |
| Rekhi et
al | 2 | 6 | Male | Tibia | Regimen
unclear | AWD | 4 months | NA | (21) |
| | | 25 | Male | Vertebra | | DOD | 8 months | | |
| Brady et
al | 5 | 14 (2-17) | Male (4) | Spine (2) | VDC-IE (4) | NED (4) | 22.4 months | NA | (22) |
| | | | Female (1) | Femur (1) | Other (1) | DOD (1) | (2-41) | | |
| | | | | Tibia (1) | | | | | |
| | | | | Pelvis (1) | | | | | |
| Present case | 1 | 12 | Male | Tibia | VDC-IE | NED | 18 months | NA | |
Imaging features of BCOR-CCNB3 sarcoma of
bone resemble those of aggressive malignant bone tumors such as
Ewing sarcoma. Due to the relatively small number of cases reported
and the frequency of case series lacking imaging findings,
radiological imaging characteristics for BCOR-CCNB3 sarcoma
of bone are difficult to summarize. However, a recent case series
and literature review by Brady et al and a review article by
Sirisena et al have reported detailed features of imaging
(22,25). The most common radiological
features were bone lysis, seen in 60%, mixed lysis and sclerosis in
25%, and sclerotic changes in 15%. Periosteal reactions were
relatively common in long bones, but to varying degrees (12,22).
In the present case, radiography showed a lytic lesion with a
moth-eaten appearance as a weak periosteal reaction; this may be
because bone tumors tend to develop around sites of
Osgood-Schlatter disease. The contralateral proximal tibia showed
Osgood-Schlatter disease (data not shown). Based on the MRI
findings reviewed by Sirisena et al, BCOR-CCNB3
sarcoma of bone commonly demonstrated intermediate signal intensity
on T1-weighted imaging and heterogeneous increased on T2-weighted
signal intensity (25).
Furthermore, a common MRI finding for some BCOR-CCNB3
sarcomas of bone, as well in the present case, is the presence of
tumor showing extraosseous soft-tissue extension (25). On MRI, the finding of extraosseous
soft tissue extension is similar to that of Ewing sarcoma (26), making that pathology difficult to
distinguish from BCOR-CCNB3 sarcoma of bone. However,
T2-weighted MRI of Ewing sarcoma typically shows homogeneous
intensity, reflecting the proliferation of small, round, blue tumor
cells (26). Another differential
diagnosis from the present case on the imaging findings is primary
bone lymphoma and osteosarcoma. However, primary bone lymphoma
shows a predilection for the fourth to sixth decades (27). Moreover, distinguishing points
between primary bone lymphoma and BCOR-CCNB3 sarcoma of bone
are the level of soluble interleukin 2 receptor (sIL-2R) in serum.
Akahane et al reported that sIL-2R showed 95% sensitivity
and 70% specificity for primary bone lymphoma, making this marker
useful for differentiating from other primary bone tumors (28). Osteosarcoma is the most common
primary malignant bone tumor in the first to second decades of
life. Among osteosarcomas, small cell osteosarcoma occasionally
presents with radiological findings similar to those of Ewing
sarcoma, showing a predominantly lytic, non-mineralized appearance
on radiography (29). On MRI,
small cell osteosarcoma appears as typically iso- to hypointense
homogeneous lesions on T1-weighted imaging and hyperintense
heterogeneous lesions on T2-weighted imaging compared to muscle
(30), and these findings
resembles BCOR-CCNB3 of bone.
Histopathological features of BCOR-CCNB3
sarcoma of bone typically present a tumor comprising a uniform
proliferation of short, spindle-shaped to round cells with scant
cytoplasm and irregular nuclei (17). Compared to typical Ewing sarcoma,
tumor cells from BCOR-CCNB3 sarcoma of bone are more likely
to be spindle-shaped. Some cases of BCOR-CCNB3 sarcoma
reportedly show variations in cellularity and myxoid changes to the
stroma (5,17). Differential diagnoses for
BCOR-CCNB3 sarcoma of bone include Ewing sarcoma,
CIC-rearrangement sarcoma, so-called Ewing-like sarcoma, and
small round cell osteosarcoma. Several reports have shown the
specificity of simple CCNB3 immunohistochemical staining for
BCOR-CCNB3 sarcoma, which is not found in Ewing sarcoma or
CIC-rearranged sarcoma. Most BCOR-CCNB3 sarcomas
exhibit strong, diffuse positivity for CCNB3 with a nuclear
positivity (12,13,15).
In addition, the pattern of CD99 immunohistochemical staining may
also prove helpful to distinguish this tumor from Ewing sarcoma.
Where typical CD99 staining in Ewing sarcoma shows a diffusely
membranous positive pattern in almost all cases, patchy, weakly
positive staining for CD99 is seen in ~70% of BCOR-CCNB3
sarcomas (13). Our case of
BCOR-CCNB3 sarcoma of the tibia showed strong, diffuse
positivity for CCNB3, including nuclear positivity, and weak
positivity for CD99. A combination of morphological,
immunohistochemical, and molecular findings allows accurate
classification in most cases.
Although BCOR-CCNB3 sarcoma of bone is
molecularly distinct from Ewing sarcoma, the clinical behaviors,
radiological features, and histopathological morphology show some
similarities with Ewing sarcoma. To date, multidisciplinary
treatment combining chemotherapy and surgery for Ewing sarcoma has
been established as standard. For the treatment of
BCOR-CCNB3 sarcoma of bone, neoadjuvant chemotherapy
followed by surgery and postoperative adjuvant chemotherapy,
representing the same protocol applied for Ewing sarcoma, has also
been proposed (12). When we
reviewed the case series of BCOR-CCNB3 sarcoma of bone
(Table I), 33 of the 72 cases had
received chemotherapy based on the standard treatment for Ewing
sarcoma. Our present case had also received neoadjuvant and
adjuvant chemotherapy based on the regimen for Ewing sarcoma, and
our patient showed no evidence of local recurrence or distant
metastasis as of 1 year after completing adjuvant chemotherapy. In
patients with localized BCOR-CCNB3 sarcoma of bone and soft
tissue, the overall survival rate at 5 years is reportedly
significantly better for patients who have received treatment
according to the Ewing protocol than for those who have received
other chemotherapeutic regimens (12). Previous reports have stated that
the 5-year overall survival rate for BCOR-CCNB3 sarcoma
ranges from 72 to 76.5% (12,13,20).
The patient and his parents were satisfied with the
favorable oncological results of the treatment according to Ewing
sarcoma. The postoperative function of the affected limb is also
good, and the patient is able to walk stably without a cane.
We reported a case of BCOR-CCNB3 sarcoma
arising in the proximal tibia and reviewed the literature for
BCOR-CCNB3 sarcoma of bone in terms of clinical features,
therapy and prognosis. BCOR-CCNB3 sarcoma requires
differentiation from Ewing sarcoma and small cell osteosarcoma
using diagnostic imaging, given the histopathological similarity
with Ewing sarcoma. Patchy, weakly positive immunohistochemical
staining for CD99 and strong, diffusely positive staining for CCNB3
are useful for diagnosing BCOR-CCNB3 sarcoma. Confirmation
of the BCOR-CCNB3 fusion gene by RT-PCR is necessary for
final definitive diagnosis. The prognosis of BCOR-CCNB3
sarcoma is expected to be relatively good with the introduction of
multidisciplinary treatment according to the protocol for Ewing
sarcoma at an early stage.
Acknowledgements
The authors are grateful to Akira Noguchi and
Professor Joji Imura, Department of Pathology, University of Toyama
(Toyama, Japan), for discussion of histopathological diagnosis.
Funding
Funding: This work was supported by Japan Society for the
Promotion of Science (JSPS) KAKENHI (grant no. JP20K09497).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KS and YH made substantial contributions to the
conception and design. TY was responsible for the acquisition or
analysis and interpretation of data. KW acquired clinical imaging
data. KS, TY and KW were involved in surgical treatment. KN was
mainly in charge of neoadjuvant and adjuvant chemotherapy. MK and
YK critically analyzed and interpreted the data. KS made a critical
revision of the article for important intellectual content. KS and
TY confirm the authenticity of all the raw data. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This report was approved by the Ethics Committee of
the Toyama University Hospital (Toyama, Japan; approval no.
‘21-22’).
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this report and accompanying images. A
copy of the written consent is available for review upon
request.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bridge JA: Undifferentiated small round
cell sarcomas of bone and soft tissue. In: WHO classification of
tumours of soft tissue and bone tumours. IARC Press, Lyon,
pp326-335, 2020.
|
|
2
|
Turc-Carel C, Aurias A, Mugneret F, Lizard
S, Sidaner I, Volk C, Thiery JP, Olschwang S, Philip I and Berger
MP: Chromosomes in Ewing's sarcoma. I. An evaluation of 85 cases of
remarkable consistency of t(11;22)(q24;q12). Cancer Genet
Cytogenet. 32:229–238. 1988.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kawamura-Saito M, Yamazaki Y, Kaneko K,
Kawaguchi N, Kanda H, Mukai H, Gotoh T, Motoi T, Fukayama M,
Aburatani H, et al: Fusion between CIC and DUX4 up-regulates PEA3
family genes in Ewing-like sarcomas with t(4;19)(q35;q13)
translocation. Hum Mol Genet. 15:2125–2137. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pierron G, Tirode F, Lucchesi C, Reynaud
S, Ballet S, Cohen-Gogo S, Perrin V, Coindre JM and Delattre O: A
new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat
Genet. 44:461–466. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Sbaraglia M, Righi A, Gambarotti M and Dei
Tos AP: Ewing sarcoma and Ewing-like tumors. Virchows Arch.
476:109–119. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Antonescu C: Round cell sarcomas beyond
Ewing: Emerging entities. Histopathology. 64:26–37. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Le Loarer F, Pissaloux D, Coindre JM,
Tirode F and Vince DR: Update on families of round cell sarcomas
other than classical Ewing sarcomas. Surg Pathol Clin. 10:587–620.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Specht K, Zhang L, Sung YS, Nucci M, Dry
S, Vaiyapuri S, Richter GH, Fletcher CD and Antonescu CR: Novel
BCOR-MAML3 and ZC3H7B-BCOR gene fusions in undifferentiated small
blue round cell sarcomas. Am J Surg Pathol. 40:433–442.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kao YC, Sung YS, Zhang L, Huang SC, Argani
P, Chung CT, Graf NS, Wright DC, Kellie SJ, Agaram NP, et al:
Recurrent BCOR internal tandem duplication and YWHAE-NUTM2B fusions
in soft tissue undifferentiated round cell sarcoma of infancy:
Overlapping genetic features with clear cell sarcoma of kidney. Am
J Surg Pathol. 40:1009–1020. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grier HE, Krailo MD, Tarbell NJ, Link MP,
Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers
PA, et al: Addition of ifosfamide and etoposide to standard
chemotherapy for Ewing's sarcoma and primitive neuroectodermal
tumor of bone. N Engl J Med. 348:694–701. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yoshida Y, Osaka S and Ryu J:
Reconstruction of the knee extensor mechanism in patients with a
malignant bone tumor of the proximal tibia. Surg Today. 40:646–649.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cohen-Gogo S, Cellier C, Coindre JM,
Mosseri V, Pierron G, Guillemet C, Italiano A, Brugières L, Orbach
D, Laurence V, et al: Ewing-like sarcomas with BCOR-CCNB3 fusion
transcript: A clinical, radiological and pathological retrospective
study from the Société Française des Cancers de L'Enfant. Pediatr
Blood Cancer. 61:2191–2198. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Puls F, Niblett A, Marland G, Gaston CL,
Douis H, Mangham DC, Sumathi VP and Kindblom LG: BCOR-CCNB3
(Ewing-like) sarcoma: A clinicopathologic analysis of 10 cases, in
comparison with conventional Ewing sarcoma. Am J Surg Pathol.
38:1307–1318. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Peters TL, Kumar V, Polikepahad S, Lin FY,
Sarabia SF, Liang Y, Wang WL, Lazar AJ, Doddapaneni H, Chao H, et
al: BCOR-CCNB3 fusions are frequent in undifferentiated sarcomas of
male children. Mod Pathol. 28:575–586. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shibayama T, Okamoto T, Nakashima Y, Kato
T, Sakurai T, Minamiguchi S, Kataoka TR, Shibuya S, Yoshizawa A,
Toguchida J and Haga H: Screening of BCOR-CCNB3 sarcoma using
immunohistochemistry for CCNB3: A clinicopathological report of
three pediatric cases. Pathol Int. 65:410–414. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ludwig K, Alaggio R, Zin A, Peron M,
Guzzardo V, Benini S, Righi A and Gambarotti M: BCOR-CCNB3
undifferentiated sarcoma-does immunohistochemistry help in the
identification? Pediatr Dev Pathol. 20:321–329. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamada Y, Kuda M, Kohashi K, Yamamoto H,
Takemoto J, Ishii T, Iura K, Maekawa A, Bekki H, Ito T, et al:
Histological and immunohistochemical characteristics of
undifferentiated small round cell sarcomas associated with CIC-DUX4
and BCOR-CCNB3 fusion genes. Virchows Arch. 470:373–380.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matsuyama A, Shiba E, Umekita Y, Nosaka K,
Kamio T, Yanai H, Miyasaka C, Watanabe R, Ito I, Tamaki T, et al:
Clinicopathologic diversity of undifferentiated sarcoma with
BCOR-CCNB3 fusion: Analysis of 11 cases with a reappraisal of the
utility of immunohistochemistry for BCOR and CCNB3. Am J Surg
Pathol. 41:1713–1721. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Krskova L, Kabickova E, Drahokoupilova E,
Kopeckova K, Plank L, Vitkova P, Mrhalova M, Zamecnik J and Kodet
R: An undifferentiated sarcoma with BCOR-CCNB3 fusion
transcript-pathological and clinical retrospective study.
Neoplasma. 65:630–636. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kao YC, Owosho AA, Sung YS, Zhang L,
Fujisawa Y, Lee JC, Wexler L, Argani P, Swanson D, Dickson BC, et
al: BCOR-CCNB3 fusion positive sarcomas: A clinicopathologic and
molecular analysis of 36 cases with comparison to morphologic
spectrum and clinical behavior of other round cell sarcomas. Am J
Surg Pathol. 42:604–615. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rekhi B, Kembhavi P, Mishra SN, Shetty O,
Bajpai J and Puri A: Clinicopathologic features of undifferentiated
round cell sarcomas of bone & soft tissues: An attempt to
unravel the BCOR-CCNB3- & CIC-DUX4-positive
sarcomas. Indian J Med Res. 150:557–574. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Brady EJ, Hameed M, Tap WD and Hwang S:
Imaging features and clinical course of undifferentiated round cell
sarcomas with CIC-DUX4 and BCOR-CCNB3 translocations. Skeletal
Radiol. 50:521–529. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khoury JD: Ewing sarcoma family of tumors.
Adv Anat Pathol. 12:212–220. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mar WA, Taljanovic MS, Bagatell R, Graham
AR, Speer DP, Hunter TB and Rogers LF: Update on imaging and
treatment of Ewing sarcoma family tumors: What the radiologist
needs to know. J Comput Assist Tomogr. 32:108–118. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sirisena UDN, Rajakulasingam R and
Saifuddin A: Imaging of bone and soft tissue BCOR-rearranged
sarcoma. Skeletal Radiol. 50:1291–1301. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Murphey MD, Senchak LT, Mambalam PK, Logie
CI, Klassen-Fischer MK and Kransdorf MJ: From the radiologic
pathology archives: Ewing sarcoma family of tumors:
Radiologic-pathologic correlation. Radiographics. 33:803–831.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Limb D, Dreghorn C, Murphy JK and Mannion
R: Primary lymphoma of bone. Int Orthop. 18:180–183.
1994.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Akahane T, Shimizu T, Isobe K, Yoshimura Y
and Kato H: Serum soluble interleukin-2 receptor levels in patients
with malignant lymphoma of bone. J Orthop Sci. 14:248–252.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yarmish G, Klein MJ, Landa J, Lefkowitz RA
and Hwang S: Imaging characteristics of primary osteosarcoma:
Nonconventional subtypes. Radiographics. 30:1653–1672.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhong J, Hu Y, Si L, Geng J, Xing Y, Jiao
Q, Zhang H and Yao W: Clarifying prognostic factors of small cell
osteosarcoma: A pooled analysis of 20 cases and the literature. J
Bone Oncol. 24(100305)2020.PubMed/NCBI View Article : Google Scholar
|