Introduction
Pulmonary leiomyoma is a rare benign tumor that may
be classified into the tracheobronchial type which arises from the
smooth muscle of the tracheobronchial wall and pulmonary
parenchymal type which is considered to arise from the smooth
muscle of the bronchial or small vessel wall, and the iceberg
growth pattern, in which the tumor extends into both the bronchial
and pulmonary cavities (1). Only
four cases of iceberg growth patterns have been reported thus far
and none of the cases captured the increase with time (1-3).
Since most cases of tracheobronchial type is symptomatic with
airway irritation, and the iceberg growth pattern tumor also
extends into the bronchial cavity, resection is indicated.
Furthermore, each type has many differential diagnoses such as
benign disease, infections, and malignant diseases based on
nonspecific radiographical findings, including CT, it is necessary
to show the pathological findings for diagnosis of leiomyoma.
Therefore, pulmonary leiomyoma requires endoscopic or surgical
resection.
In particular, ice growth pattern tumor should
resect surgically because it is located in the bronchial cavity as
well as in the lung (4). We
present a rare case of pulmonary leiomyoma with an iceberg tumor
growth pattern expanding over time, which was resected by
thoracoscopic surgery.
Case report
A 41-year-old man was referred to the National
Center for Global Health and Medicine (Tokyo, Japan) in April 2020
for further examination because of an abnormal shadow on chest
radiography and computed tomography (CT) images. He was a current
smoker, and complained of sputum and discomfort during swallowing.
His past medical history included prostatitis and hemorrhoid.
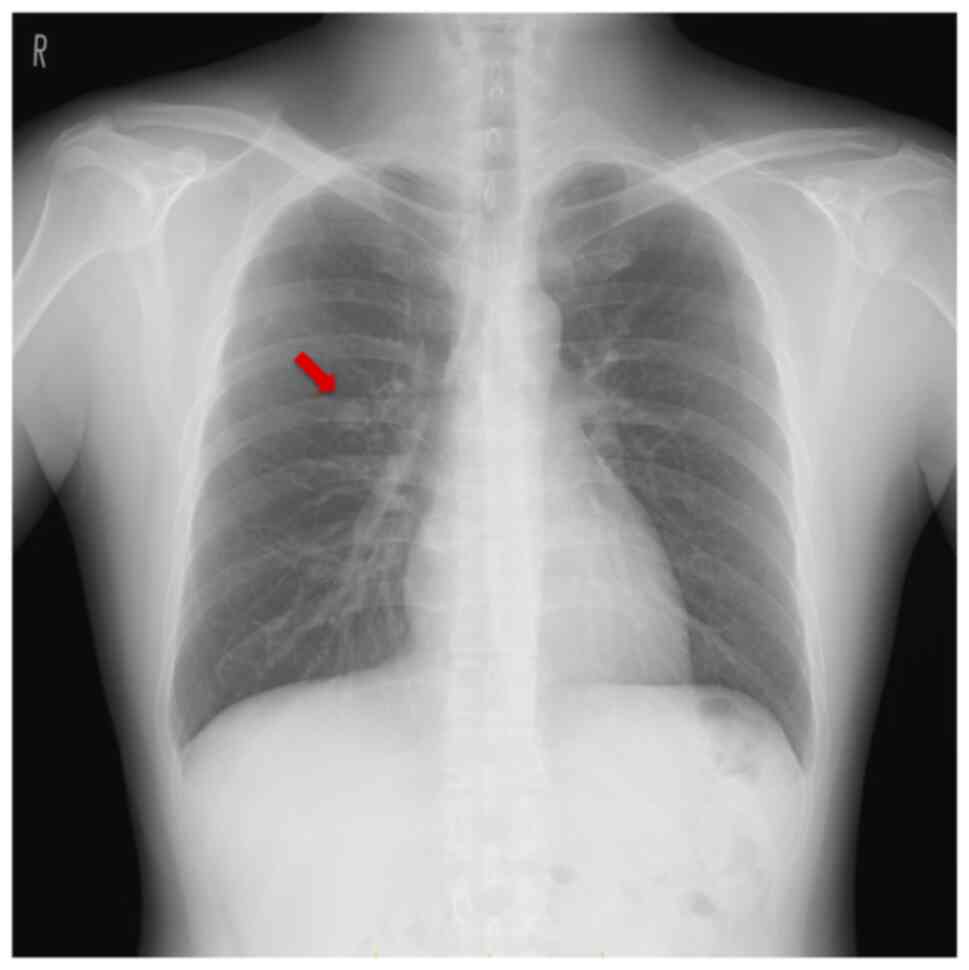
The chest radiograph revealed a smooth-surfaced
nodule in the right middle lung field (Fig. 1), and CT revealed a 10-mm
smooth-surfaced nodule on the right lung segment 3, which was
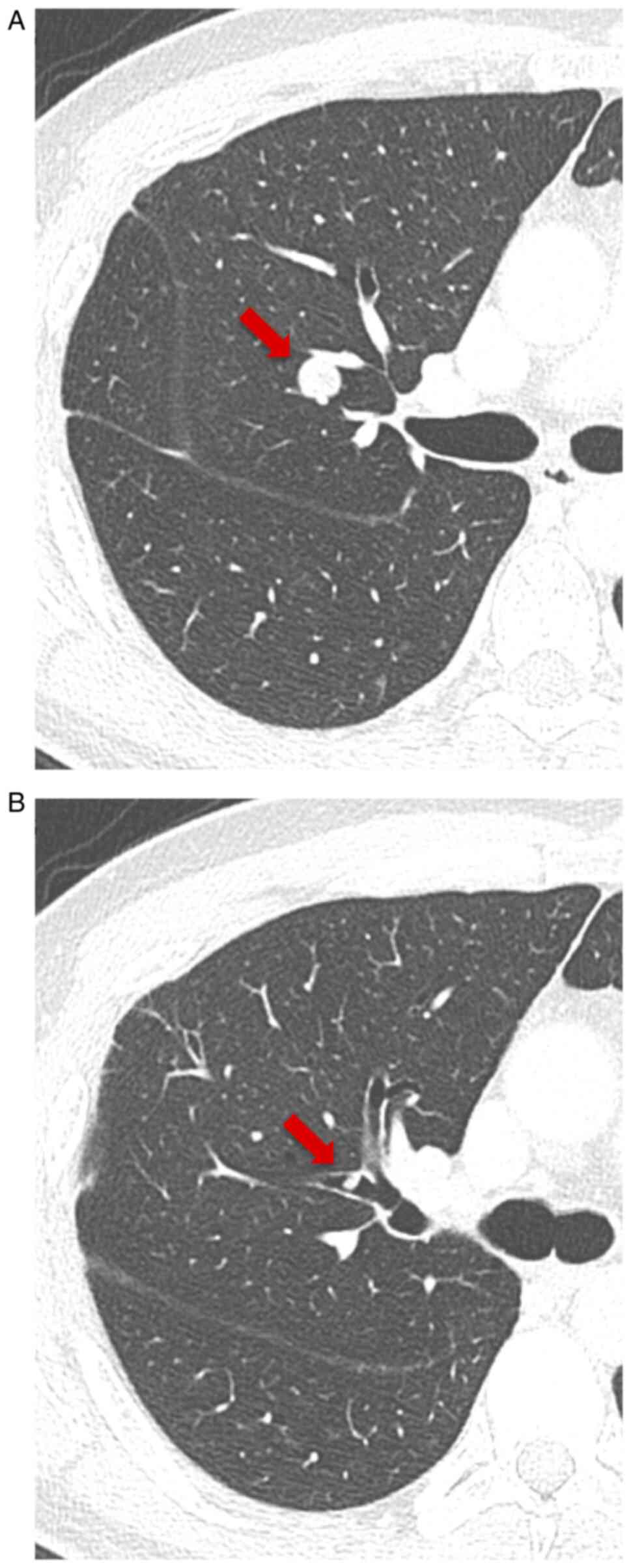
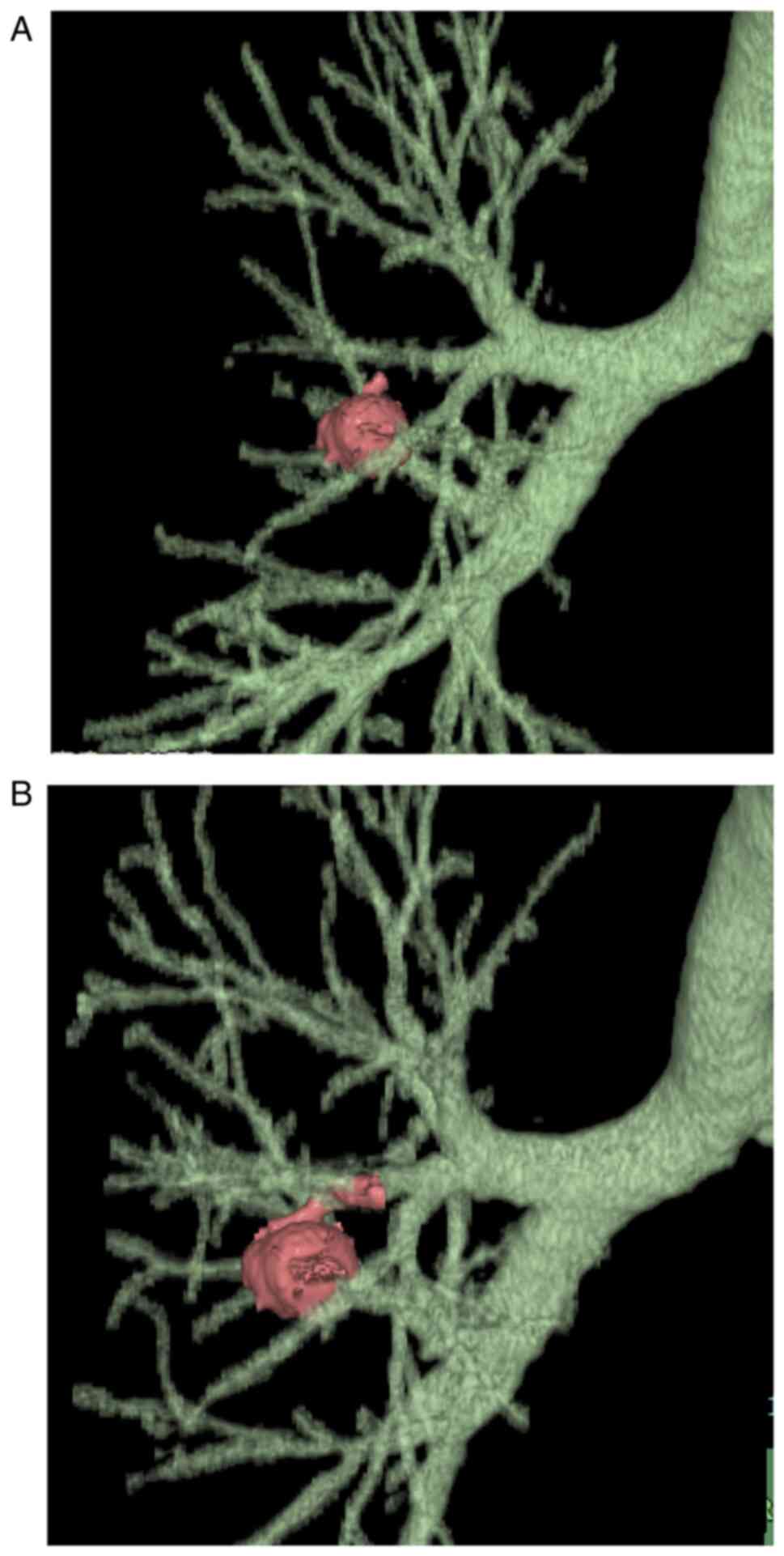
protruding to B3b with a length of 4.6 mm. After 3 and 6 months,
follow-up CTs revealed that the tumor had enlarged to 15 mm, was
crawling along the intra-bronchus, and had eventually reached the
bifurcation between B3a and B3b (Figs.
2A and B, and 3A and B). Neither enlarged lymph nodes nor
metastases were detected on CT. Laboratory examinations revealed no
renal (Cre 0.81 mg/dl) or hepatic dysfunction (AST 27 U/l, ALT 29
U/l) and tumor marker levels within the normal ranges, including
the carcinoembryonic antigen (1.5 ng/ml), carbohydrate antigen 19-9
(8.0 U/ml), and squamous cell carcinoma antigen (0.8 ng/ml). Only
mildly elevated levels of total cholesterol (221 mg/dl) and mild
prolongation of prothrombin activation (125%) were observed.
Although an intrapulmonary or bronchogenic benign tumor was
suspected (e.g., pulmonary sclerosing hemangioma, hamartoma,
leiomyoma, and lipoma), malignant diseases such as pulmonary
carcinoid tumors could not be completely excluded because of the
tumor enlargement. Therefore, we performed a robotic portal right
upper lobectomy for tumor resection and diagnosis.
Intraoperatively, loose adhesions were observed
throughout the entire thoracic cavity, with no disseminated
disease. Adhesiotomy and right upper lobectomy was performed, and
teared-polypoid lesion in the right upper bronchus was detected on
bronchoscopy during the manipulation around the upper bronchus.
Although en bloc resection would have been an ideal approach, the
tumor was found to be fragmented and compressed in the airway
during tracheal processing; hence, it was removed intraoperatively
by bronchoscopy. The operative time was 5 h 7 min, and blood loss
volume was 15 ml.
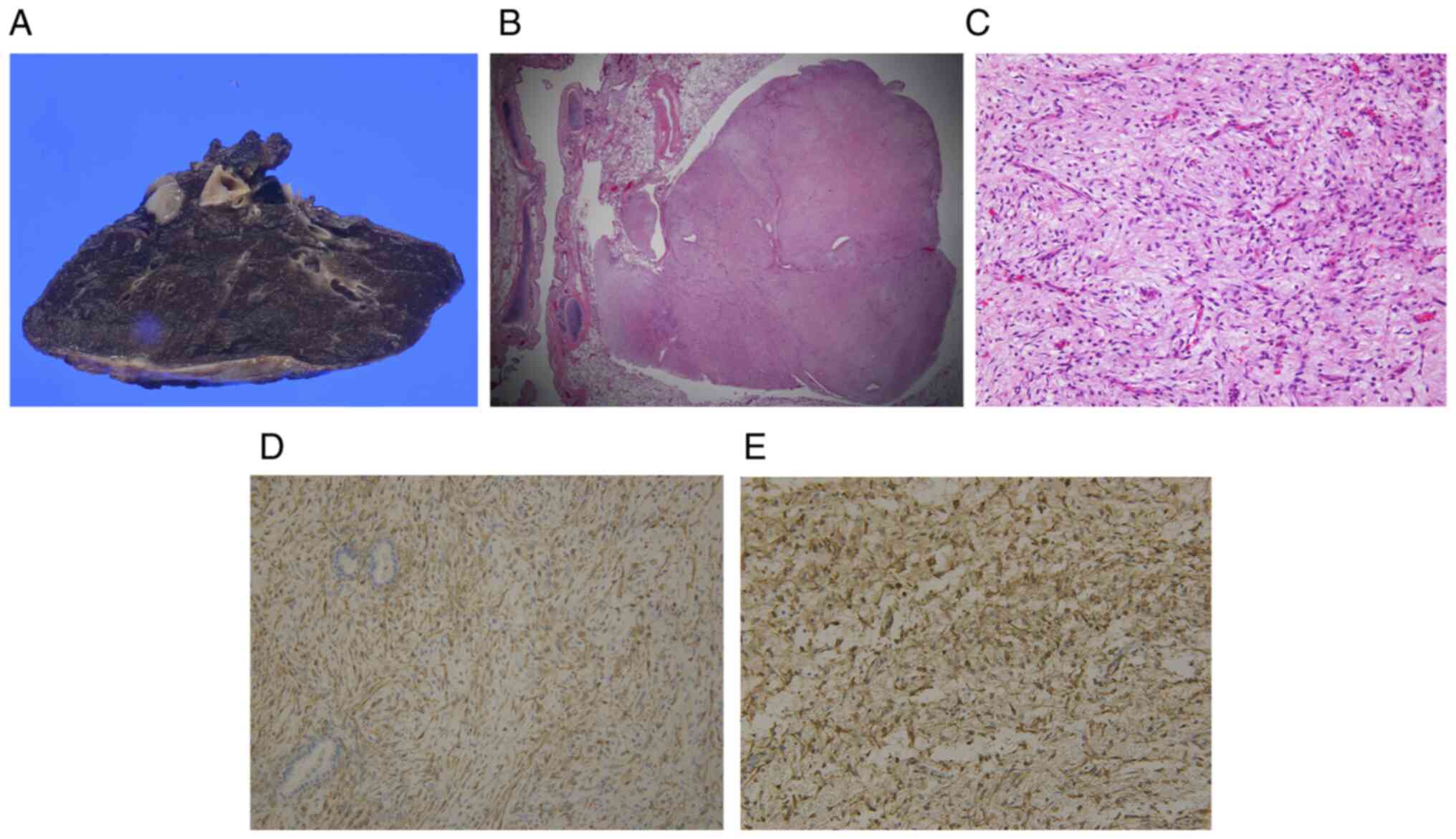
Pathological examination revealed that the white,
smooth and lobular well-defined intrapulmonary tumor had a pedicle
and was facing the bronchus (Fig.
4A-C). Both the intrapulmonary tumor and bronchial polypoid
lesion had spindle-shaped stromal cell proliferation.
Immunohistochemically, both showed diffuse cytoplasmic reactivity
for desmin and smooth muscle actin (Fig. 4D and E), while testing negative for CD34,
myogenin, and S100, indicating mesenchymal differentiation.
Ki67-positive cells accounted for approximately 5% of the cells in
a hot-spot. From the histopathological and immunohistochemical
findings, the intrapulmonary tumor and the bronchial polypoid
lesion had continuity; thus, the patient was diagnosed with
pulmonary leiomyoma. He underwent pleurodesis because of prolonged
air leakage, and he was discharged from the hospital on
postoperative day 11. He had an uneventful course after hospital
discharge without recurrence for three months.
Discussion
Pulmonary leiomyoma is a rare disease, accounts for
approximately 2% of benign lung tumors (5). It can be classified by the
localization of the tumor because of its different clinical
features (1). The major type of
leiomyoma arises from the smooth muscle of the tracheobronchial
wall and is classified as the tracheobronchial type. Conversely,
the pulmonary parenchymal type is considered to arise from the
smooth muscle of the bronchial or small vessel wall (1). A rare population with only four
reported cases, including two in the Japanese literature (Table I), have exhibited tumor extension
into both bronchial and pulmonary cavities, and is called the
‘iceberg tumor growth pattern’ (1-3).
Herein, we present an extremely rare case of leiomyoma with an
iceberg tumor growth pattern. To the best of our knowledge, this is
the first case of a leiomyoma with an iceberg growth pattern of the
tumor with a process of tumor growth.
 | Table IReported cases of pulmonary leiomyoma
with iceberg tumor growth pattern. |
Table I
Reported cases of pulmonary leiomyoma
with iceberg tumor growth pattern.
| First author,
year | Age, years | Sex | Location | Preoperative
diagnosis | Size, mm | Operation | Outcome | Follow-up duration,
months | (Refs.) |
|---|
| White et al,
1985 | 21 | M | Left main
bronchus | Pulmonary leiomyoma
recurrence | 22x11 | Segmentally resected
distal trachea and main bronchus (sleeve resection) | NA | NA | (5) |
| White et al,
1985 | 55 | F | Main bronchus, right
lung | NA | 40x25 | Tracheal segmental
resection | NA | NA | (5) |
| Kim et al,
2007 | 34 | M | Right lung segment
4 | Spindle-celled tumor
by transbronchial biopsy | 27x19 | Thoracoscopic right
middle lobectomy | No recurrence | 20 | (3) |
| Mizuno et al,
2014 | 39 | M | Right middle lobe
entrance area (B4) | Pulmonary leiomyoma
by transbronchial biopsy | 15x10 | Thoracoscopic right
middle lobectomy | No recurrence | 7 | (2) |
| Present case | 41 | M | Right lung segment
3 | - | 12 | Robotic-assisted
right upper lobectomy | No recurrence | 3 | - |
Clinically, although patients with pulmonary
parenchymal leiomyomas are typically asymptomatic, patients with
the tracheobronchial type may experience airway irritation symptoms
such as cough, sputum, blood sputum, dyspnea, fever, chest pain,
and wheezing. Moreover, tumor extension into the bronchial cavity
may cause partial or complete airway obstruction, which may
eventually result in bronchiectasis and recurrent lung infection
(6,7). Therefore, endoscopic or surgical
resection is indicated for the tracheobronchial type. Although an
endoscopic resection should be attempted for pedunculated central
airway lesions, an incomplete resection or tracheobronchial type
lesions in the peripheral airway requires surgical resection
(1-3,8).
Since the iceberg growth pattern tumor extends into the bronchial
cavity, resection is required. Moreover, this tumor should resect
surgically because it is located in the bronchial cavity as well as
in the lung (4). Past reports have
described sectional resection, lobectomy, and pneumonectomy as well
as bronchotomy and tracheoplasty to preserve respiratory function.
In our case, the tumor showed an iceberg growth pattern in b3 and
caused airway irritation symptoms, which improved soon after right
middle lobectomy.
Radiologically, the leiomyoma is located intra- or
extratracheally on chest radiographs. On CT, the leiomyoma presents
as a smooth-surfaced solitary nodule with homogeneous or
heterogeneous enhancement (9).
Differential diagnoses for the tracheobronchial type are benign
disease (e.g., granulomatous disease, sarcoidosis, amyloidosis,
fibroepithelial polyp, and broncholith), infections (e.g., fungal,
endobronchial tuberculosis, and hydatid disease), and malignant
diseases such as lung cancer and bronchial carcinoid (10). Differential diagnoses of the
pulmonary parenchymal type are benign tumors (e.g., hamartoma,
pulmonary sclerosing hemangioma, leiomyoma, fibroid tumor, and
lipoma), malignant diseases (e.g., lung cancer, metastatic lung
tumor, and pulmonary carcinoid), infection (tuberculosis,
non-tuberculosis mycobacterial infection, bacterial abscess, and
aspergilloma), and pulmonary arteriovenous malformation (11). Because radiographical findings,
including CT, are nonspecific with the tracheobronchial and
pulmonary parenchymal types, pathological examination via
bronchoscopy and surgical resection is necessary to diagnose
pulmonary leiomyoma. Regarding the diagnosis of the iceberg growth
pattern tumor, malignant diseases such as pulmonary carcinoid and
mucocutaneous carcinoma should be considered (2). Our patient had a smooth-surfaced
nodule in the right middle lung field on chest radiography, and CT
revealed that the tumor had enlarged and crawled to the central
bronchus over time. Consequently, we performed a right upper
lobectomy for tumor resection and diagnosis.
Macroscopically, the tumor was white, smooth, oval,
or lobular. Histologically, spindle-shaped cells were arranged in
an intricate bundle-like arrangement without cytological atypia or
mitosis. Sometimes, fibrous stromal proliferation or calcification
was present, but no necrosis or hemorrhage was observed (12). Furthermore, we confirmed that no
mitotic activity, cytological atypia, or necrosis were present.
Immunohistochemically, smooth muscle markers, such
as actin and desmin, are diffusely positive in leiomyomas.
Moreover, Ki-67, a marker for proliferative cells, is negative in
leiomyomas (12). If nuclear
palisading is detected histologically, as seen in schwannoma,
immunostaining of s-100, Leu-7, actin, and desmin are valuable for
the definitive diagnosis (13). In
our case, the histopathological and immunohistochemical findings
confirmed that the intrapulmonary tumor and bronchial polypoid
lesion were the same leiomyomas, which indicated a tumor with an
iceberg growth pattern.
We present a case of symptomatic iceberg growth
pattern leiomyoma with a growing trend, which was removed by right
upper lobectomy. After resection, the symptoms of airway irritation
improved. Iceberg growth pattern leiomyoma extends into both the
bronchial and pulmonary cavities; thus, surgical resection should
be considered.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YU, RS, TI and SN conceived and designed the current
study, acquired the data and analyzed the data. YU and RS confirm
the authenticity of all the raw data. HM carried out the microscopy
and contributed to the interpretation of the results. YU and RS
drafted the manuscript and revised it critically. TI and SN
supervised the conduct of this report. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
As this is a case report, approval from the
institutional review board was not required.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Minegishi K, Tsubochi H, Negishi H, Endo
T, Otani S, Sohara Y and Endo S: A case of bronchial leiomyoma
presenting with an iceberg tumor growth pattern. J Jpn Soc Respir
Endoscopy. 39:308–311. 2017.(In Japanese).
|
|
2
|
Mizuno Y, Mitta S, Yamamoto H, Shirahashi
K, Iwata H and Takemura H: A case of thoracoscopic right middle
lobectomy for bronchial leiomyoma presenting iceberg tumor growth
pattern. Jpn J Chest Surg. 28:933–936. 2014.(In Japanese).
|
|
3
|
Kim YK, Kim H, Lee KS, Han J, Yi CA, Kim J
and Chung MJ: Airway leiomyoma: Imaging findings and
histopathologic comparisons in 13 patients. AJR Am J Roentgenol.
189:393–399. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Oka S, Yamada S, Uramoto H, Mitsuhiro M
and Hanagiri T: Surgical resection of tracheal carinal leiomyoma:
Report of a case. Jpn J Chest Surg. 25:392–396. 2011.
|
|
5
|
White SH, Ibrahim NB, Forrester-Wood CP
and Jeyasingham K: Leiomyomas of the lower respiratory tract.
Thorax. 40:306–311. 1985.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ayabe H, Tsuji H, Tagawa Y, Tomita M,
Tsuda N and Chen J: Endobronchial leyomyoma: Report of a case
treated by broncoplasty and a review of the literature. Surg Today.
25:1057–1060. 1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stevic R and Milenkovic B:
Tracheobronchial tumors. J Thorac Dis. 8:3401–3413. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Park JS, Lee M, Kim HK, Choi YS, Kim K,
Kim J, Kim H and Shim YM: Primary leiomyoma of the trachea,
bronchus, and pulmonary parenchyma-a single-institutional
experience. Eur J Cardiothorac Surg. 41:41–45. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nam SW, Jeong YJ, Lee G, Lee JW, Eom JS,
Lee CH and Park SM: A rare case of tracheal leiomyoma: Role of
digital tomosynthesis in diagnosis and treatment. J Korean Soc
Radiol. 81:225–230. 2020.
|
|
10
|
Cárdenas-García J, Lee-Chang A, Chung V,
Shim C, Factor S and Tibb A: Bronchial leiomyoma, a case report and
review of literature. Respir Med Case Rep. 12:59–62.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gould MK, Fletcher J, Iannettoni MD, Lynch
WR, Midthun DE, Naidich DP and Ost DE: American College of Chest
Physicians. Evaluation of patients with pulmonary nodules: When is
it lung cancer?: ACCP evidence-based clinical practice guidelines
(2nd edition). Chest. 132 (Suppl 3):108S–130S. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chiba N, Chang SS, Saito M, Ueda Y,
Ishikawa S and Nakagawa T: A case of endobronchial leiomyoma
suspected of being a malignant tumor. Jpn J Chest Surg. 29:141–145.
2015.(In Japanese).
|
|
13
|
Wilson RW and Kirejczyk W: Pathological
and radiological correlation of endobronchial neoplasms: Part I,
Benign tumors. Ann Diagn Pathol. 1:31–46. 1997.PubMed/NCBI View Article : Google Scholar
|