Introduction
The glomus tumor (GT) is a distinctive neoplasm that
resembles the normal glomus body, and accounts for approximately
1.6% of all soft tissue tumors (1). GTs are rare, and usually present in
adults between the third and fourth decade of life. The classical
triad of symptoms is localized tenderness, severe pain and cold
hypersensitivity, and usually present as a small solitary tumor
(2).
When first described as a distinct clinical entity
by Wood in 1812(3), it was
considered a form of angiosarcoma until its histopathology was
accurately described by Masson in 1924 (4,5).
These lesions show varying proportions of glomus cells, blood
vessels and smooth muscle cells and are classified accordingly into
solid glomus tumor (25% of cases), glomangioma (60% of cases) and
glomangiomyoma (15% of cases) (6).
In this study, we report a rare form of glomus
tumor: a glomangiomyoma of the neck. To our knowledge this is the
second glomangiomyoma of the neck reported in the literature and
the first described in an adult.
Case report
A 31-year-old woman presented to the Ear Nose Throat
department of Son Espases University Hospital in Palma de Mallorca,
Spain with a 4-year history of a growing right submandibular tumor
with localized non-irradiated pain. The physical examination
revealed a localized tenderness and a well-defined ovoid mass of
approximately 4x3 cm, with no neurological deficit. The oral
cavity, oropharynx and larynx examination were unremarkable. She
reported no previous personal or family history of glomus tumor, no
evidence of cold sensitivity and no previous history of trauma.
Before she was sent to our hospital, a core needle
biopsy had been performed in another center in 2018, suggesting the
diagnosis of glomangioma. The histopathology showed a homogenous
cellularity with cells arranged diffusely and perivascularly.
Immunohistologically, the tumor cells featured a cytoplasmic
staining for SMA (smooth muscle actin), H-caldesmon, vimentin and
collagen IV, whereas the reactions with antibodies against CD3,
CD20, CD138, desmin, S-100, CD138, CD1 and CD34 were negative. No
mitotic activity was observed, and Ki-67 cell proliferation index
was 2%.
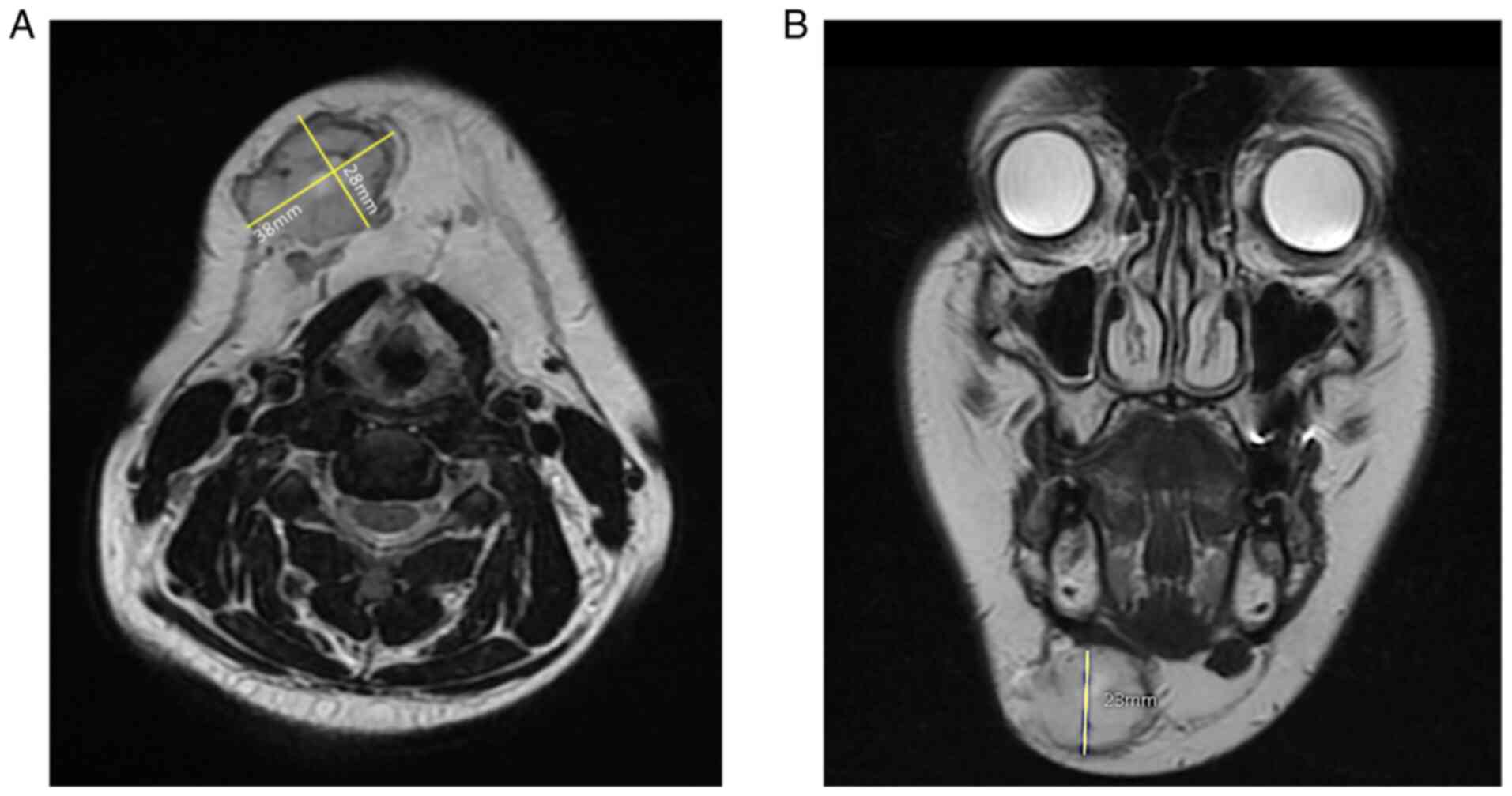
The first MRI was performed in June 2019, showing a
solid nodular lesion measuring 25x21x20 mm, with a decreased signal
intensity on T1 and increased signal intensity on T2-weighted
images. After the diagnosis, the patient decided not to proceed
with the surgery. She returned in October 2020 due to increased
pain and a second MRI was performed, showing a significant growth
of the tumor, measuring 38x28x23 mm; the enhancement pattern
remained unchanged (Fig. 1).
She underwent excision of the tumor under general
anesthesia and facial nerve monitoring with no complications.
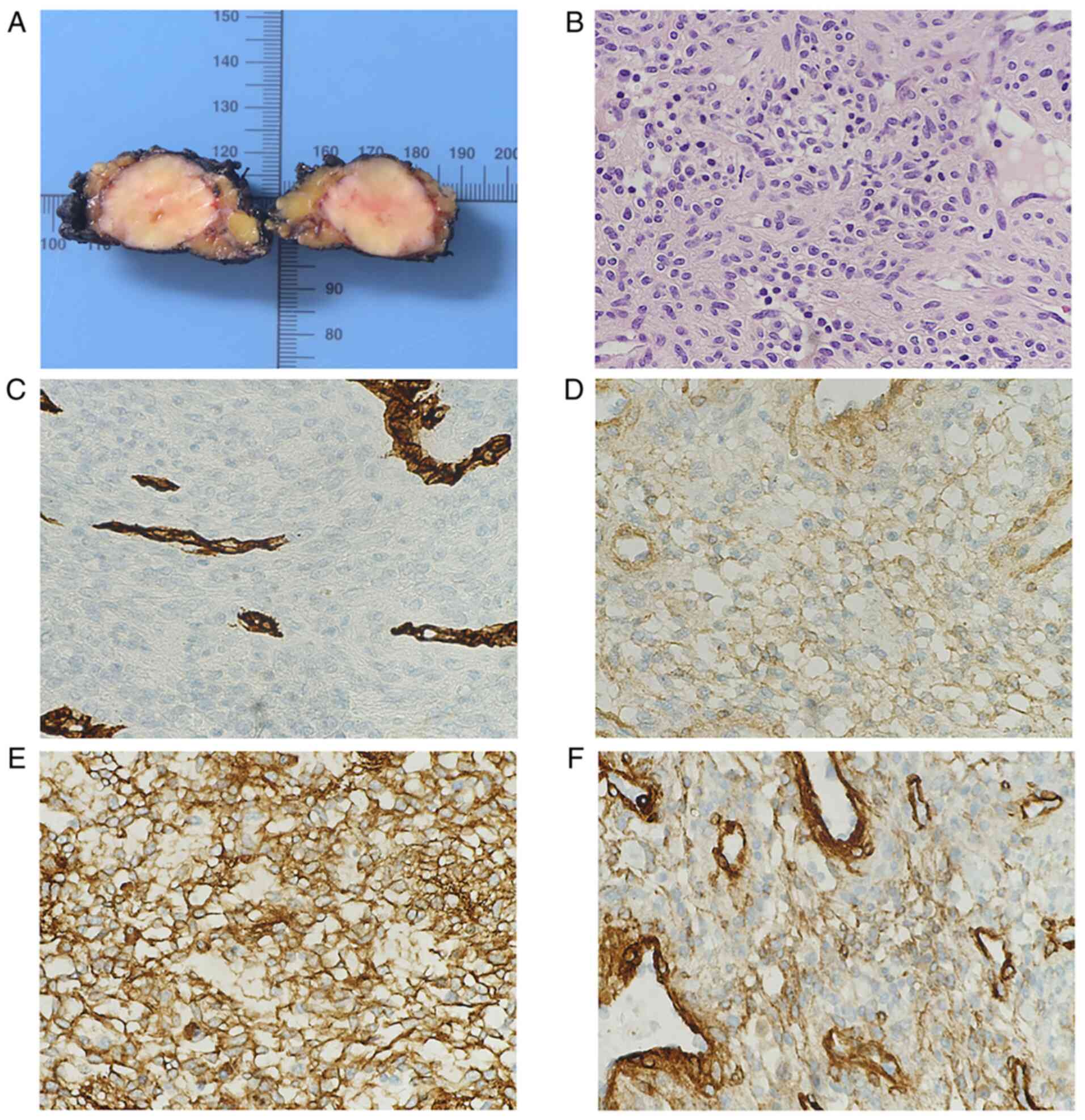
Intraoperatively the tumor was solid and well delimited, with no
sign of tumor spread into the adjacent tissue (Fig. 2A). A meticulous dissection was
performed preserving the marginal nerve. Postoperatively the
patient was free of complications.
The histopathology exhibited a well circumscribed
solid lesion, composed of uniform elongated cells with a round and
ovoid central nuclei with no signs of atypia, and lightly
eosinophilic cytoplasm with well-defined cell margins. These cells
were distributed near vascular spaces.
Immunohistochemical analysis was performed showing a
positive stain for smooth muscle actin, H-caldesmon, muscle
specific actin and collagen type IV. The tumor cells were negative
to desmin, calponin, melan-A, Sox-10, S100 and CD34. Ki-67
proliferation index was 1% (Fig.
2B-F).
Discussion
GTs are usually benign perivascular neoplasms, most
likely derived from modified smooth muscle cells of the
neuromyoarterial glomus body. The normal glomus body is a
specialized form of arteriovenous anastomosis that regulates heat,
and is located in the stratum reticularis of the dermis and most
frequently encountered in the subungual region, the lateral areas
of the digits and the palm (7).
The most affected site is the hands (particularly
the subungual region and palm), followed by the foot and forearm
(6). These lesions can also be
found in regions where no normal glomus have been identified,
including deeper tissues such as joint capsule and striated muscle
(8).
A 20-year-long series published by Schiefer et
al (9) shows that the extra
digital location could be more common than we thought, comprising
approximately 61% of glomus tumors seen at the Mayo Clinic during
this time frame. This finding is also supported by the experience
of Heys et al (10) in a
43-patient-series (1992), where 67% of tumors were extradigital,
like the findings reported by Chou et al (2).
In our literature review, we found that only a few
extradigital locations of glomagiomyoma (less frequent histological
variant of glomus tumor) have been reported: Laryngeal (11), trachea (12,13),
pancreas (14), kidney (15,16),
forearm (17,18), vagina (19), knee (20), periurethral (21), lung (22), gastrointestinal (23), chest wall (24), nasal cavity (25) and only one other case of neck
glomagiomyoma in a child in Nepal (26).
Classically described, the glomus tumor presents as
a solitary, small (<1 cm), blue-red nodule with paroxysmal pain,
worsened by cold temperature and pressure (2,27).
In a patient series reported by Schiefer et al (9) and Heys et al (10) the most common symptom was localized
pain, while pain elicited by cold temperature was rare A prior
history of trauma in the affected area has also been described
(9).
Our patient presented a growing large neck mass,
differing from the classical presentation described in the
literature. We think that the patient may not have noticed the mass
during the first years of presentation due to its submandibular
localization, and the only symptom she presented was localized
non-irradiated pain, which worsened with growth.
While most glomus tumors are benign; malignant, and
aggressive tumors have been published in the literature. In 2001,
Folpe et al (28) published
a classification of atypical GTs identifying a size larger than 2
cm, deep location, nuclear atypia, mitotic activity, and diffuse
growth as potentially malignant features.
In our case, even though the tumor fulfills the
malignancy criteria proposed by Folpe et al due to its deep
location and size, intraoperatively it was a well circumscribed
lesion with no macroscopic signs of adjacent tissue infiltration.
The histopathology of the core needle biopsy and the surgical
specimen showed no signs of atypia or mitotic activity and a low
Ki-67 cell proliferation index. In deep soft tissues, the
differential diagnosis of an atypical GT should consider
hemangiopericytoma, leiomyosarcoma with epithelioid change,
rhabdomyosarcoma, and pPNET (peripheral primitive neuroectodermal
tumor) (28). Given the patient's
characteristics we also considered HPV associated oropharyngeal
cancer, salivary gland neoplasm and lymphoma. Distinctive
characteristics of these lesions are described in Table I.
 | Table IDifferential diagnosis and its
distinctive cytological and inmmunohistochemical features (42-60). |
Table I
Differential diagnosis and its
distinctive cytological and inmmunohistochemical features (42-60).
| Differential
diagnosis | History/physical
exam | Cytological
features on fine needle aspiration biopsy/biopsy |
Immunohisto-chemistry |
|---|
| Atypical glomus
tumor | Large size mass
>2 cm, deeply located. Presents with paroxysmal pain elicited by
cold | Glomus cells, blood
vessels and smooth muscle cells | H-caldesmon, MSA,
SMA, collagen type IV, +/- CD34 |
| Salivary gland
malignancy | Painless
unilateralgrowing mass. That appears immobile without defined
borders. Marginal nerve palsy. Cervical lymphadenopathy | Atypical mitotic
figures and mitotic activity | ACC: CK7, CAM 5.2,
calponin, p63, SOX10, S100, SMA MEC: CK5, CK6, CK7, CK8, CK14,
CK18, CK19, EMA, CEA, and p63 |
| Hodgkin
lymphoma | Painless
lymphadenopathy. Constitutional symptoms | Large neoplastic
cells may be mononucelated (hodgkin cell)or bi or mutilobated
(Reed-Sternberg cell) with prominent nucleoli and abundant
cytoplasm | CD30, CD15, PAX5,
CD20 |
| Non-Hodgkin
lymphoma | Painless
lymphadenopathy. Constitutional symptoms | DLBCL: lymphoid
cells with nuclear size more than twice the size of normal
lymphocytes | CD19, CD22,
CD79a |
| | | FL: small
mature-appearing lymphocytes with angulated, elongated, or cleaved
nuclei and inconspicuous nucleoli, corresponding to the
centrocytes. Large, non-cleaved cells corresponding to the
centroblasts are present | CD19, CD20, CD10,
BCL-6 |
| HPV oropharyngeal
squamous cell carcinoma | Painless neck mass
and sore throat in usually non-smoker patients | Squamoid cytoplasm
and cohesive streaming groups | Keratin, p63, p40
HR-HPV by PCR or ISH |
| Metastatic
melanoma | History of exposure
to intense UVR at a young age. Large number of naevi. Painless
fixed adenopathy | Nuclear molding and
nuclear crush artifact, necrosis, mitosis and apoptosis | S100, HMB-45,
Melan-A, tyrosinase |
|
Hemangioperycitoma | Usually large
painless mass. Present in several anatomical sites | Homogenous vascular
pattern, uniform cell population with ovoid/round cells enmeshed
but reticulin and collagen fibers | Vimentin, STAT6,
BCL2 +/- CD34 CD57, CD99 |
| Leiomyosarcoma | Nonspecific
symptoms caused by displacement of structures, slowly enlarging,
discrete, firm, non-ulcerated painless mass | Intersecting,
sharply marginated fascicles of spindle cells with abundant
eosinophilic cytoplasm and elongated and hyperchromatic nuclei | SMA, desmin, CK,
EMA |
|
Rhabdomyosarcoma | Children and
adolescents presenting with a visible or palpable mass, symptoms
develop from compression or invasion of adjacent structures | Primitive
mesenchymal cells recapitulating various stages of myogenesis with
variable presence of rhabdomyoblasts | Desmin, Myogenin,
CD56, muscle-specific actin, Myoglobin, Vimentin and MyoD1 |
| Peripheral
primitive neuroectodermal tumor | Young patients with
rapidly enlarging, often painful mass | Sheets of small,
round to oval cells, often arranged in lobules, separated by
fibrous septa Homer-Wright rosettes | MIC2, Vimentin, NSE
(neuron specificenolase), synaptophysin |
The molecular mechanisms that may lead to a glomus
tumor have also been researched. In their series of 93 patients
with GTs, Agaram et al observed that 54% of GTs harbor
NOTCH-gene fusions. NOTCH2-MIR143 was the most common fusion,
detected in 76% of the cases (29). BRAF V600E mutation has also been
studied potentially related to malignancy and tumor progression
(30).
During diagnostic evaluation an image test should be
performed, with MRI being shown to be the most sensitive imaging
modality for diagnosing glomus tumors (9,31).
Most lesions are surrounded by a capsule and are iso- or slightly
hyper intense on T1 and strongly hyperintense on T2-weighted images
relative to the muscle, as seen on this patient (Fig. 1A). Vascular predominant GT could
show a stronger contrast enhancement (31-34).
These lesions immunohistochemically and
ultrastructurally exhibit smooth muscle characteristics. Cells
usually stain for smooth muscle actin, H-caldesmon, muscle-specific
actin, and myosin. Staining for collagen type IV shows prominent
pericellular positivity. Desmin has occasionally been found to be
positive and S100 positivity is rare. Studies show conflicting
results for CD34 positivity, classically considered as an
endothelial marker, but its role in glomus tumors remains unclear
(27,28,35-40).
The immunohistochemical analysis of our patient
showed positivity for smooth muscle actin, H-caldesmon,
muscle-specific actin and collagen IV, similar to what has been
classically described in the literature.
Glomangiomyomas share the architectural pattern of a
classic glomus tumor showing transitions between glomus cells and
cells with partial smooth muscle features (4). It has been proposed that glomangiomas
and glomangiomyomas designate the same lesion; the latter with
transitional areas from glomus cells to well defined smooth muscle
cells, and to identify these areas, extensive sampling and analysis
should be made (38,41). We believe this is why our case was
initially diagnosed as a glomangioma through a core needle biopsy
with the final diagnosis of glomangiomyoma only being established
after the final specimen had been obtained.
A glomus tumor is a rare neoplasm, more so if
localized in the head and neck region. It should be considered in
an adult presenting with a neck mass and localized tenderness
especially if no other risk factors for head and neck tumors are
present. Imaging technique and fine needle aspiration biopsy are
mandatory to characterize the mass properly as complete surgical
excision continues to be the treatment of choice.
Acknowledgements
This article has been revised by Mr. Jonathan
McFarland (Associate Professor at Autonomous University of Madrid,
and Senior Lecturer at Sechenov University, Moscow).
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
PSE and GTP are the main surgeons and provided
substantial contributions in the design of this article. CMO and CC
drafted the final manuscript, acquired all data, revised it
critically and wrote the final version to be published. EMH
performed the immunohistochemical staining and histopathology. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided oral informed consent for the
use of their surgical samples in scientific research and the use of
images for publication (November 5, 2020).
Competing interests
The authors declare that that they have no competing
interests.
References
|
1
|
Shugart RR, Soule EH and Johnson EW Jr:
Glomus Tumor. Surg Gynecol Obstet. 117:334–340. 1963.PubMed/NCBI
|
|
2
|
Chou T, Pan SC, Shieh SJ, Lee JW, Chiu HY
and Ho CL: Glomus tumor: Twenty-year experience and literature
review. Ann Plast Surg. 76 (Suppl 1):S35–S40. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wood W: On painful subcutaneous tubercle.
Edinb Med Surg J. 8:283–291. 1812.PubMed/NCBI
|
|
4
|
Enzinger FM and Weiss SW: Perivascular
tumors. Chapter 24. In: Soft Tissue Tumors. 7th edition. Enzinger
FM and Weiss SW (eds). Elsevier, Philadelphia, pp837-862, 2020.
|
|
5
|
Masson P: Le glomus neuromyoarterial des
regions tactiles et ses tumeurs. Lyon Chir. 21(257)1924.
|
|
6
|
Calonje E and Brenn T: Vascular tumors.
Tumors and tumor-like conditions of blood vessels and lymphatics.
In: Lever's Histopathology of the Skin. 11th edition. Elder David
E, Elenitsas R, Rosenbach M, Murphy G, Rubin A and Xu X (eds).
Lippincott Williams & Wilkins, Philadelphia, 2015.
|
|
7
|
Popoff NW: The digital vascular system
with reference to the state of glomus in inflammation,
arte-riosclerotic gangrene, diabetic gangrene, thromboangiitis
obliterans and supernumerary dig-its in man. Arch Pathol.
18:295–330. 1934.
|
|
8
|
Murray MR and Stout AP: The glomus tumor:
Investigation of its distribution and behavior, and the identity of
its ‘epithelioid’ cell. Am J Pathol. 18:183–203. 1942.PubMed/NCBI
|
|
9
|
Schiefer TK, Parker WL, Anakwenze OA,
Amadio PC, Inwards CY and Spinner RJ: Extradigital glomus tumors: A
20-year experience. Mayo Clin Proc. 81:1337–1344. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Heys SD, Brittenden J, Atkinson P and
Eremin O: Glomus tumour: An analysis of 43 patients and review of
the literature. Br J Surg. 79:345–347. 1992.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee WT, Murthy SC, Gildea TR and Lorenz
RR: First case of laryngeal glomangiomyoma. Laryngoscope.
115:2038–2040. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Baek SH, Huh DM, Park JH, Kwak EK, Kim BH
and Han WK: Glomangiomyoma of the trachea. Korean J Thorac
Cardiovasc Surg. 44:440–443. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guibert N, Mazieres J, Didier A, Porte SJ,
Projetti F and Hermant C: Tracheal glomangioleiomyoma treated by
multimodal interventional bronchoscopy. Ann Thorac Surg.
101:1591–1594. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Miliauskas JR, Worthley C and Allen PW:
Glomangiomyoma (glomus tumour) of the pancreas: A case report.
Pathology. 34:193–195. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Siddiqui NH, Rogalska A and Basil IS:
Glomangiomyoma (glomus tumor) of the kidney. Arch Pathol Lab Med.
129:1172–1174. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Al-Ahmadie HA, Yilmaz A, Olgac S and
Reuter VE: Glomus tumor of the kidney: A report of 3 cases
involving renal parenchyma and review of the literature. Am J Surg
Pathol. 31:585–591. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Deger AN, Deger H, Tayfur M, Balcioglu MG
and Kadioglu E: Acquired solitary glomangiomyoma on the forearm: A
rare case report. J Clin Diagn Res. 10:ED10–ED11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ning X, Wang N, Yan H, Feng Y and Zhang Y:
A nodule on the forearm. Dermatol Online. J
26(13030/qt7x251867)2020.PubMed/NCBI
|
|
19
|
Rahimi S, Marani C, Balega J and
Hirschowitz L: Glomangiomyoma of the vagina: A report of 2 cases
and literature review. Int J Gynecol Pathol. 36:334–338.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hustings N, Vanhoenacker F and De Backer
A: Correction: Glomangiomyoma of the knee: A rare juxtasynovial
presentation. J Belg Soc Radiol. 104(26)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Blandamura S, Florea G, Brotto M, Salmaso
R and Castellan L: Periurethral glomangiomyoma in women: Case
report and review of the literature. Histopathology. 36:571–572.
2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Katabami M, Okamoto K, Ito K, Kimura K and
Kaji H: Bronchogenic glomangiomyoma with local intravenous
infiltration. Eur Respir J. 28:1060–1064. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lo AW, Chow LT, To KF and Yu MY: Gastric
glomangiomyoma: A pedunculated extramural mass with a florid
angiomyomatous pattern. Histopathology. 44:297–298. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schneller J: Multifocal glomangiomyomas in
the chest wall of a young man. Arch Pathol Lab Med. 125:1146–1147.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shek TW and Hui Y: Glomangiomyoma of the
nasal cavity. Am J Otolaryngol. 22:282–285. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tulachan B and Borgohain BN:
Glomangiomyoma of the neck in a child in Nepal: A rare case report
and literature review. BMC Ear Nose Throat Disord.
17(8)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mravic M, LaChaud G, Nguyen A, Scott MA,
Dry SM and James AW: Clinical and histopathological diagnosis of
glomus tumor: An institutional experience of 138 cases. Int J Surg
Pathol. 23:181–188. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Folpe AL, Fanburg-Smith JC, Miettinen M
and Weiss SW: Atypical and malignant glomus tumors: Analysis of 52
cases, with a proposal for the reclassification of glomus tumors.
Am J Surg Pathol. 25:1–12. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Agaram NP, Zhang L, Jungbluth AA, Dickson
BC and Antonescu CR: A molecular reappraisal of glomus tumors and
related pericytic neoplasms with emphasis on NOTCH-gene fusions. Am
J Surg Pathol. 44:1556–1562. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Karamzadeh Dashti N, Bahrami A, Lee SJ,
Jenkins SM, Rodriguez FJ, Folpe AL and Boland JM: BRAF V600E
mutations occur in a subset of glomus tumors, and are associated
with malignant histologic characteristics. Am J Surg Pathol.
41:1532–1541. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Al-Qattan MM, Al-Namla A, Al-Thunayan A,
Al-Subhi F and El-Shayeb AF: Magnetic resonance imaging in the
diagnosis of glomus tumours of the hand. J Hand Surg Br.
30:535–540. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Drapé JL, Idy-Peretti I, Goettmann S,
Wolfram-Gabel R, Dion E, Grossin M, Benacerraf R, Guérin-Surville H
and Bittoun J: Subungual glomus tumors: Evaluation with MR imaging.
Radiology. 195:507–515. 1995.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jablon M, Horowitz A and Bernstein DA:
Magnetic resonance imaging of a glomus tumor of the fingertip. J
Hand Surg Am. 15:507–509. 1990.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee S, Le H, Munk P, Malfair D, Lee ChH
and Clarkson P: Glomus tumour in the forearm: a case report and
review of MRI findings. JBR-BTR. 93:292–295. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mentzel T, Hügel H and Kutzner H:
CD34-positive glomus tumor: Clinicopathologic and
immunohistochemical analysis of six cases with myxoid stromal
changes. J Cutan Pathol. 29:421–425. 2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kaye VM and Dehner LP: Cutaneous glomus
tumor. A comparative immunohistochemical study with
pseudoangiomatous intradermal melanocytic nevi. Am J Dermatopathol.
13:2–6. 1991.PubMed/NCBI
|
|
37
|
Porter PL, Bigler SA, McNutt M and Gown
AM: The immunophenotype of hemangiopericytomas and glomus tumors,
with special reference to muscle protein expression: An
immunohistochemical study and review of the literature. Mod Pathol.
4:46–52. 1991.PubMed/NCBI
|
|
38
|
Watanabe K, Kusakabe T, Hoshi N, Saito A
and Suzuki T: h-Caldesmon in leiomyosarcoma and tumors with smooth
muscle cell-like differentiation: Its specific expression in the
smooth muscle cell tumor. Hum Pathol. 30:392–396. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hatori M, Aiba S, Kato M, Kamiya N and
Kokubun S: Expression of CD34 in glomus tumors. Tohoku J Exp Med.
182:241–247. 1997.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu YY, Luo XM, Zhou SH and Zheng ZJ:
CD34-positive expression in benign nasal glomus tumour: Two case
reports and a literature review. J Int Med Res. 38:2169–2177.
2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Calduch L, Monteagudo C, Martínez-Ruiz E,
Ramón D, Pinazo I, Cardá C and Jordá E: Familial generalized
multiple glomangiomyoma: Report of a new family, with
immunohistochemical and ultrastructural studies and review of the
literature. Pediatr Dermatol. 19:402–408. 2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Boulagnon-Rombi C, Fleury C, Fichel C,
Lefour S, Marchal Bressenot A and Gauchotte G: Immunohistochemical
approach to the differential diagnosis of meningiomas and their
mimics. J Neuropathol Exp Neurol. 76:289–298. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gerner RE, Moore GE and Pickren JW:
Hemangiopericytoma. Ann Surg. 179:128–132. 1974.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Han Y, Zhang Q, Yu X, Han X, Wang H, Xu Y,
Qiu X and Jin F: Immunohistochemical detection of STAT6, CD34, CD99
and BCL-2 for diagnosing solitary fibrous
tumors/hemangiopericytomas. Int J Clin Exp Pathol. 8:13166–13175.
2015.PubMed/NCBI
|
|
45
|
Enzinger FM and Smith BH:
Hemangiopericytoma. An analysis of 106 cases. Hum Pathol. 7:61–82.
1976.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Viray H, Bradley WR, Schalper KA, Rimm DL
and Gould Rothberg BE: Marginal and joint distributions of S100,
HMB-45, and Melan-A across a large series of cutaneous melanomas.
Arch Pathol Lab Med. 137:1063–1073. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ahmed OA and Kelly C: Head and neck
melanoma (excluding ocular melanoma): United Kingdom national
multidisciplinary guidelines. J Laryngol Otol. 130 (S2):S133–S141.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hanna DC and Clairmont AA: Submandibular
gland tumors. Plast Reconstr Surg. 61:198–203. 1978.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shaha AR, Webber C, DiMaio T and Jaffe BM:
Needle aspiration biopsy in salivary gland lesions. Am J Surg.
160:373–376. 1990.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Meyer MT, Watermann C, Dreyer T, Ergün S
and Karnati S: 2021 Update on diagnostic markers and translocation
in salivary gland tumors. Int J Mol Sci. 22(6771)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lee RJ, Tan AP, Tong EL, Satyadev N and
Christensen RE: Epidemiology, prognostic factors, and treatment of
malignant submandibular gland tumors: A population-based cohort
analysis. JAMA Otolaryngol Head Neck Surg. 141:905–912.
2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Pusztaszeri MP and Faquin WC: Cytologic
evaluation of cervical lymph node metastases from cancers of
unknown primary origin. Semin Diagn Pathol. 32:32–41.
2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chen YH and Gong Y: Cytopathology in the
diagnosis of lymphoma. Cancer Treat Res. 160:211–240.
2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Serrano C and George S: Leiomyosarcoma.
Hematol Oncol Clin North Am. 27:957–974. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Iwata J and Fletcher CD:
Immunohistochemical detection of cytokeratin and epithelial
membrane antigen in leiomyosarcoma: A systematic study of 100
cases. Pathol Int. 50:7–14. 2000.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yadav J, Bakshi J, Chouhan M and Modi R:
Head and neck leiomyosarcoma. Indian J Otolaryngol Head Neck Surg.
65 (Suppl 1):S1–S5. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bishop JA, Thompson LD, Cardesa A, Barnes
L, Lewis JS Jr, Triantafyllou A, Hellquist H, Stenman G, Hunt JL,
Williams MD, et al: Rhabdomyoblastic differentiation in head and
neck malignancies other than rhabdomyosarcoma. Head Neck Pathol.
9:507–518. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Freedman A and Jacobsen E: Follicular
lymphoma: 2020 Update on diagnosis and management. Am J Hematol.
95:316–327. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhou DN, Yang QQ, Li ZL, Pan ZY and Deng
YF: Head and neck rhabdomyosarcoma: Follow-up results of four cases
and review of the literature. Int J Clin Exp Pathol. 8:4277–4283.
2015.PubMed/NCBI
|
|
60
|
Nikitakis NG, Salama AR, O'Malley BW Jr,
Ord RA and Papadimitriou JC: Malignant peripheral primitive
neuroectodermal tumor-peripheral neuroepithelioma of the head and
neck: A clinicopathologic study of five cases and review of the
literature. Head Neck. 25:488–498. 2003.PubMed/NCBI View Article : Google Scholar
|