Introduction
Novel technologies in cancer immunotherapy have made
significant strides with the emergence of therapeutic approaches,
including blocking checkpoint molecules, including cytotoxic
T-lymphocyte (CTL)A-4, PD-1, TIM-3, LAG-3, TIGIT, BTLA (1-5)
and genetically modifying effector cells, including CAR-T and TCR-T
(6,7). Nevertheless, overcoming the
limitations appearing and the cost reduction of such technologies
is a subject that remains to be elucidated In addition, the study
of all possible therapeutic approaches to the induction of an
immune response is still highly relevant (8,9). To
overcome the negative effects of the tumors on the formation of
immune response, cytokines including IL-12, IL-18 and IL-2
(10,11), antibodies against immunosuppressive
molecules (CTLA-4 and PD-1) expressed on the surface of tumor cells
(12) and enzyme inhibitors
(indoleamine 2,3-dioxygenase 1, prostaglandin-endoperoxide synthase
2) (13,14). The use of cellular immunotherapy is
suggested at different stages of cancer development, in combination
with the main complex antitumor therapy (15-18).
The effectiveness of cellular immunotherapy is dependent on the
stage of the disease, the type of tumor and the severity of
systemic immunosuppression (19).
One of the most important aspects of efficient
immune response formation is proper antigen presentation, in order
to enable an endogenous antigen-specific cytotoxic T-cell response
(20,21). Dendritic cells (DCs) play a crucial
role in the activation of the antitumor immunity (22-24).
The functional activity of DCs has been demonstrated to be
significantly reduced in cancer patients (24-26).
In cancer, the ability of DCs to capture tumor antigens and present
them to the T-cells, as well as to mount an effective cellular
response, is impaired (27,28).
The main reason is considered to be the impairment of the DC
maturation process (7), as well as
the T-cell activation mechanisms (27,28).
The activation of T cell-related endogenous immune
response ex vivo, outside the influence of the
immunosuppressive tumor microenvironment, has been demonstrated to
help succeed in obtaining efficient T-cells for tumor elimination
when administered to patients as part of combination therapy
(29,30). Several studies have confirmed the
effectiveness of DC antigen loading using DNA constructs (31-33).
In relation to this, the search for an effective combination of the
aforementioned therapeutic approaches is of utmost scientific
interest for further study and development of protocols for wide
clinical use for immunotherapy in cancer patients.
Tumor-associated antigens (TAAs) are usually
presented in a wide range of epithelial tumors, and each tumor can
express a wide range of known antigens (34-37).
Previously, the efficiency of in vitro generation antitumor
immune response with the use of DCs transfected with DNA constructs
encoding epitopes of particular tumor-associated antigen
determinants was demonstrated by the authors (38,39).
In the present study, this therapeutic approach was optimized and
the hypothesis about whether the efficiency of using genetic
constructs, encoding a wide range of TAAs for the activation of
T-cell cytotoxicity against autologous tumor cells from tumors of
different localization was examined.
The novelty of the present study, to the best of our
knowledge, may be attributed to the demonstration the effectiveness
of this approach by using a DNA construct which encodes epitopes of
tumor-associated antigens of a group of oncological pathologies,
not only limited to one nosology. In the present study, it was
demonstrated that the possibility of using cell technology based on
autologous DCs transfected with this DNA construct could
effectively induce a cytotoxic antitumor immune response in the
cell culture of patients with various oncological diseases [breast
cancer (BC), colorectal cancer (CRC) and non-small cell lung cancer
(NSCLC)]. This allows for the expansion of the scope of cellular
immunotherapy based on antigen-primed DCs.
Materials and methods
Patients
Heparinized venous blood and tumor samples obtained
between June, 2021 and March, 2022 from 9 patients with CRC, 13
patients with NSCLC and 18 patients with BC receiving treatment at
the City Clinical Hospital No. 1 (Novosibirsk) and Novosibirsk
Regional Oncology Center (Novosibirsk, Russia) were used in the
present study (Table I). The
inclusion criterion was the lack of history concerning surgery,
chemotherapy and/or radiation therapy. Adenocarcinomas of the
colon, lung or breast were histologically verified in all patients
in accordance with the pathology. The presence of the
HLA-A*02:01 allele was confirmed by genotyping DNA
isolated from peripheral blood cells, using an ALLSET™ GOLD HLA A
LOW RES SSP kit (54310D; Invitrogen; Thermo Fisher Scientific,
Inc.). Voluntary written informed consent was obtained from all
patients. All subjects gave their informed consent prior to their
participation in the study. The study was conducted in accordance
with the Declaration of Helsinki, and the study was approved by the
local Research Institute of Fundamental and Clinical Immunology
(RIFCI) Ethics Committee (No. 132 from June 4, 2021).
 | Table IClinical characteristics of the
cancer patients in the present studya. |
Table I
Clinical characteristics of the
cancer patients in the present studya.
| Cancer type
(n) | Colorectal cancer
(n=9) | Non-small cell lung
cancer (n=13) | Breast cancer
(n=18) |
|---|
| Age (years), median
(min; max) | 45 (41; 55) | 54 (43; 60) | 45 (35; 58) |
| Sex | | | |
|
Male | 7 | 12 | 0 |
|
Female | 2 | 1 | 18 |
| Diseases stage,
n | | | |
|
Stage
II | 6 | 4 | 10 |
|
Stage
III-IV | 3 | 9 | 8 |
| Localization
(n) | Rectum (2) Sigmoid
colon (5) Colon (2) | Peripheral
Localization (13) | Ductal (18) |
Polyepitope DNA constructs
Original DNA vaccine constructs were developed using
the corresponding artificial genes based on the pmax plasmid
(Addgene) (40). In particular,
the following designs were applied: The pmax-CTL_1 construct
containing epitopes from MAGE-A10, NY-ESO-1 and MUC-1; the
pmax-CTL2 construct containing epitopes from MAGE-A3, PRAME, EpCAM
and MUC-1; the pmax-CTL3 construct containing epitopes from EpCAM,
CEA, GuanylylCyclase C and 5T4; the pmax-CTL4 construct containing
epitopes from Legumain, VEGFR-1, VEGFR-2, FAP and Fos-related
antigen-1; the pmax-CTL5 construct containing epitopes from
Brachyury, SOX2, Snail1 and Snail2; the pmax-PolyTh construct
containing epitopes from HER2, hTERT, p53, WT1, NY-ESO-1, VEGFR-2,
survivin and MAGE-A3 (Table II).
For DC transfection, a mixture of an equimolar amount of all DNA
constructs was used, thereby providing a wide range of antigenic
information.
 | Table IIAmino acid sequences of the DNA
constructs. |
Table II
Amino acid sequences of the DNA
constructs.
| Construct name | Aminoacidic
sequence of the DNA constructs |
|---|
| Poly-CTL-epitope
construct 1 |
LMWITQCFLLLMWITQCFLGLYDGMEHLIAQIACSSPSVQLSLLMWITSLAQD
APPLVLVCVLVALRLLEFYLAMGPGPGFLLLLLTVLSLSYTNPAVFLSFHISNLIL
ILILSIVSLGLTYDGMLAASLLMWITQVSLLMWITQCFLFLWGPRAHASLLKFL
AKVGLYDGMEHLSLLMWITQCALGSTAPPVAILILSIVFIAALLLLTVLTVSTAP
PAHGVMLLVFGIDVYLFLWGPRAFLLLLLTVLTVVYLEYRQVPVLLLTVLTVV
WITQCFLPVGPGPGA KFVAAWTLKAAA |
| Poly-CTL-epitope
construct 2 |
VLVCVLVALLLLTVLTVVGLSNLTHVLLLLLTVLTVAKVAELVHFLALSRKVAE
LALGSTAPPVAAAVVAGIVVLVKIWEELSVLALQSLLQHLVLDGLDVLLAAYL
FLWGPRAFLLLLLTVLKVAELVHFLLSLSYTNPAVAAAYIFATCLGLYLEYRQV
PVQLLALLPSLAYLGLSYDGLLAAYNLTHVLYPVFLWGPRALVRLRELLCELA
ALYVDSLFFLAAYRTYWIIIELGPGPGFLLLLLTVLTVVILYENNVITIFLSFHIS
NLSLLQHLIGLMLTDVSPEPLALVETSYVKVAAKMILKMVQLATLAKFSPYLS
TAPPAHGVYLHARLRELCLGLSYDGLLGLKAGVIAVGPGPGAKFVAAWTL KAAA |
| Poly-CTL-epitope
construct 3 |
LMIAVFTLVLYGPDTPIVLYGPDTPVHMADMVTWLYVCGIQNSVAAVVAGIVV
LVAAYLWWVNNQSLFQMYLDTELFLTGNQLAVYLPRDVLAQLLLDLALWSLG
AFEHLPSLKLDTMIFGVSLQTSYVFLGIVLALIGAIFLLVALLFGHMLKIGLKAG
VIAVRTYWIIIELGIMIGVLVGVSLQTSYVFLAAYVFLGIVLAYLSGANLNLATV G
IMIGVMISAGSFGLVLLTYDTHVAYMDTLIRRLILYENNVITIALIGAIFLLYL
WWVNGQSLGQFRVYPELGLSAGATVGIYLNALEASVAAYIMIGVLVGVYLHSS
KTEVLMMGNSAFAGPGPGAKFVAAWTLKAAA |
| Poly-CTL-epitope
construct 4 |
YMISYAGMVAALQWMVQPHFLNLCEKPYPLYVYQNNIYLAALVCYGPGIAAY
VLLWEIFSLGPGPGMVWKVAVFLATLHKQYHLVLLAASEAEVNLSDHTVAIGL
FKCGIAVAATIFDRVYTIKQFCSTLTLKLLRGHTLVGLQREIEELSLTPFTPSLVK
LWRYSYTAMQSKVLLAVGPGPGSLLAASEAEVAAVLLAVALWLAADLISYSFQ
VSLTPFTPSLASSWEYYASVVLLWEIFSLILIHIGHHLTLNLTIMNVAGLLTCEAT
VKMYRKMVFYIGLSPDRQFVAYSYTATYYIAFLYRDVTWIAAAMFFWLLLVA
YQYGTTQTLFAVNWISYLFLQAETDKLAHLICYSFQVIMDPDEVPLYLGYPPPE
IFIIDTTYPAAATLFWLLLTLMMYDDIAYSGPG PGAKFVAAWTLKAAA |
| Poly-CTL-epitope
construct 5 |
SLPMLIWDSVAAYQNEEITALRMFPVLKVNVAAAYLYESYSMPVQLPNGLSPL
ILSSGAYSPIAASMLPVSHNAALLSAVENELLLAAIPPPEIMLIWDSVLAAASQY
PSLWSVRLIASWTPVAAYMNGSPTYSMAWLLPGTSTLGMALGSMGSVALQYR
VDHLLVLAPQAQPIRVDHLLSAVAAYFLVKKHFNASMYLPGAEVMISMYLPG
AGPGPGAKFVAAWTLKAAA |
| Poly-Th-epitope
construct |
MAAPGSARRPLLLLLLLLLLGLMHCASAPVIFSKAFSSLQLVFGSPYVSRLLGIC
LLTDLQPYMRQFVAHLAKFVAAWTLKAAAHNQVRQVPLQRLRIVKVRRAIEQ
LAAMDGFRLGFLHSGTAKSVLLPENNVLSPLRLGFLHSGTAKSVTCQARMFP
NAPYLPSCLVLLKEFTVSGNILTIRLTAADHRQLEKRFVPDGNRISWDSISTFKN
WPFLEGCACQFEELTLGEFLKLDRLGEFLKLDRERAKNKFNNFTVSFWLRVP KVSASHLE
QYIKANSKFIGITELRKRSHAGYQTI |
Effective antigenic determinants recognized by
cytotoxic T-lymphocytes were predicted using using NetMHC (41) and TEpredict software (42). Fragments containing epitopes
capable of binding to the largest number of allomorphs of human MHC
class II molecules (HLA-DR) were selected when designing the DNA
constructs. The fact that amino acid residues flanking the epitope
in the protein (the target antigen) can be important for
interaction with the corresponding T-cell receptor was taken into
account during fragment selection. The prediction of cytotoxic
T-cell epitopes was carried out for the allelic variant of
HLA-A*0201 class I molecules. Peptides for which the
predicted pIC50 value (a characteristic of the affinity of the
interaction of a peptide with an MHC molecule) was >6.8 were
selected for further analysis.
Generation of mature DCs
Mononuclear cells (MNCs) were isolated from the
peripheral blood of patients with CRC, BC and NSCLC using the
standard method of Ficoll-Urografin density gradient centrifugation
(ρ=1.077 g/ml) (43). The obtained
MNCs were then incubated for 30 min in 5% CO2 at 37˚C in
order to isolate cells with increased adhesion ability.
Non-adherent MNCs were isolated with the medium, precipitated by
centrifugation at 415 x g for 10 min at room temperature, and
cultured at 37˚C and 5% CO2 in a 75 cm2
culture flask (TPP Techno Plastic Products AG) to be further used
at a concentration of 2x106 cells/ml, in complete
RPMI-1640 medium containing 10% fetal calf serum (FCS; HyClone;
Cytiva), 2 mM L-glutamine (BioloT Ltd.), 10 mM HEPES buffer
(MilliporeSigma), 5x10-4 M 2-mercaptoethanol
(MilliporeSigma), 40 µg/ml gentamicin (Krka, d. d.) and 200 U/ml
benzylpenicillin (OJSC Biosintez) prior to seeding. The adherent
fraction was cultured in a 48-well plate (Greiner Bio One; Merck
KGaA) at a concentration of 1x106 cells/ml in 0.5 ml of
complete RPMI-1640 medium, supplemented with 50 ng/ml recombinant
human (rh) granulocyte-macrophage colony-stimulating factor and 100
ng/ml rhIL-4 (PeproTech, Inc.) for 96 h to obtain immature DCs. In
order to obtain mature DCs, rhTNF-α (25 ng/ml) (PeproTech, Inc.)
was added to the culture of immature DCs, and the cells were
incubated for 2 days. In the resulting DCs, the expression of
maturation and differentiation markers was assessed using flow
cytometry (flow cytometer BD FACS Aria, BD FACS Diva v.6.0
software, BD Biosciences), using primary antibodies labeled with
various fluorochromes [CD11c-PE-Cy7 (cat. no. 337216), CD83-APC
(cat. no. 305312) and HLA-DR-FITC (cat. no. 327006)] (BioLegend,
Inc.).
Magnetic transfection and evaluation
of the efficiency of dendritic cell transfection
The magnetic transfection method was used as a
method for delivering the DNA construct; unlike the electroporation
method, it permits the acquisition of a cell culture with high
viability (85.4±6.2%). The essence of magnetic transfection is the
preliminary sorption of the DNA construct on the surface of
magnetic beads, which penetrate into the cells in the culture of
dendritic cells under the influence of a magnetic field. The
magnetic transfection of mature DCs was performed in a total volume
of 0.5 ml in a 48-well plate. Plasmid pmax (the control plasmid)
and pmax-CTL1-8 (the target plasmid) were used for transfection.
Magnetic transfection was performed using PromoKine reagents; the
procedure was performed according to the manufacturer's protocol.
Plasmids were dissolved in DMEM (State Research Center of Virology
and Biotechnology ‘Vector’) in separate tubes; component was added
in the ratio of 0.3 µg of DNA plasmid per 0.3 µl of MATra-A
reagent, and the mixture was incubated at room temperature for 20
min. In parallel, DCs were precipitated by centrifugation at 266 x
g for 5 min at room temperature, and RPMI-1640 medium was replaced
with 250 µl of DMEM medium. Subsequently, the plasmid-MATra-A
complex was added to the cells (25 µl per well); the plate was
placed on a magnetic stand for 15 min. The medium was replaced
after transfection: the DMEM was removed, and 300 µl complete
RPMI-1640 medium were added. The transfected cells were incubated
overnight in 5% CO2 at 37˚C. The obtained dendritic
cells were then plated with MNCs. The transfection efficiency was
assessed using the Promo-Fluor-500 Nick Translation Labeling kit
(PromoKine), with further analysis on a flow cytometer BD FACS
Aria, BD FACS Diva v.6.0 software (BD Biosciences) using the
Flow-Fish method (44,45).
Co-culture of DCs and MNCs
The obtained DCs transfected with pmax and
pmax-CTL1-8 plasmids were co-cultured with the fraction of
non-adherent MNCs (at a concentration of 1x106 cells/ml)
for 120 h at 37˚C and 5% CO2 to prime specific antigens
(at a 1:10 DC to MNC ratio). Non-adherent MNCs cultured under the
same conditions, as well as cells cultured in the presence of DCs
not transfected with plasmids [MNCs+DC(0) group], were used as the
controls.
Generation of autologous tumor
cells
Tumor cells were obtained by cold trypsinization of
the tumor samples obtained from the patients during the planned
surgical intervention. A tumor sample was divided into small
fragments in RPMI-1640 medium containing 80 µg/ml gentamicin, 400
U/ml benzylpenicillin and 5 µg/ml amphotericin B (PanEco Ltd.),
placed in trypsin (Biolot Ltd.), minced with scissors, and left
overnight at >4˚C. To inhibit trypsin, RPMI-1640 medium
containing 10% FBS was added and mixed thoroughly; the homogeneous
suspension was precipitated (185 x g, 10 min, room temperature),
and the cell count was calculated in a Goryaev chamber (Minimed).
The cells were frozen in FCS with 10% DMSO and stored at -70˚C. The
cells were thawed and cultured in complete RPMI-1640 medium 24 h
prior to the cytotoxicity assay. Additionally, the viability of the
tumor cell culture was assessed by staining with erythrosin (0.5
mg/ml) (MilliporeSigma) at room temperature within 1 min, prior to
the cytotoxicity assay, once the cell suspension has been mixed
with the dye, which averaged 67.6±15.4%, and the settlement was
calculated based on the number of living cells.
Evaluation of the cytotoxic activity
of mononuclear cells against tumor cells
The cytotoxic activity was analyzed by assessing the
level of lactate dehydrogenase (LDH) in a conditioned medium in the
co-culture of the DC and MNC cell population and autologous tumor
cells from patients with CRC, BC and NSCLC. The procedure was
carried out according to the instructions of the manufacturer of
the kit ‘CytoTox96® Non-Radioactive Cytotoxicity Assayʼ
(Promega Corporation). Following the co-culture of the non-adherent
fraction of MNCs and DCs transfected with the plasmids, as well as
the culture of the cells of the control groups for 120 h, the cell
suspension was washed with RPMI-1640 culture medium (Biolot), and
the resulting cells were seeded at a cell concentration of
1x106 cells/ml into round-bottom plates (TPP Techno
Plastic Products AG; well volume, 50 µl) containing pre-thawed
autologous tumor cells at a 10:1 ratio and incubated for 16-18 h at
37˚C and 5% CO2. Cell seeding, and the experimental
protocol were performed in accordance with the instructions for the
‘CytoTox 96 Non-Radioactive Cytotoxicity Assay’ kit (Promega
Corporation). The optical density (OD) was measured on an Anthos
2020 spectrophotometer (Biochrom, Ltd.) at a single wavelength (490
nm). The cytotoxic effect was calculated according to the formula
proposed by the kit manufacturer and expressed as a percentage: %
cytotoxicity={[OD(experimental lysis)-OD(spontaneous lysis of
effector cells)-OD(spontaneous lysis of tumor cells)]/[(OD(maximum
lysis of tumor cells)-OD(spontaneous lysis of tumor cells)]} x100%.
Thus, the formula considered the natural death of the tumor cells
and MNCs.
Statistical analysis
Statistical data analysis was carried out using the
GraphPad Prism software (GraphPad Software, Inc.). The normality of
the sample distribution was assessed using the Kolmogorov-Smirnov
test. The non-parametric Kruskal-Wallis test was used for
statistical verification in the case of a non-normal distribution.
An appropriate post hoc test was used after the Kruskal-Wallis test
(e.g., Dunn's test). When comparing only 2 groups, a non-parametric
Mann-Whitney U test was used. A value of P<0.05 was considered
to indicate a statistically significant difference. Data are
presented as the median ± standard deviation. The number of
individuals per group is indicated by ‘n’ in the figure
legends.
Results
Generation of mature DCs and effector
cells
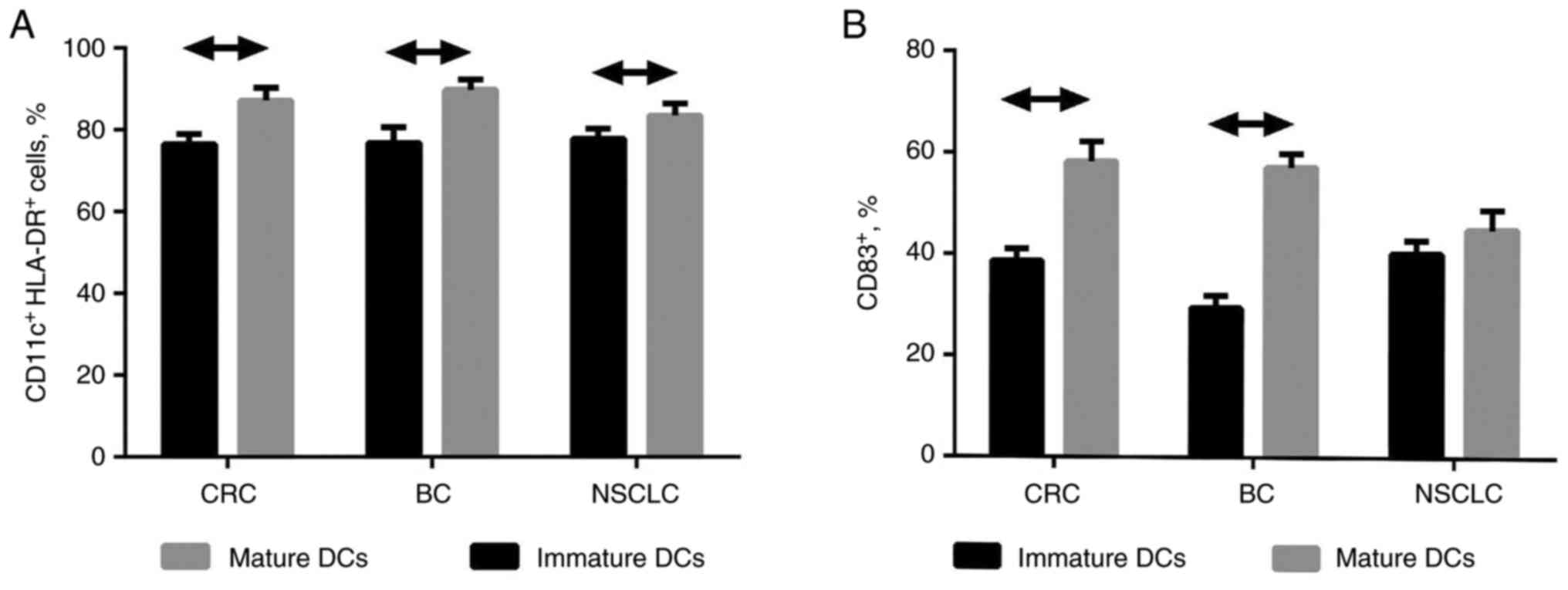
The mature DCs of patients with CRC, BC and NSCLC
with a >80% content of CD11c+HLA-DR+ cells
(Fig. 1A) and a >40% content of
CD83+ cells (Fig. 1B)
were obtained in vitro using the protocol described above.
The unified protocol for DC generation was used for CRC-, BC- and
NSCLC-derived cells, and it was observed to be effective for CRC-
and BC-derived dendritic cell maturation. At the same time, in case
of NSCLC-derived cells, the absence of a significant increase in
the number of CD83+ cells was demonstrated.
The described cell-based protocol allows the
generation of mature DCs derived from peripheral blood monocytes of
patients with CRC, BC and NSCLC. The resulting DCs are
characterized by high expression levels of the
CD11c+HLA-DR+ and CD83+ markers
(Fig. S1).
The following stage involved the magnetic
transfection of the obtained DCs with an original polyepitope DNA
construct. An average efficiency of the DNA transfection of DCs was
~70%. An example of the evaluation of the efficiency of
transfection using flow cytometry is presented in Fig. S2.
Following co-culture of the transfected DCs with the
autologous MNCs obtained from cancer patients, final studies were
conducted to evaluate the efficacy of the developed cell-based
vaccine, which consisted of antigen-activated MNCs (effector cells)
and mature DCs transfected with a polyepitope DNA construct. The
content of CD4+ and CD8+ lymphocytes between
groups of cancer patients did not exhibit significant differences
and amounted to 52-62% (CD4+ lymphocytes) and 24-27%
(CD8+ lymphocytes) (Table
III). The efficiency of mounting a specific cytotoxic immune
response in the co-culture of MNCs and DCs was evaluated using the
cytotoxicity assay against autologous tumor cells.
 | Table IIIRelative count of
CD3+CD4+ and CD3+CD8+
cells in the co-culture of the transfected DCs with the autologous
MNCs of patients with colorectal cancer, breast cancer and
non-small cell lung cancer. |
Table III
Relative count of
CD3+CD4+ and CD3+CD8+
cells in the co-culture of the transfected DCs with the autologous
MNCs of patients with colorectal cancer, breast cancer and
non-small cell lung cancer.
| Cancer type
(n) | Colorectal cancer
(n=9) | Non-small cell lung
cancer (n=13) | Breast cancer
(n=18) |
|---|
|
CD3+CD4+, % | 60.0±4.7 | 53.0±4.7 | 54.5±2.1 |
|
CD3+CD8+, % | 27.0±2.6 | 25.5±1.9 | 25.5±2.6 |
Evaluation of the cytotoxic activity
of MNCs against tumor cells
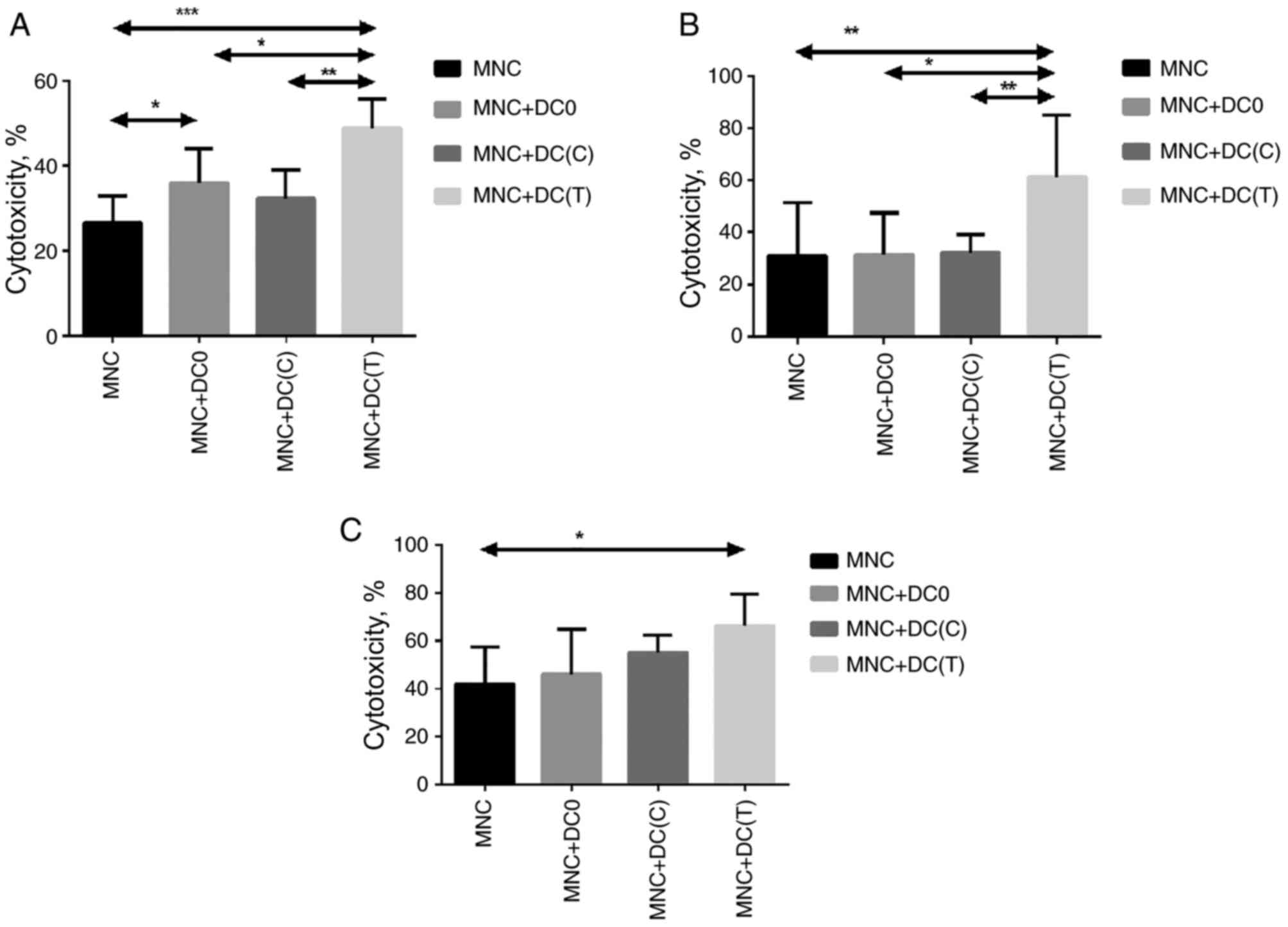
The LDH-releasing assay revealed that the
cytotoxicity of the MNCs activated by DCs transfected with a
polyepitopic DNA construct reached its values (45.5±7.1, 51.0±23.7
and 66.2±11.5% for patients with BC, NSCLC and CRC, respectively)
when co-cultured with mature antigen-activated DCs transfected with
the original DNA construct (Fig.
2). A significant increase in the cytotoxicity of cells in the
culture of MNCs co-cultured with antigen-primed DCs, compared with
groups of DCs and without transfection with the DNA construct of
DCs, indicates the induction of an antigen-mediated cytotoxic
immune response in vitro. These results directly demonstrate
the effectiveness of the described approach.
Discussion
The emergence and development of the tumor
development process is associated with a violation of the antitumor
immune defense of the body (19,28,29).
To effectively destroy tumor cells, there must be a sufficient
number of effector cells that are able to recognize and destroy
tumor cells. The aim of immune cell therapy is to obtain autologous
antigen-primed dendritic cells capable of stimulating antitumor
immunity. Hitherto, DNA constructs encoding epitopes of
tumor-associated antigens have been successfully used previously
(46). The following step in the
development of this therapeutic approach is the creation of DNA
constructs that can cover a wide range of tumor antigens, which is
crucial, considering that the expression of tumor antigens may
change qualitatively and quantitatively during chemotherapy and
radiation therapy (47).
The use of a DNA construct encoding epitopes of TAAs
of oncological diseases of general histogenesis will prevent tumor
‘avoidance’ when the antigenic composition of the effector cells
changes. It may also permit the DNA construct to be used for the
stimulation an antigen-specific anti-tumor immune response in
oncological diseases that share a common histogenesis (e.g., BC,
CRC and NSCLC). The cultivation and transfection of DCs in
vitro makes it possible to overcome the tolerance of T-cells to
tumor antigens and induces a complete cytotoxic immune response
(48). In the present study, the
ability of DCs, transfected with a DNA construct encoding epitopes
of TAAs of neoplasms, to use a DNA construct, encoding epitopes of
TAAs of various epithelial malignant neoplasms that have common
antigens and are more specific for each specific pathology, was
investigated. The use of transfection for the efficient delivery of
antigens for the presentation of DC MNCs is a novel option for
obtaining immune cells to stimulate antitumor immunity in
vitro. HLA-A02:01-specific constructs were created and tested,
the design of which was aimed at activating cytotoxic T-lymphocytes
by presenting the corresponding TAAs on the surface of DCs. An
analysis of cytotoxic activity revealed that the use of DCs
transfected with an allele-specific DNA construct encoding epitopes
of TAAs of BC, CRC and NSCLC induced an antitumor immune response
of MNCs against autologous tumor cells. It should be noted that
when using immunotherapies based on the use of TAAs as a target,
one should always be aware of safety issues, namely, the risks of
developing unwanted side effects (damage to normal cells). Provided
that TAAs are expressed on the surface of normal cells, there is
also a possibility of autoimmune damage. The risk of developing
such an undesirable effect increases with the use of immunotargeted
drugs that are highly sensitive to one tumor-associated antigen
(48-51).
Cell therapy based on the use of DCs as key cells in the
implementation of natural mechanisms for the induction and
development of an antigen-specific cytotoxic immune response
against tumor cells does not have a radical therapeutic effect,
such as surgical, chemo- and radiation, immunotargeted therapy and,
accordingly, does not have pronounced side-effects (31,52,53).
The use of polyepitopic DNA constructs for the antigen priming of
DCs renders it possible to induce a cytotoxic immune response
against tumor cells that express a wide range of TAAs, and of which
the expression levels are much higher compared to those in normal
cells. Thus, the use of DNA constructs encoding epitopes of the
main TAAs for tumors of different localization, and also having
similar antigenic characteristics for the antigen-priming of DCs,
can effectively stimulate the antitumor cytotoxic immune response.
This therapeutic approach renders it possible to address the
treatment of tumors not only of different localization, and also to
act on tumor cells which are characterized by antigenic escape
during chemotherapy and radiation therapy.
Thus, due to the versatility of the composition of
the DNA construct used, which contains the main most immunogenic
epitopes of TAAs, new directions become available for the use of
cell therapy based on mature DCs for the treatment of various
epithelial malignant neoplasms.
In the present study, the use of immunogenic
polyepitopes of TAAs as part of a DNA construct was successfully
examined, considering also the most common antigenic profile of the
studied tumors. It is known that the immunogenicity of a tumor is
dependent on factors associated with the tumor microenvironment,
including the functioning of antigen-presenting cells (DCs) and it
is not determined by the tumor cell only (54). Key prerequisites for tumor
immunogenicity include tumor antigenicity and the efficiency of
antigen processing and presentation (55). The proposed approach to the
production of mature antigen-primed DCs in vitro facilitates
the solution of the problem of low antigen processing and
presentation efficiency to a large extent. The use of a polyepitope
DNA construct also facilitates covering for the most common tumor
antigens. By contrast, the antigenicity of a tumor can vary, even
within the same type of cancer. It has been demonstrated for a
number of tumors (CRC, BC and NSCLC) that the immunogenicity of the
tumor depends on the molecular profile of the antigens expressed by
the tumor cells (56-59).
Considering these differences in the expression of antigenic
molecules, it is important to seriously consider this, since it is
indicative of the intracellular mechanisms and patterns of
development of various cancer subtypes. The development of cell
therapy approaches in the future will be directed to the tumor
cells of patients, considering the individual molecular profile of
the tumor.
The present study bears two main limitations: In the
framework of the study, the evaluation of changes in the
morphological shape during the maturation of DCs was not
photographed, and no in vivo experiments were conducted. The
absence of images of mature DCs does not cancel the results of the
evaluation of the expression of DC maturation markers using flow
cytometry, which is a more objective and visual method for
assessing the maturity of DCS. The study of the effectiveness of
the developed approach under in vivo conditions is a logical
continuation of the present study and justifies future research
perspectives a separate publication.
In conclusion, the use of a DNA construct encoding
epitopes of TAAs of various oncological diseases for priming
autologous DCs of oncological patients enables the induction of a
cytotoxic immune response in a culture of MNCs against autologous
tumor cells of patients with breast cancer, colorectal cancer, and
non-small cell lung cancer. This is of utmost importance for future
practical application of polyepitope DNA constructs more
universally, due to the greater representation of tumor antigens,
towards cellular immunotherapy of various oncological diseases and
the secondary prevention of relapse.
Supplementary Material
General scheme of gated dendritic
cells, in order to evaluate their differentiation and maturation by
flow cytometry using antibodies CD11c-PE-Cy7, HLA-DR-FITC,
CD83-PerCP-Cy5.5. HLA-DR, human MHC class II.
Typical scatter plots, evaluating the
efficiency of transfection of mature dendritic cells with the DNA
plasmid with the MaTRa-A reagent, using a probe derived from the
same plasmid using the Flow-Fish hybridization method. (A) Sample
without transfection. (B) Sample with transfection. On the Y axis,
lateral light scattering of cells; on the X axis, Atto488
fluorescence via the FITC channel. A probe was added to each sample
at a concentration of 3 μg/ml.
Acknowledgements
The authors are grateful to the staff of the
Laboratory of Molecular Immunology, Federal State Budgetary
Scientific Institution Research Institute of Fundamental and
Clinical Immunology.
Funding
Funding: The present study was funded by the Russian Science
Foundation grant no. 21-65-00004,
https://rscf.ru/project/21-65-00004/.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The conceptualization of the present study was
performed by SSe, AM and HS. Patient selection, clinical data
collection, experimentation and statistical analysis were performed
by VKu, EK, JS, JL, IO, JK, MK, AK, NK, SSi, AS, AV and VKo.
Project administration was assigned to SSe and VKu. The acquisition
of resources was made by AK, NK, VKo, SSi, AS and AV. SSe and HS
supervised this study. Data validation was performed by EK, JS, JL,
IO, AK, NK and JK. Figures and tables were prepared by VKu, JS, JK,
EK, and IO. Writing and original draft preparation were performed
by EK, VKu, SSi, AS, AV and MK. Additionally, SSe, MK and VKu
performed writing, review and editing of the manuscript. The
control of data collection and validation of the statistical
analysis of the data was carried out by VKu. VKu and SSe confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All subjects provided their informed consent to
inclusion prior to participation in the study. It was carried in
accordance with the Declaration of Helsinki, and was approved by
the ethics committee of the local Research Institute for
Fundamental and Clinical Immunology (RIFCI) (No. 132 of June 4,
2021). Voluntary written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Korman AJ, Peggs KS and Allison JP:
Checkpoint blockade in cancer immunotherapy. Adv Immunol.
90:297–339. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chowdhury PS, Chamoto K and Honjo T:
Combination therapy strategies for improving PD-1 blockade
efficacy: A new era in cancer immunotherapy. J Intern Med.
283:110–120. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Leach DR, Krummel MF and Allison JP:
Enhancement of antitumor immunity by CTLA-4 blockade. Science.
271:1734–1736. 1996.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shi X, Li CW, Tan LC, Wen SS, Liao T,
Zhang Y, Chen TZ, Ma B, Yu PC, Lu ZW, et al: Immune co-inhibitory
receptors PD-1, CTLA-4, TIM-3, LAG-3, and TIGIT in medullary
thyroid cancers: A large cohort study. J Clin Endocrinol Metab.
106:120–132. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ou X, Ma Q, Yin W, Ma X and He Z:
CRISPR/Cas9 gene-editing in cancer immunotherapy: Promoting the
present revolution in cancer therapy and exploring more. Front Cell
Dev Biol. 9(674467)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pettitt D, Arshad Z, Smith J, Stanic T,
Holländer G and Brindley D: CAR-T cells: A systematic review and
mixed methods analysis of the clinical trial landscape. Mol Ther.
26:342–353. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li S, Zhang Z, Lai WF, Cui L and Zhu X:
How to overcome the side effects of tumor immunotherapy. Biomed
Pharmacother. 130(110639)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Medler TR, Cotechini T and Coussens LM:
Immune response to cancer therapy: Mounting an effective antitumor
response and mechanisms of resistance. Trends Cancer. 1:66–75.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li Q, Carr AL, Donald EJ, Skitzki JJ,
Okuyama R, Stoolman LM and Chang AE: Synergistic effects of IL-12
and IL-18 in skewing tumor-reactive T-cell responses towards a type
1 pattern. Cancer Res. 65:1063–1070. 2005.PubMed/NCBI
|
|
11
|
Mortara L, Balza E, Bruno A, Poggi A,
Orecchia P and Carnemolla B: Anti-cancer therapies employing IL-2
cytokine tumor targeting: Contribution of innate, adaptive and
immunosuppressive cells in the anti-tumor efficacy. Front Immunol.
9(2905)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dubensky TW Jr and Reed SG: Adjuvants for
cancer vaccines. Semun Immunol. 22:155–161. 2010.PubMed/NCBI View Article : Google Scholar : Mantia-Smaldone GM
and Chu CS: A review of dendritic cell therapy for cancer: Progress
and challenges. BioDrug 27, 453-468, 2013.
|
|
13
|
Higgins JP, Bernstein MB and Hodge JW:
Enhancing immune responses to tumor-associated antigens. Cancer
Biol Ther 8: 1440-1449, 2009; Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tang K, Wu YH, Song Y and Yu B:
Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials
for cancer immunotherapy. J Hematol Oncol. 14(68)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu B, Kusmartsev S, Cheng F, Paolini M,
Nefedova Y, Sotomayor E and Gabrilovich D: Effective combination of
chemotherapy and dendritic cell administration for the treatment of
advanced-stage experimental breast cancer. Clin Cancer Res.
9:285–294. 2003.PubMed/NCBI
|
|
16
|
Teitz-Tennenbaum S, Li Q, Davis MA,
Wilder-Romans K, Hoff J, Li M and Chang AE: Radiotherapy combined
with intratumoral dendritic cell vaccination enhances the
therapeutic efficacy of adoptive T-cell transfer. J Immunother.
32:602–612. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Belderbos RA, Aerts JGJV and Vroman H:
Enhancing dendritic cell therapy in solid tumors with
immunomodulating conventional treatment. Mol Ther Oncolytics.
13:67–81. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kawano M, Tanaka K, Itonaga I, Iwasaki T,
Miyazaki M, Ikeda S and Tsumura H: Dendritic cells combined with
doxorubicin induces immunogenic cell death and exhibits antitumor
effects for osteosarcoma. Oncol Lett. 11:2169–2175. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bai R, Chen N, Li L, Du N, Bai L, Lv Z,
Tian H and Cui J: Mechanisms of cancer resistance to immunotherapy.
Front Oncol. 10(1290)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Santos PM and Butterfield LH: Dendritic
cell-based cancer vaccines. J Immunol. 200:443–449. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee C, Lee M and Rhee I: Distinct features
of dendritic cell-based immunotherapy as cancer vaccines. Clin Exp
Vaccine Res. 7:16–23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Giovanelli P, Sandoval TA and
Cubillos-Ruiz JR: Dendritic cell metabolism and function in tumors.
Trends Immunol. 40:699–718. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Steinman RM and Nussenzweig MC: Dendritic
cells: Features and functions. Immunol Rev. 53:127–147.
1980.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gardner A, de Mingo Pulido Á and Ruffell
B: Dendritic cells and their role in immunotherapy. Front Immunol.
11(924)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Satthaporn S, Robins A, Vassanasiri W,
El-Sheemy M, Jibril JA, Clark D, Valerio D and Eremin O: Dendritic
cells are dysfunctional in patients with operable breast cancer.
Cancer Immunol Immunother. 53:510–518. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gallois A and Bhardwaj N: Dendritic
cell-targeted approaches to modulate immune dysfunction in the
tumor microenvironment. Front Immunol. 4(436)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mastelic-Gavillet B, Sarivalasis A, Lozano
LE, Wyss T, Inoges S, de Vries IJM, Dartiguenave F, Jichlinski P,
Derrè L, Coukos G, et al: Quantitative and qualitative impairments
in dendritic cell subsets of patients with ovarian or prostate
cancer. Eur J Cancer. 135:173–182. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bandola-Simon J and Roche PA: Dysfunction
of antigen processing and presentation by dendritic cells in
cancer. Mol Immunol. 113:31–37. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang L and Carbone DP: Tumor-host immune
interactions and dendritic cell dysfunction. Adv Cancer Res.
92:13–27. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Restifo NP, Dudley ME and Rosenberg SA:
Adoptive immunotherapy for cancer: Harnessing the T cell response.
Nat Rev Immunol. 12:269–281. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shevchenko JA, Khristin AA, Kurilin VV,
Kuznetsova MS, Blinova DD, Starostina NM, Sidorov SV and Sennikov
SV: Autologous dendritic cells and activated cytotoxic T-cells as
combination therapy for breast cancer. Oncol Rep. 43:671–680.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ophir E, Bobisse S, Coukos G, Harari A and
Kandalaft LE: Personalized approaches to active immunotherapy in
cancer. Biochim Biophys Acta. 1865:72–82. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sennikov SV, Khantakova JN, Kulikova EV,
Obleukhova IA and Shevchenko JA: Modern strategies and capabilities
for activation of the immune response against tumor cells. Tumour
Biol. 39(1010428317698380)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Brossart P, Schneider A, Dill P, Schammann
T, Grünebach F, Wirths S, Kanz L, Bühring HJ and Brugger W: The
epithelial tumor antigen MUC1 is expressed in hematological
malignancies and is recognized by MUC1-specific cytotoxic
T-lymphocytes. Cancer Res. 61:6846–6850. 2001.PubMed/NCBI
|
|
35
|
Cloosen S, Arnold J, Thio M, Bos GM,
Kyewski B and Germeraad WT: Expression of tumor-associated
differentiation antigens, MUC1 glycoforms and CEA, in human thymic
epithelial cells: Implications for self-tolerance and tumor
therapy. Cancer Res. 67:3919–3926. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Baeuerle PA and Gires O: .: EpCAM (CD326)
finding its role in cancer. Br J Cancer. 96:417–423.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Criscitiello C: Tumor-associated antigens
in breast cancer. Breast Care (Basel). 7:262–266. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sennikov SV, Shevchenko JA, Kurilin VV,
Khantakova JN, Lopatnikova JA, Gavrilova EV, Maksyutov RA, Bakulina
AY, Sidorov SV, Khristin AA and Maksyutov AZ: Induction of an
antitumor response using dendritic cells transfected with DNA
constructs encoding the HLA-A*02:01-restricted epitopes
of tumor-associated antigens in culture of mononuclear cells of
breast cancer patients. Immunol Res. 64:171–180. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kuznetsova M, Lopatnikova J, Shevchenko J,
Silkov A, Maksyutov A and Sennikov S: Cytotoxic activity and memory
T cell subset distribution of in vitro-stimulated CD8+ T cells
specific for HER2/neu epitopes. Front Immunol.
10(1017)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sennikov SV, Maksyutov AZ, Maksyutov RA,
Bakulina AY, Gavrilova EV, Kurilin VV, Lopatnikova YA, Shevchenko
YA, Obleukhova IA, Kulikova EV, et al: The polyepitope antitumor
vaccine construct containing epitopes of tumor-associated antigens,
pharmaceutical composition, and its application to elicit the
specific antitumor immune response. RF Patent 2684235. Filed
November 29, 2016; issued April 4, 2019.
|
|
41
|
Lundegaard C, Lamberth K, Harndahl M, Buus
S, Lund O and Nielsen M: NetMHC-3.0: Accurate web accessible
predictions of human, mouse and monkey MHC class I affinities for
peptides of length 8-11. Nucleic Acids Res. 36 (Web Server
Issue):W509–W512. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Antonets DV and Maksiutov AZ: TEpredict:
Software for T-cell epitope prediction. Mol Biol. 44:119–127.
2010.PubMed/NCBI
|
|
43
|
Böyum A: Separation of leukocytes from
blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl.
97(7)1968.PubMed/NCBI
|
|
44
|
Rufer N, Brümmendorf TH, Kolvraa S,
Bischoff C, Christensen K, Wadsworth L, Schulzer M and Lansdorp PM:
Telomere fluorescence measurements in granulocytes and T lymphocyte
subsets point to a high turnover of hematopoietic stem cells and
memory T cells in early childhood. J Exp Med. 190:157–167.
1999.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Arrigucci R, Bushkin Y, Radford F, Lakehal
K, Vir P, Pine R, Martin D, Sugarman J, Zhao Y, Yap GS, et al:
FISH-flow, a protocol for the concurrent detection of mRNA and
protein in single cells using fluorescence in situ hybridization
and flow cytometry. Nat Protoc. 12:1245–1260. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Schneider T, Hoffmann H, Dienemann H,
Schnabel PA, Enk AH, Ring S and Mahnke K: Non-small cell lung
cancer induces an immunosuppressive phenotype of dendritic cells in
tumor microenvironment by upregulating B7-H3. J Thorac Oncol.
6:1162–1168. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hu Y, Qi W, Sun L, Zhou H, Zhou B and Yang
Z: Effect of TGF-β1 on blood CD4+CD25 high regulatory T cell
proliferation and Foxp3 expression during non-small cell lung
cancer blood metastasis. Exp Ther Med. 16:1403–1410.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Obleukhova I, Kiryishina N, Falaleeva S,
Lopatnikova J, Kurilin V, Kozlov V, Vitsin A, Cherkasov A, Kulikova
E and Sennikov S: Use of antigen-primed dendritic cells for
inducing antitumor immune responses in vitro in patients with
non-small cell lung cancer. Oncol Lett. 15:1297–1306.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Fiala O, Šorejs O, Šustr J and Fínek J:
Side effects and efficacy of immunotherapy. Klin Onkol. 33:8–10.
2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Blidner AG, Choi J, Cooksley T, Dougan M,
Glezerman I, Ginex P, Girotra M, Gupta D, Johnson D, Shannon VR, et
al: Cancer immunotherapy-related adverse events: Causes and
challenges. Support Care Cancer. 28:6111–6117. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kichloo A, Albosta M, Dahiya D, Guidi JC,
Aljadah M, Singh J, Shaka H, Wani F, Kumar A and Lekkala M:
Systemic adverse effects and toxicities associated with
immunotherapy: A review. World J Clin Oncol. 12:150–163.
2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Boudewijns S, Westdorp H, Koornstra RH,
Aarntzen EH, Schreibelt G, Creemers JH, Punt CJ, Figdor CG, de
Vries IJ, Gerritsen WR and Bol KF: Immune-related adverse events of
dendritic cell vaccination correlate with immunologic and clinical
outcome in stage III and IV melanoma patients. J Immunother.
39:241–248. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chen C, Ma YH, Zhang YT, Zhang F, Zhou N,
Wang X, Liu T and Li YM: Effect of dendritic cell-based
immunotherapy on hepatocellular carcinoma: A systematic review and
meta-analysis. Cytotherapy. 20:975–989. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mellman I and Steinman RM: Dendritic
cells: Specialized and regulated antigen processing machines. Cell.
106:255–258. 2001.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Blankenstein T, Coulie PG, Gilboa E and
Jaffee EM: The determinants of tumour immunogenicity. Nat Rev
Cancer. 12:307–313. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Picard E, Verschoor CP, Ma GW and Pawelec
G: Relationships between immune landscapes, genetic subtypes and
responses to immunotherapy in colorectal cancer. Front Immunol.
11(369)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Denkert C, von Minckwitz G, Darb-Esfahani
S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen
F, Furlanetto J, et al: Tumour-infiltrating lymphocytes and
prognosis in different subtypes of breast cancer: A pooled analysis
of 3771 patients treated with neoadjuvant therapy. Lancet Oncol.
19:40–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
de Vries NL, Swets M, Vahrmeijer AL,
Hokland M and Kuppen PJ: The immunogenicity of colorectal cancer in
relation to tumor development and treatment. Int J Mol Sci.
17(1030)2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhou J, Yu X, Hou L, Zhao J, Zhou F, Chu
X, Wu Y, Zhou C and Su C: Epidermal growth factor receptor tyrosine
kinase inhibitor remodels tumor microenvironment by upregulating
LAG-3 in advanced non-small-cell lung cancer. Lung Cancer.
153:143–149. 2021.PubMed/NCBI View Article : Google Scholar
|