Introduction
Breast cancer is the most commonly occurring
neoplasm in women. In 2018 alone, 2.1 million new cases were
reported worldwide, rendering breast cancer the malignancy with the
second highest overall incidence and the first cause of
cancer-related death in women. Risk factors for breast cancer range
from hormonal and anthropometric to dietary (1).
Triple negative breast cancer (TNBC) does not
express hormone receptors and lacks human epidermal growth factor
receptor 2 (HER2) overexpression (2). It accounts for 12-17% of all breast
tumors (3). In a study of breast
cancer patients in Mexico, TNBC was reported to represent between
16 and 23% of breast tumors. The frequency of diagnosis is 72% in
stages II and III, and 12.9% in stage IV (4,5).
Clinically, triple negative tumors are more common
in young, Hispanic and African-American patients. They frequently
present as high-grade tumors with a high risk of recurrence (HR of
4.2 for recurrence compared with non-triple negative tumors), and,
similarly, with a high prevalence of BRCA mutations (up to 20% of
TNBCs) (2,6-8).
TNBC, along with HER2 positive tumors, present a
greater risk of developing distant metastasis at the time of
diagnosis (7,9). These metastases preserve the
molecular characteristics of the primary tumor that originated them
(10). Main sites for TNBC
metastasis are the liver, lung and brain. These are associated with
a worse prognosis and have a median survival of 9.0 (3.0-17.0),
11.0 (4.0-20.0) and 6.0 (2.0-13.0) months, respectively. A
distinctive feature of TNBC is a significantly lower rate of bone
metastasis (10,11).
The risk of brain metastasis in TNBC is up to 3.5
times higher than in luminal tumors. A total of ~3.5 to 4.7% of
TNBC patients will develop brain metastases as the first site of
recurrence, compared with 1.3% in patients with non-TNBC. Moreover,
TNBC has the shortest time interval between diagnosis of early
stage disease and brain metastasis (12). Certain clinical characteristics,
including young age, lymph node disease, large or high grade tumors
and multiple visceral metastases, have been associated with a
higher incidence of brain metastasis (13,14).
The cerebellum and basal ganglia are the most common brain
metastasis locations, representing 33% of cases. This may be due to
the high blood flow in these areas (13). This is relevant as patients with
symptomatic brain metastases present a worse prognosis, with a
median survival of 3 to 9 months after diagnosis (15).
TNBC presents a higher risk of leading to death
whenever a recurrence takes place (6). A distinctive pattern of recurrence
has been identified among TNBCs: in the first two years after
diagnosis, there is a rapid increase in the rate of recurrence with
a peak at 3 years, followed by a rapid decrease in the following 5
years, and a very low risk of subsequent recurrences (16).
All the aforementioned factors contribute to the
fact that TNBC has a clearly lower overall survival (OS) and
cancer-specific survival, as well as a worse prognosis, with an
increase in mortality after 2 years of diagnosis, compared with
other subtypes of breast cancer (6-9).
Furthermore, treatment of TNBC is often complex. The lack of tumor
markers to direct treatment, along with an increased resistance to
conventional treatments, make TNBC a challenge for clinicians
(8). There is consequently a
growing need for the identification and development of risk
profiles to guide management.
TNBC tumors have distinct patterns of genetic
expression. A previous study showed that levels of COX1, COX2,
ALOX5 and ALOX5AP expression were high in TNBC, but low in other
subtypes. It should be noted that this report also showed that
there is overlap in gene expression across breast cancer subtypes.
For instance, CYP19A1, which encodes for aromatase, is expressed in
all subtypes. However, its expression is correlated to different
genes (17).
A study published in 2011 by Lehman et al
(18) identified 6 subtypes of
gene expression in TNBC: basal-like-1 (BSL1), basal-like-2 (BSL2),
immunomodulatory (IM), mesenchymal (M), mesenchymal stem-like (MSL)
and luminal androgen receptor (LAR).
The BSL subtypes (BSL1 and BSL2) represent 47% of
TNBC cases and express high levels of cell cycle genes. In the BSL1
subtype there are high levels of DNA damage response gene
expression, while the BSL2 subtype displays unique gene ontologies
involving growth factor signaling. The IM subtype is enriched for
gene ontologies involved in immune cell processes. M and MSL
subtypes both express genes involved in cell motility and cell
differentiation. However, the MSL subtype additionally expresses
genes linked to growth factor signaling pathways. The LAR subtype
expresses genes involved in hormonally regulated pathways, such as
those related to the androgen receptor (AR) (18).
Regarding the clinicopathological characteristics,
the Lehman et al (18)
study suggested that tumor size and histological type did not
differ significantly between TNBC subtypes, while the age at
diagnosis was higher in the LAR subtype. In terms of prognosis, the
study showed that recurrence-free survival (RFS) differed
significantly between subtypes. RFS was lower in the M subtype
compared with BSL1 and IM. In another study by Masuda et al
(19), BSL1 had the highest
pathologic complete response (pCR) rate, while BSL2 and LAR had the
lowest.
Hispanics are more likely to develop TNBC compared
with non-Hispanic whites (20).
However, little is known about the role of TNBC molecular subtypes
in this population. Hispanics have often been underrepresented in
studies defining TNBC subtypes and their clinical, pathological and
prognostic characteristics (21).
In the present study, it was aimed to identify
differences in prognosis across TNBC molecular subtypes in a
Mexican based cohort. Additionally, it was aimed to describe the
clinical and pathological characteristics and identify differences
in treatment response and incidence of metastasis per TNBC
molecular subtype in this population. Finally, the present study
examined the behavior of metastatic [central nervous system (CNS)
and visceral non-CNS] and non-metastatic TNBC.
Materials and methods
The present study retrospective cohort study was
approved (approval no. CLAVE SALUD-2013-01-201336) by the bioethics
and scientific committee of Mexico's National Institute of Cancer
(INCan; Mexico City, Mexico) and conducted at the aforementioned
institute. Written consent was obtained from all included patients
before being included in the study.
Patients
Female patients (n=55) with a histopathological
diagnosis of TNBC and a viable tissue sample were included. TNBC
diagnosis was defined as having an immunohistochemical report (IHC)
indicating estrogen receptor (ER)-negative, progesterone receptor
(PR)-negative, and HER-2-negative in the initially performed
Tru-Cut biopsy at INCan (Mexico City, Mexico). A viable tissue
sample was defined as the availability of a formalin-fixed
paraffin-embedded breast tumor specimen with 70% or higher
neoplastic cellularity and 200 ng of RNA.
For hematoxylin-eosin staining,
Tissue-Tek® Glas™ g2 Glass Coverslipper was used. The
wax was first dissolved with xylene, which was later removed by
passing the slide through ethanol and thoroughly rinsing with
water. The slide was first stained for 3 min at room temperature
with Harris hematoxylin and was ‘blued’ by treatment with a weakly
alkaline solution. The section was later stained for 30 sec at room
temperature with eosin and was lastly rinsed with alcohol and
xylene.
Samples were assessed by IHC according to the 2020
ASCO/CAP guidelines. The antibodies used were ER (clone SP1; cat.
no. 760-4324), PR (clone 1E2; cat. no. 790-4296), HER2 (4B5; cat.
no. 760-4324; all from Ventana Medical Systems, Inc.) and Ki-67
(clone SP6; cat. no. CRM 325B Biocare Medical, LLC;). An
independent batch of tumor tissues, processed in the same manner as
our samples, was used for determining staining specificity. Serial
titrations were performed in order to obtain optimal concentrations
for every antibody: anti-CK14 (1:500; clone SP53; cat. no.
760-4805), anti-CK17 (1:150; clone EP98; cat. no. 317R-16; both
from Cell Marque; MilliporeSigma), anti-AR (1:80; clone 441; cat.
no. Mob245; Diagnostic BioSystems, Inc.), anti-p63 (1:100; clone
cm163c; cat. no. CM 163C; Biocare Medical, LLC) and anti-CK5/6
(1:500; clone 16B4; cat. no. GA780; DAKO; Agilent Technologies,
Inc.). Additionally, antigen retrieval using Tris-EDTA or citrate
was performed.
For chromogenic immunodetection, DAKO Envision
systems or MACH 1 Universal HRP Polymer and diaminobenzidine were
used. Afterwards, samples were counterstained for 3 min at room
temperature with hematoxylin. All samples were reviewed with an
Olympus BX53 microscope (phase contrast and fluorescence) at low
magnification by a breast cancer pathologist who was blinded to
patient characteristics and outcomes, and the positivity/negativity
was determined following the College of American Pathologists
guidelines (22): Any given
biomarker was reported as positive when its expression was ≥1% in
neoplastic cells even at low intensity in either cytoplasm,
nucleus, or cytoplasmic membrane. On the contrary, when the
expression was <1%, the biomarker was reported as negative.
All included patients were first treated at Mexico's
National Institute of Cancer between 2007 and 2011. Follow-up for
each patient began at the date when treatment was first
administered, and continued until: i) Loss of follow-up, ii) Death
or iii) Last visit before our cut-off date (June 2019). Patient
data was obtained from electronic medical records. Collected
information included administered drugs, treatment response,
presence of metastatic disease and/or recurrence, as well as other
clinical and pathological characteristics.
Gene expression profile analysis
Ultrasound-guided Tru-Cut needle biopsy samples were
obtained, formalin fixed for 1 h at 56˚C and paraffin embedded.
These biopsies were later subjected to microarray analysis using
the Human Gene ST 2.0 microarray platform (Affymetrix; Thermo
Fisher Scientific, Inc.). It should be noted that the corresponding
microarray data was used in a previous study (23) and is publicly available (accession
information is available in the ‘Availability of data and
materials’ section). Microarray data was analyzed with the R
software tool (24) and
Bioconductor libraries (25).
Based on the gene expression results, each patient was classified
into one of the 6 TNBC molecular subtypes reported by Lehman et
al in 2011(18).
For technical quality control, results were
processed before performing the differential expression analysis.
This so-called low-level processing is performed at the probe level
and corresponds to background correction using Robust Multiarray
Average. This eliminates nonspecific hybridization, normalizes to
remove systematic variations, and allows fluorescence intensity
signals to be comparable with each other (Quantile Normalization
method) (26,27).
RNA was extracted with TRIzol (Ambion; Thermo Fisher
Scientific, Inc.) from paraffin-embedded samples. Later, cDNA was
obtained using the AffymetrixTM SensationPlus kit following the
manufacturer's protocol. This technique allows for a high and good
quality of pure cDNA for microarray analysis.
In order to increase the number of samples used for
subgroup identification, previously processed and classified (by
PAM50) Mexican women breast cancer samples (106 tumor samples and
35 controls) from the National Institute of Genomic Medicine
(INMEGEN) in Mexico City, were utilized in addition to our
collected samples (66 samples). These samples were assessed in a
previously published work and their data are publicly available (in
the dbGaP repository, accession no. phs000369.v1.p1) (28). In order to combine all samples,
data was normalized using the batch effect adjustment method with
the ComBat algorithm (29)
implemented in the Bioconductor library.
Statistical analysis
Chi-squared tests or Fisher's exact tests were used
to compare the distribution of categorical variables (patient
characteristics and treatment-related characteristics) between
groups. For continuous variables, differences were analyzed using
unpaired Student's t-test. These tests were performed as
two-tailed, and P<0.05 was considered to indicate a
statistically significant difference. Survival curves were
generated using the Kaplan-Meier method, and differences between
groups were analyzed with the log-Rank test. All analyses were
performed using SPSS version 20.0 (IBM Corp.).
Results
Our cohort initially comprised 80 female patients
with a diagnosis of TNBC, of whom, 66 had a viable
paraffin-embedded sample (as aformentioned). Representative images
of the pathological TNBC specimens used in the present study are
shown in Fig. 1.
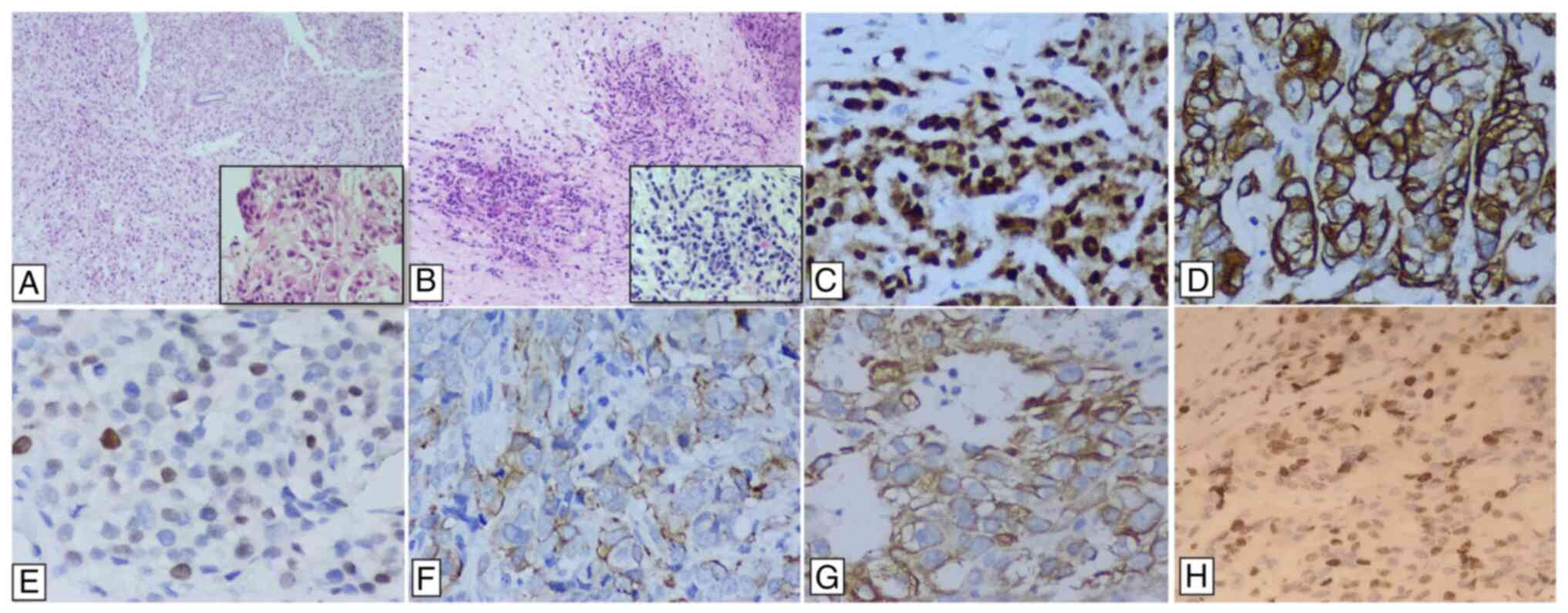 | Figure 1Representative images of
immunohistochemical assay by biomarker. (A) H&E staining, which
shows a ductal carcinoma (magnification, x10) and tumor detail
(square) (magnification, x100). (B) H&E staining of lobular
carcinoma (magnification, x100) and tumor detail (square)
(magnification, x100). (C) Immunoperoxidase staining of androgen
receptor with nuclear expression in 100% of neoplastic cells with
high intensity (magnification, x400). (D) Immunoperoxidase staining
of CK of low molecular weight (CK14) with cytoplasm membrane
staining and high intensity (magnification, x400). (E) p63 nuclear
staining (magnification, x400). (F) CK17 staining (magnification,
x400). (G) Immunoperoxidase staining of CK 5/6, with cytoplasm
membrane staining 60% of neoplastic cells with high intensity
(magnification, x400). (H) Immunoperoxidase staining of ki-67,
nuclear expression in the 30% of neoplastic cells with high
intensity (magnification, x400). CK, cytokeratin. |
After molecular testing, 11 samples were reported as
‘unspecified’ molecular subtype. Therefore, the final cohort was
made up of 55 patients. Based on the gene expression results, each
patient was classified into one of the TNBC molecular subtypes
reported by Lehman et al (18) in 2011, obtaining the following
results: 16 patients with the IM subtype, 12 of M subtype, 11 of
BSL2 subtype, 9 of BSL1 subtype, 6 of LAR subtype and 1 of MSL
(Table I). Mean patient follow-up
time was 47.1 months (range, 3-137 months).
 | Table ITriple-negative breast cancer
molecular subtypes prevalence in a 55-patient cohort at Mexico's
National Institute of Cancer between 2007 and 2011. |
Table I
Triple-negative breast cancer
molecular subtypes prevalence in a 55-patient cohort at Mexico's
National Institute of Cancer between 2007 and 2011.
| Molecular
subtype | Number of patients
(%) |
|---|
| Basal-like 1 | 9 (16.4) |
| Basal-like 2 | 11(20) |
|
Immunomodulatory | 16 (29.1) |
| Mesenchymal | 12 (21.8) |
| Androgen-like
receptor | 6 (10.9) |
| Mesenchymal
Stem-Like | 1 (1.8) |
Gene expression profile analysis
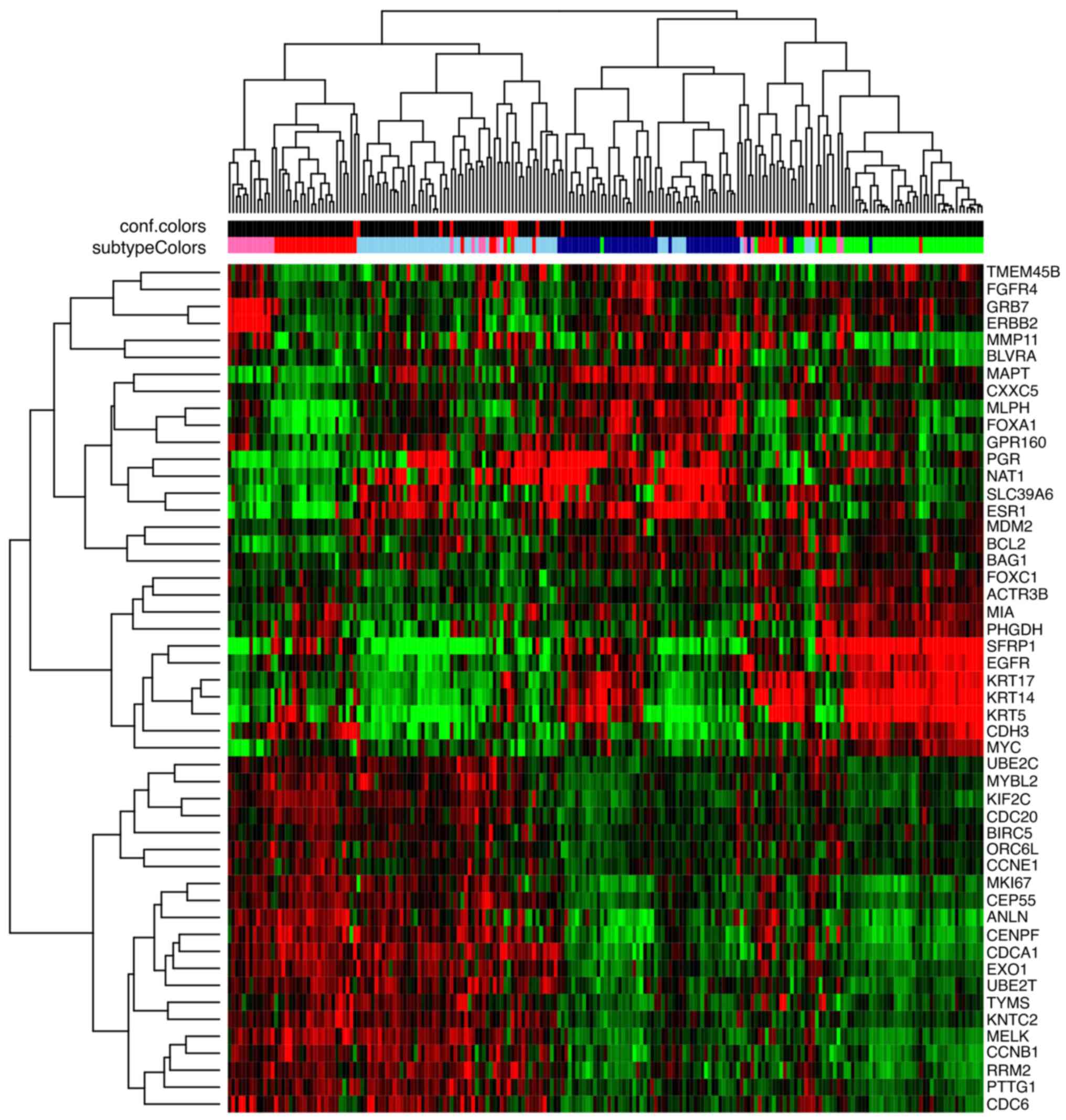
A heat map demonstrating hierarchical unsupervised
clustering is observed in Fig. 2.
Centroids, which were defined by Parker et al (30) using Caucasian women samples, were
used. Therefore, slight differences may be encountered compared
with Hispanic women. In the dendrogram, it is revealed that samples
are clustered by their molecular profile; very few samples are
clustered in intermediate positions.
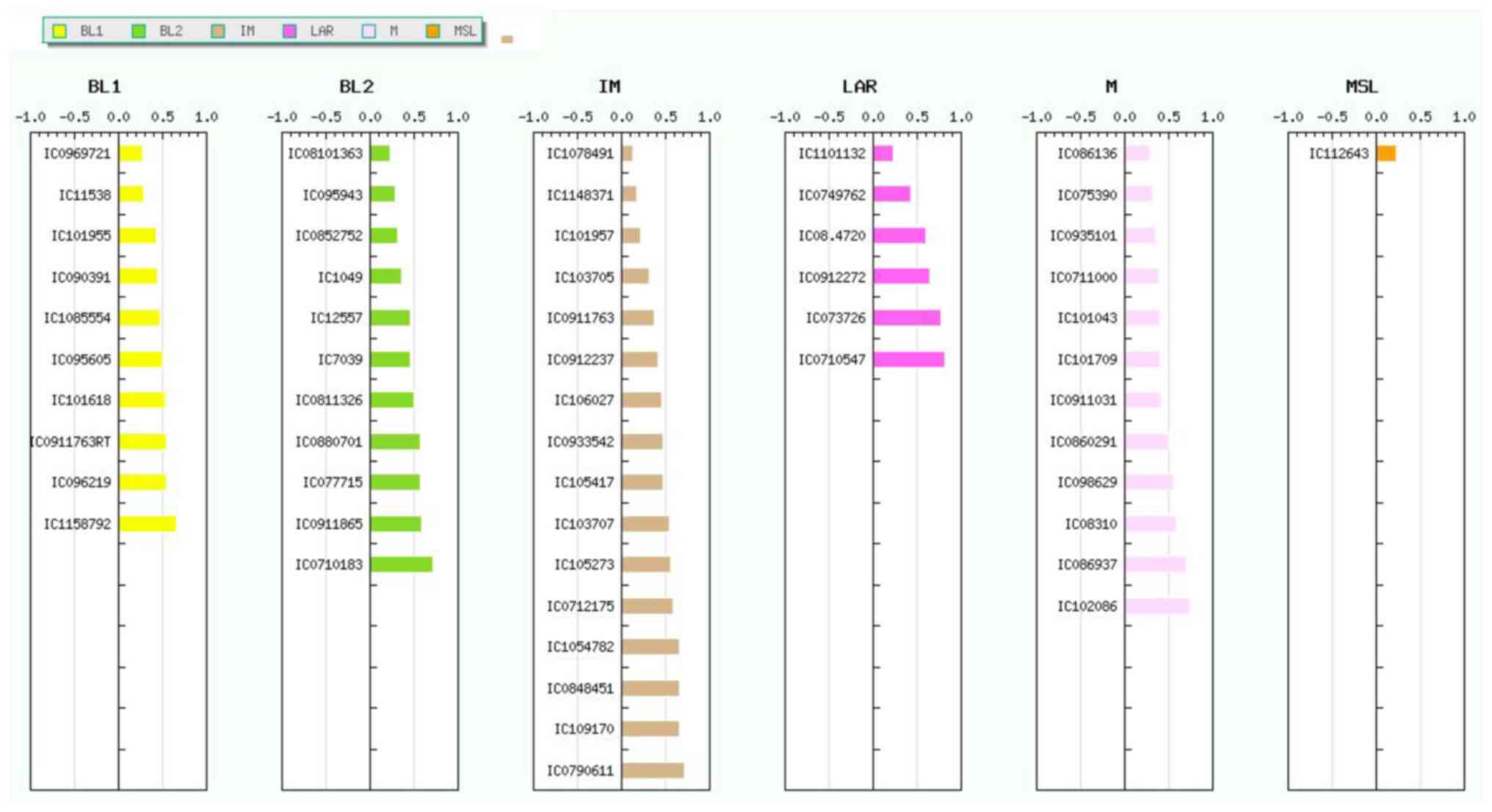
All samples were analyzed using the TNBCtype
web-based tool (developed by Vanderbilt University). This algorithm
is based on 3,247 gene expression profiles from 21 breast cancer
data sets, from which the 6 aforementioned TNBC subtypes were
discovered. The 55 samples which were classified into TNBC subtypes
(9 BL1, 11 BL2, 16 IM, 6 LAR, 12 M and 1 ML) are revealed in
Fig. 3.
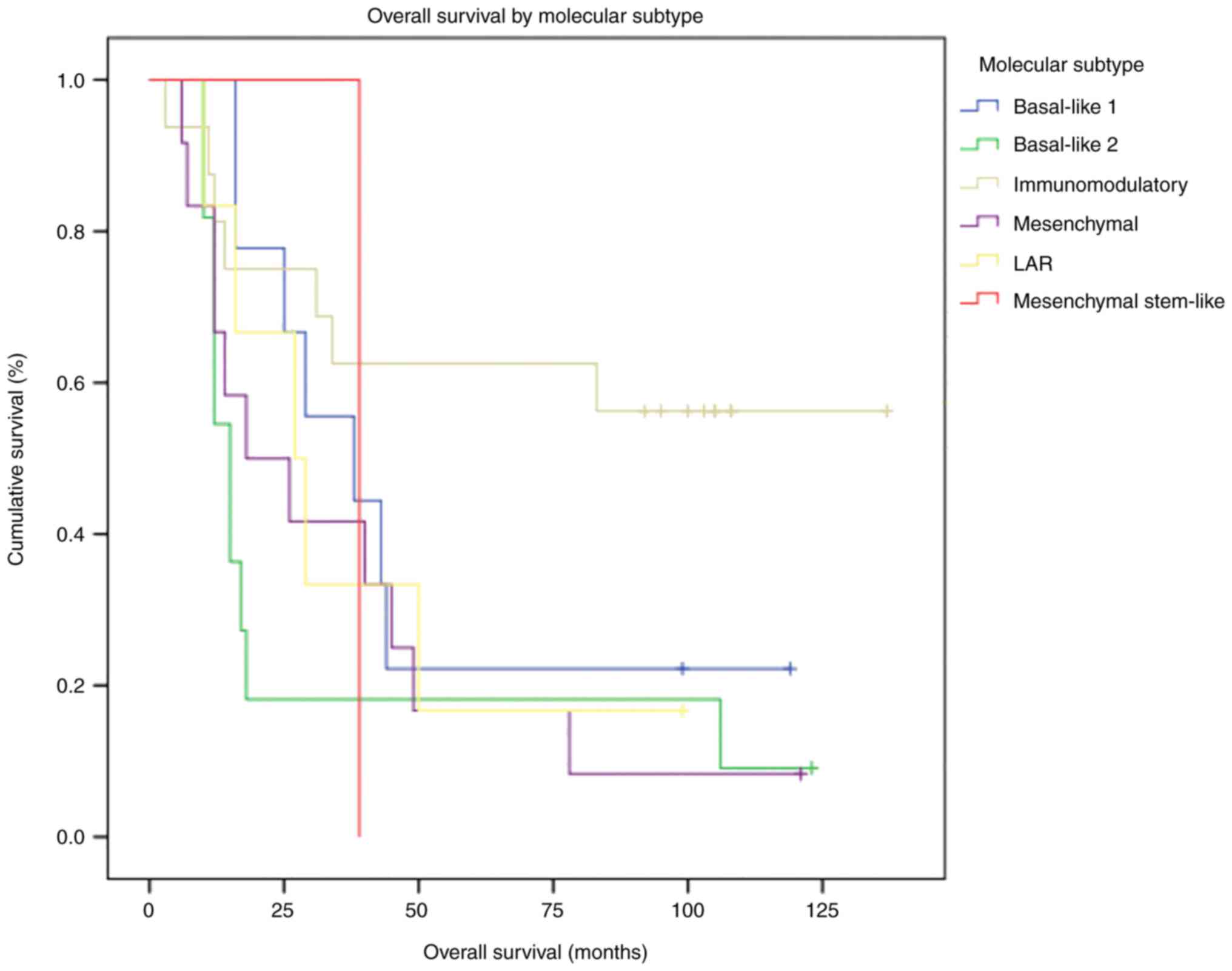
OS and survival analysis by TNBC
molecular subtype
As aforementioned, the cut-off date for follow-up
was June 2019. Up to that moment, only 14 of the 55 patients (25%)
were reported alive. The entire cohort's median OS was 29 months.
OS by molecular subtype is presented in Table II. The best OS was observed in the
IM subtype, as median OS was not reached at the cut-off date. The
worst median OS was observed in the BSL2 subtype (15 months).
Differences in OS between molecular subtypes did not reach
statistical significance (P=0.064).
 | Table IITriple-negative breast cancer
molecular subtypes median OS. |
Table II
Triple-negative breast cancer
molecular subtypes median OS.
| Molecular
subtype | Median OS,
months |
|---|
|
Immunomodulatory | NA |
| Mesenchymal
Stem-Like | 29 |
| Basal-like 1 | 38 |
| Androgen-like
receptor | 27 |
| Mesenchymal | 18 |
| Basal-like 2 | 15 |
Most patients reported alive at the cut-off date
(64.3%) had the IM subtype. In the survival analysis, the
difference in survival between the IM subtype and other molecular
subtypes met statistical significance (P=0.034; Fig. 4).
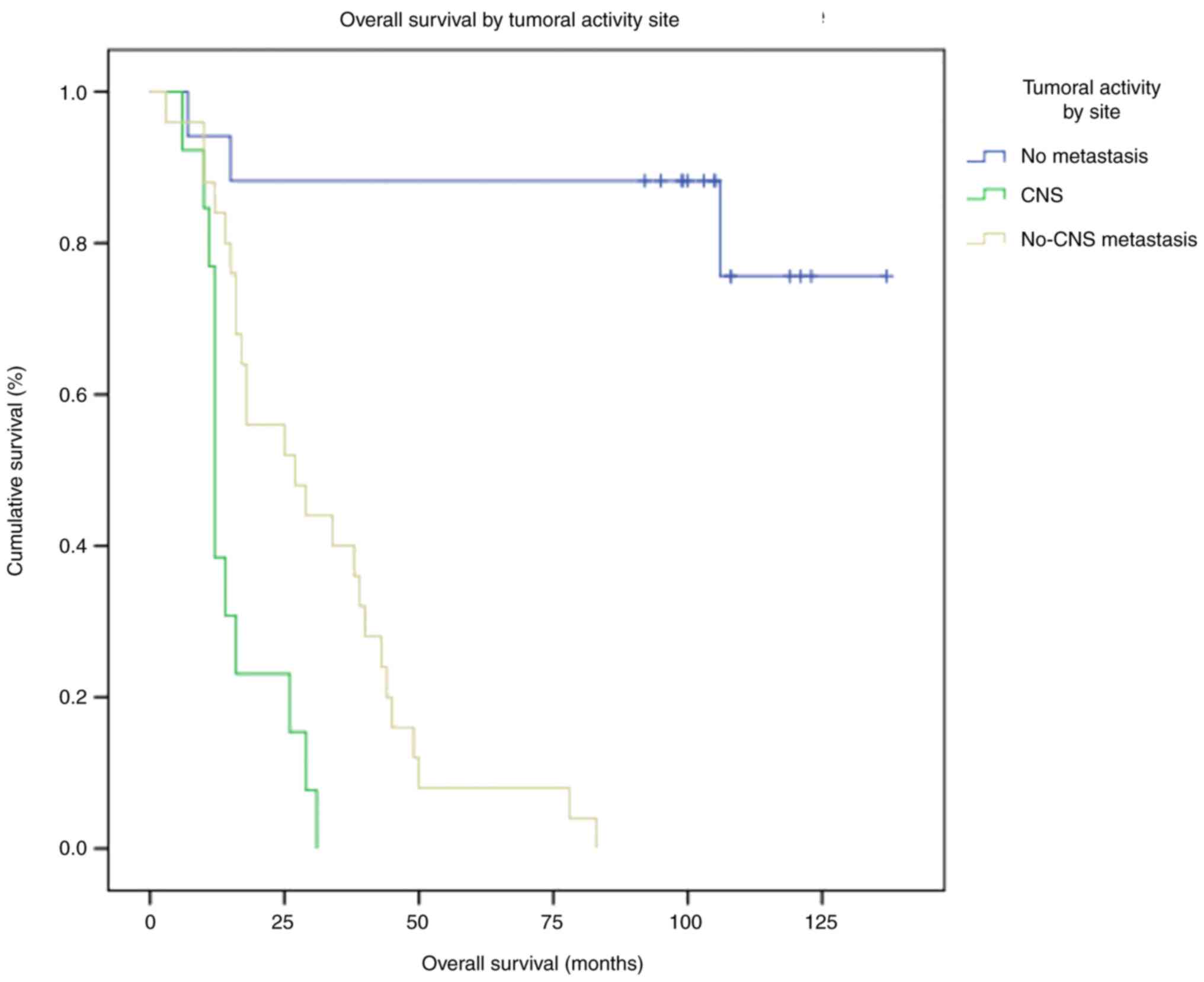
OS according to metastatic disease
status
At the cutoff date, 14 patients were alive, all
without metastatic disease. All patients who had metastasis
succumbed. These patients were stratified according to their
metastatic disease status (CNS, visceral non-CNS, no metastasis),
and a survival analysis was performed (Fig. 5). It was observed that the group of
patients with CNS metastasis had a lower OS than patients with
visceral non-CNS metastatic activity: 12 months vs. 27 months.
Among patients who did not develop metastatic disease, the median
OS was not reached. More than half of the patients from the latter
group (56.25%) belonged to the IM molecular subtype.
Clinicopathological characteristics of
TNBC molecular subtypes
The main analyzed clinicopathological
characteristics per molecular subtype are summarized in Table III. It should be noted that
several of the patient characteristics initially intended to be
analyzed were not recorded in the medical notes and therefore could
not be examined. None of the assessed characteristics met
statistical significance. As for BMI, most molecular subtypes had a
majority of patients that were either obese or overweight, as
opposed to the BSL2 molecular subtype, where 63% had a low to
normal BMI. However, this difference did not meet statistical
significance (P=0.498). Regarding the use of hormones, BSL1 was the
molecular subtype where a higher proportion of patients was exposed
(P=0.162). In the present study, 66.7% of the patients reported to
have used hormone-containing drugs, mainly in the form of oral
contraceptives.
 | Table IIITriple-negative breast cancer
molecular subtypes main clinical and pathological
characteristics. |
Table III
Triple-negative breast cancer
molecular subtypes main clinical and pathological
characteristics.
| | BSL1 | BSL2 | IM | M | LAR | MSL | P-value |
|---|
| | n (%) |
|---|
| BMI | | | | | | | 0.498 |
|
<25 | 2 (22.2) | 7 (63.6) | 6 (37.5) | 6(50) | 1 (16.7) | 0 | |
|
25-29.9 | 4 (44.4) | 2 (18.2) | 6 (37.5) | 2 (16.7) | 3(50) | 0 | |
|
>30 | 3 (33.3) | 2 (18.2) | 4(25) | 4 (33.3) | 2 (33.3) | 1(100) | |
| Hormone use | | | | | | | 0.194 |
|
Yes | 6 (66.7) | 1 (9.1) | 6 (37.5) | 5 (41.7) | 2 (33.3) | 0 | |
|
No | 2 (22.2) | 9 (81.8) | 8(50) | 7 (58.3) | 2 (33.3) | 1(100) | |
|
Unknown | 1 (11.1) | 1 (9.1) | 2 (12.5) | 0 | 2 (33.3) | 0 | |
| Parity | | | | | | | 0.681 |
|
<2 | 2 (22.2) | 7 (63.6) | 4(25) | 4 (33.3) | 3(50) | 0 | |
|
>2 | 7 (77.8) | 4 (36.4) | 12(75) | 8 (66.6) | 3(50) | 1(100) | |
| Hormonal
status | | | | | | | 0.125 |
|
Postmenopausal | 4 (44.4) | 3 (27.3) | 4(25) | 8 (66.7) | 4 (66.7) | 1(100) | |
|
Premenopausal | 5 (55.6) | 8 (72.7) | 12(75) | 4 (33.3) | 2 (33.3) | 0 | |
| Ki 67 (%) | | | | | | | 0.275 |
|
0 | 4 (44.4) | 6 (54.5) | 5 (31.2) | 7 (58.3) | 3(50) | 0 | |
|
10-19 | 0 | 0 | 0 | 2 (16.7) | 0 | 0 | |
|
>20 | 5 (55.6) | 5 (45.5) | 11 (68.8) | 3(25) | 3(50) | 1(100) | |
| Androgen
Receptor | | | | | | | 0.076 |
|
Negative | 8 (88.9) | 6 (54.5) | 6 (37.5) | 7 (58.3) | 3(50) | 0 | |
|
Positive | 1 (11.1) | 1 (9.1) | 0 | 1 (8.3) | 2 (33.3) | 0 | |
|
Unknown | 0 | 4 (36.4) | 10 (62.5) | 4 (33.3) | 1 (16.7) | 1(100) | |
| CK 17 | | | | | | | 0.171 |
|
Negative | 8 (88.9) | 6 (54.5) | 4 (54.5) | 6(50) | 4 (66.7) | 0 | |
|
Positive | 1 (11.1) | 1 (9.1) | 2 (12.5) | 2 (16.7) | 1 (16.7) | 0 | |
|
Unknown | 0 | 4 (36.4) | 10 (62.5) | 4 (33.3) | 1 (16.7) | 1(100) | |
| CK 14 | | | | | | | 0.194 |
|
Negative | 7 (77.8) | 5 (45.5) | 4 (54.5) | 5 (41.7) | 5 (41.7) | 0 | |
|
Positive | 2 (22.2) | 2 (18.2) | 2 (12.5) | 3(25) | 1 (16.7) | 0 | |
|
Unknown | 0 | 4 (36.4) | 10 (62.5) | 4 (33.3) | 1 (16.7) | 1(100) | |
| p63 | | | | | | | 0.127 |
|
Negative | 9(100) | 6 (54.5) | 6 (37.5) | 5 (41.7) | 4 (66.7) | 0 | |
|
Positive | 0 | 0 | 0 | 1 (8.3) | 0 | 0 | |
|
Unknown | 0 | 5 (45.5) | 10 (62.5) | 6(50) | 2 (33.3) | 1(100) | |
| pCR | | | | | | | 0.011 |
|
Yes | 1 (11.1) | 1 (9.1) | 9 (56.2) | 1 (8.3) | 0 | 0 | |
| Neoadjuvant CT
scheme | | | | | | | 0.092 |
|
Anthracycline
and taxane | 3 (33.3) | 6 (54.5) | 3 (18.8) | 8 (66.7) | 2 (33.3) | 0 | |
|
Platinum | 6 (66.7) | 4 (36.4) | 9 (56.2) | 3(25) | 1 (16.7) | 0 | |
|
Platinum and
taxane | 0 | 0 | 1 (6.2) | 0 | 0 | 0 | |
|
No
neoadjuvant CT | 0 | 1 (9.1) | 3 (18.8) | 1 (8.3) | 3(50) | 1(100) | |
| Status | | | | | | | 0.034 |
|
Alive | 2 (22.2) | 1 (9.1) | 9 (56.2) | 1 (8.3) | 1 (16.7) | 0 | |
|
Dead | 7 (77.8) | 10 (90.9) | 7 (43.8) | 11 (91.7) | 5 (83.3) | 1(100) | |
More than half of the patients were premenopausal
(56.3%), and this proportion was increased in the IM (75%) and BSL2
(72.7%) subtypes. Although a trend was identified, statistical
significance was not met (P=0.125). Most patients had a parity of 3
or greater. However, 63.6% of the BSL2 subtype patients presented a
parity of 2 or less (P=0.681). Breastfeeding was not assessable,
since more than half of patient files did not report it.
In addition to clinical characteristics,
pathological variables and their association with TNBC molecular
subtypes were also analyzed. Histological type (luminal or ductal),
histological grade, lymphovascular infiltration, CK 5/6, CK 17,
CK14 and p63 were not associated with any molecular subtype. Ki-67
was not significantly associated with any subtype (P=0.27).
However, it was lower in the M subtype; 75% of patients presented
Ki-67 <20. Only 5 patients were reported as positive for the
androgen receptor, but positivity for this receptor was not
assessed for most patients. BRCA status was non-assessable since
its status was only reported for 3 patients (one each for the BSL1,
BSL2 and IM subtypes).
Response to treatment by TNBC
molecular subtype
Out of the 55 patients, only 36 (65.5%) received
neoadjuvant chemotherapy (CT), with the platinum-based scheme being
the one most frequently administered (58.3% of CTs). This was
followed by the anthracycline- and taxane-based scheme (38.2%). A
total of 12 patients undergoing neoadjuvant CT (33.3%) had a pCR,
of whom, 9 (75%) were identified as IM subtype. This association
was statistically significant (P=0.011). A total of 8 of the
patients who had a pCR (66.6%) belong to the group of patients who
were reported to have no metastatic activity and who are currently
alive. This association was also statistically significant
(P=0.032). There was no statistically significant association
between achieving a pCR and the type of scheme used, nor between
prognosis and type of scheme: out of the 14 patients who were
reported to still be alive, 8 received a platinum scheme, 2 did not
receive neoadjuvant and 4 received a scheme with anthracyclines and
taxanes.
Development of metastatic disease by
TNBC molecular subtype
Patients who had CNS metastases were mainly of the
BSL1 and Ml subtypes, 30.4 and 21.7%, respectively. Patients who
had non-CNS visceral metastasis were mainly of the BSL2 and M
subtypes, 37.5 and 31.2%, respectively. Finally, patients who did
not have metastases belonged mainly to the IM molecular subtype
(56%). Although this association was not statistically significant,
it showed a trend (P=0.057).
Discussion
Breast cancer is the neoplasm with highest incidence
among women in Mexico. Hormone receptors and/or HER2 overexpression
in the surface of the tumor's cells allow for breast cancer
characterization. In turn, breast cancer classification per
receptor expression implies different behaviors and prognoses,
which now has been exploited through therapeutic targets.
Globally, between 13 and 20% of all cases of breast
cancer correspond to triple negative tumors, that is, tumors
without expression of either hormone or HER2 receptors. Through
Mexico's National Institute of Cancer reports, it has been
identified that the incidence of TNBC in Mexico is ~23% (5).
TNBC is well known for being an aggressive disease
with a greater capacity to develop metastasis. This, and
particularly CNS metastases, deteriorates prognosis of patients.
Unlike hormone-sensitive or HER2 overexpressing breast tumors, in
TNBC there is still no biomarker that enables the use of targeted
therapy, which would improve prognosis. For this reason, CT
continues to be the mainstay of treatment.
In an attempt to gain improved understanding of the
behavior of TNBC, throughout the years, gene expression in these
tumors has been studied. Based on this, different gene expression
profiles have been identified as possible prognosticators for the
variability among different TNBC tumors. This way, TNBC could be
regarded as a set of diseases, which can have different behaviors,
prognoses and treatment sensitivities. One of the best described
and most complete models is the one proposed by Lehman et al
in 2011(18), in which 6 different
molecular subtypes were described, each one with a specific
behavior, prognosis and treatment response profile. Since this is a
rather complete and practical model, in the present study, our
patients were classified according to it.
The present study builds upon a previous assessment
of TNBC patients being treated between 2007 and 2011 at Mexico's
National Institute of Cancer. That study aimed at identifying the
role of gene expression profiles in the CNS metastasis process. The
current study, by contrast, has a clinical approach. Herein, the
information regarding the gene expression profiles, which was
obtained previously, was used to find an association with clinical
characteristics. This focus places the present project within the
realm of translational medicine.
The intention of the current study was to identify
the differences in survival across TNBC molecular subtypes.
Additionally, it aimed to define clinical and pathological
characteristics to help characterize them. This could eventually
lead to an increasing effectiveness of treatments by aiding in the
development of personalized treatment guidelines.
In the present study, differences in OS across TNBC
molecular subtypes were identified. The best OS was reported in the
IM subtype (median OS not reached), followed by BSL1 (38 months).
Furthermore, until June 2019, 64.3% of patients reported to be
alive belonged to this molecular subtype. By contrast, the worst OS
was reported in patients identified with the BSL2 subtype (15
months), followed by the M subtype (18 months) (P=0.64). Up to the
cut-off date, none of the patients reported as alive (14 of the 55)
had metastatic disease and/or recurrence. One of these patients,
however, had a double metachronous primary in the contralateral
breast, which was successfully treated.
In total, 36 patients received neoadjuvant CT. A
third of them (12 patients) had a pCR. Remarkably, the IM molecular
subtype represented 75% of these patients (P=0.032). A total of 8
of these 12 patients belonged to the group of patients who did not
develop metastasis.
Our analyses showed a tendency for BSL2 and M
molecular subtypes to present with a higher rate of metastatic
disease. The BSL2 subtype tended to present with CNS metastasis
more often, and the M subtype with non-CNS visceral metastatic
disease. On the contrary, the IM molecular subtype had a lower
probability of presenting metastasis.
Previous studies have found BSL2 and M subtypes to
have the worst prognosis (18,21).
These two subtypes were reported to have the worst OS and distant
metastasis-free survival (19).
This is in accordance with the present findings. On the other hand,
current knowledge suggests that either BSL1 or IM have the best
prognosis, with studies favoring one or the other (18,30).
In our population, IM consistently showed the best prognosis,
followed by BSL1.
The IM subtype is highly enriched for genes involved
in the immune cell signaling process (18). Recently, this subtype was found to
present with a higher proportion of intratumoral infiltrating
lymphocytes (23). This is
relevant as different studies have demonstrated that tumors with
high lymphocytic infiltrate have an improved prognosis. The
presence of tumor-infiltrating lymphocytes is associated with an
improved OS and decreased metastasis incidence (32). These phenomena could be a source of
rationalization for our findings.
A total of 31 variables (clinical and pathological)
were assessed in an attempt to clinically characterize the TNBC
molecular subtypes in our population. Unfortunately, electronic
medical records were highly heterogeneous and often did not report
these variables. Hence, the elucidation of the relationship between
certain of these variables and the TNBC molecular subtypes could
not be accomplished.
Mean age in our cohort was 49 years, which is in
accordance with studies of TNBC in the Hispanic population
(5). It should be noted that the
age range was wide (30-80 years old). There was no significant
relation between age and either development of metastatic disease
or molecular subtype.
Having a high body mass index (BMI) has been linked
to the development of this disease. In this cohort, 60% of patients
were either overweight or obese. However, there was no significant
association between BMI and the development of metastasis, or BMI
and molecular subtype.
Hormone exposure (either exogenous or endogenous)
does not seem to have a relationship with the development of TNBC
over other subtypes of breast cancer. In the present study, the age
of menarche and the use of hormonal drugs were not associated with
metastasis or a molecular subtype. Hormonal status showed a slight
tendency towards an association with the development of metastasis
(P=0.149). Most patients with CNS metastasis (56.5%) were
postmenopausal, while most patients with non-CNS visceral
metastasis (56%) and without metastasis (75%) were premenopausal.
Furthermore, premenopausal patients consisted mainly of patients
who had the BSL2 or IM molecular subtype (25 and 38% of this group,
respectively) (P=0.125).
Parity was not significantly associated with any
molecular subtype. Neither was the age of the first and last
deliveries, or breastfeeding. The BSL2 molecular subtype presented
the lowest parity (63.6% had 2 or less deliveries).
As part of the study, a possible relationship
between 10 histopathological characteristics and the development of
metastasis and molecular subtype was also investigated. However,
this analysis was limited due to lack of reporting of these
variables in the medical records.
The development of high grade tumors, with a high
completion rate (determined by Ki-67, mostly >20%) are
well-known features of TNBC. Accordingly, in the present study
these same features were observed, exempting the M molecular
subtype, where 75% of the patients had tumors with a Ki 67
<20%.
Metastatic disease resulted in lower survival
regardless of the molecular subtype. CNS metastasis in particular
represented the worst prognosis. Of the 55 studied patients, 23 had
metastatic CNS disease, 16 patients had non-CNS visceral metastatic
disease and 16 patients did not develop metastatic disease.
Patients with CNS metastasis belonged mainly to the
molecular subtypes BSL1 and M (30.4 and 21.7%, respectively). The
non-CNS visceral metastasis group consisted mainly of patients with
the BSL2 and M molecular subtypes (37.5 and 31.2%, respectively).
Lastly, the group of patients without metastases was made up mainly
of patients with the IM molecular subtype (56%). Even though
statistical significance was not met, differences between groups
showed a trend.
Patients with metastatic CNS disease had the worst
median OS (12 months), followed by patients with non-CNS visceral
metastatic disease (27 months). Even when only analyzing patients
with metastatic disease, differences across TNBC molecular subtypes
were observed. The best survival still occurred in patients with
the IM subtype. Patients without metastatic disease had the best
survival; up to the cut-off date, the survival median had not yet
been reached.
Overall, these results clearly revealed that CNS
metastasis is associated with the worst prognosis in TNBC patients,
which is consistent with previous studies (33). Factors such as brain inflammation
and edema have classically been proposed to lead to potentially
deadly complications (brain herniation, neurological deficit and
seizures) that may account, at least partially, to the worsening of
prognosis (34). Certain breast
cancer genes have been individually associated with CNS metastasis,
and therefore, it is reasonable to consider that gene expression
profiles may affect CNS metastasis incidence (35). Our findings suggested that patients
with the BSL-1 subtype have a higher likelihood of developing CNS
metastasis. Consequently, future research should evaluate BSL-1
genes as mechanistic factors leading to CNS metastasis. Moreover,
efforts to detect brain metastasis opportunely should be made in
breast cancer patients with this subtype.
It was not possible to analyze the implications of
BRCA mutations in TNBC molecular subtypes in the present study, as
these mutations were seldom investigated and reported in medical
records. It would be interesting to study elsewhere the
implications of these mutations in TNBC subtypes in Hispanics.
One strength of the current study is the fact that a
large number of clinical and pathological variables were collected
and considered for each patient. No variable met statistical
significance for its association with a molecular subtype. Another
strength is that included patients have maintained adherence to the
surveillance consultations throughout the study period. These
patients were closely monitored by a multidisciplinary team in
Mexico's National Institute of Cancer.
The main limitation of the present study was the
heterogeneity and occasional lack of reporting in the electronic
medical records. This limited the power to analyze all the desired
variables. This problem was encountered since numerous of the
collected variables were previously not considered a standard in
patient evaluation at the Institute, hence they were often not
collected and reported in medical records. For instance, the
acquisition of negative surgical margins or the evidence of
lymphatic spread at diagnosis, which could have been useful to
compare groups who did and did not develop metastasis, were not
evaluated. However, it should be mentioned that patients from
stages II to III were included to ensure that the progression of
disease was evaluated without being influenced by the time of
diagnosis. Additionally, no clinical differences were identified
between patients who did and did not develop metastasis, apart from
the molecular subtype.
Another limitation to the current study was the
diversity in administered treatments between patients. This
hindered our ability to analyze results regarding individual
patient response to treatment. It should also be mentioned that our
microarray data were not validated using RT-qPCR, which would be
ideal, due to lack of sampled tissue. Future studies assessing TNBC
subtypes should aim to validate their microarray results using
methods such as RT-qPCR in order to ensure accuracy. One final
limitation is the relatively small gathered sample size in the
present study. This may have limited the ability to elucidate
associations and to discuss interesting but rare subtypes such as
MSL (only 1 patient with MSL was included in our study).
Information obtained in the present study may help
to incorporate a clinical-molecular model for TNBC patient care.
Particularly, this information is valuable for decision making in
Hispanic-based populations, where TNBC represents a larger
proportion of cases and information is lacking. This way, treatment
and monitoring can be individualized to the patient's risk. For
instance, whenever managing a patient with the BSL2 or M molecular
subtypes in populations similar to ours, a more rigorous diagnostic
approach could be initiated to rule out metastatic disease.
Subclinical disease could be diagnosed in an early manner, highly
benefiting patients. The implementation of measures such as these,
which personalize management, utilizing existing and forthcoming
information, may ultimately contribute to a substantial improvement
in prognosis and quality of life of patients with TNBC.
In conclusion, patients with the TNBC IM molecular
subtype had a longer OS, with a median survival not reached during
the span of our study. By contrast, the lowest OS was observed in
patients with the BSL2 molecular subtype, followed by the M
subtype. No significant associations were found between any of the
clinical or pathological characteristics and TNBC molecular
subtypes. Patients with the IM molecular subtype presented a pCR to
neoadjuvant CT more often (75% of patients who had a pCR belonged
to this subtype). Patients with the BSL2 and M molecular subtypes
had a higher probability of developing CNS metastatic disease and
visceral non-CNS metastatic disease, respectively. The IM molecular
subtype had the lowest probability of developing metastatic
disease. Patients with CNS metastatic disease had the lowest
survival. The results obtained in the present study should be
considered to implement a clinical-molecular model for TNBC patient
care in Hispanic-based populations.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Fondo
SS/IMSS/ISSSTE-CONACYT (grant no. SALUD-2013-1-201336).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request. Microarray data are available at the Gene Expression
Omnibus (GEO) repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE176128).
Authors' contributions
AOG, OA, CVG, JAMS and EOV conceptualized and
designed the study. EOV, CRE, RRB and CHCS performed data
collection. RVR performed the statistical analysis. AOG, EOV, CRE,
JAMS, RVR, AG, RRB and CHCS analyzed and interpreted data. EOV, AOG
and AG drafted the manuscript. JAMS, AOG, EOV, CVG and OA reviewed
and revised the manuscript. AOG, EOV, CHCS and JAMS confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no. CLAVE
SALUD-2013-01-201336) by the local bioethics and scientific
committee of Mexico's National Institute of Cancer (INCan, Mexico
City, Mexico). Written consent was obtained from all included
patients before being included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sharma P: Biology and management of
patients with triple-negative breast cancer. Oncologist.
21:1050–1062. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reynoso-Noverón N, Villarreal-Garza C,
Soto-Perez-de-Celis E, Arce-Salinas C, Matus-Santos J,
Ramírez-Ugalde MT, Alvarado-Miranda A, Cabrera-Galeana P,
Meneses-García A, Lara-Medina F, et al: Clinical and
epidemiological profile of breast cancer in mexico: Results of the
seguro popular. J Glob Oncol. 3:757–764. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lara-Medina F, Pérez-Sánchez V,
Saavedra-Pérez D, Blake-Cerda M, Arce C, Motola-Kuba D,
Villarreal-Garza C, González-Angulo AM, Bargalló E, Aguilar JL, et
al: Triple-negative breast cancer in Hispanic patients. Cancer.
117:3658–3669. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de Ruijter TC, Veeck J, de Hoon JPJ, van
Engeland M and Tjan-Heijnen VC: Characteristics of triple-negative
breast cancer. J Cancer Res Clin Oncol. 137:183–192.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li X, Yang J, Peng L, Sahin AA, Huo L,
Ward KC, O'Regan R, Torres MA and Meisel JL: Triple-negative breast
cancer has worse overall survival and cause-specific survival than
non-triple-negative breast cancer. Breast Cancer Res Treat.
161:279–287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Martin AM, Cagney DN, Catalano PJ, Warren
LE, Bellon JR, Punglia RS, Claus EB, Lee EQ, Wen PY, Haas-Kogan DA,
et al: Brain metastases in newly diagnosed breast cancer: A
population-based study. JAMA Oncol. 3:1069–1077. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xiao W, Zheng S, Yang A, Zhang X, Zou Y,
Tang H and Xie X: Breast cancer subtypes and the risk of distant
metastasis at initial diagnosis: A population-based study. Cancer
Manag Res Volume. 10:5329–5338. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ignatov A, Eggemann H, Burger E and
Ignatov T: Patterns of breast cancer relapse in accordance to
biological subtype. J Cancer Res Clin Oncol. 144:1347–1355.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jin J, Gao Y, Zhang J, Wang L, Wang B, Cao
J, Shao Z and Wang Z: Incidence, pattern and prognosis of brain
metastases in patients with metastatic triple negative breast
cancer. BMC Cancer. 18(446)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rostami R, Mittal S, Rostami P, Tavassoli
F and Jabbari B: Brain metastasis in breast cancer: A comprehensive
literature review. J Neurooncol. 127:407–414. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim YJ, Kim JS and Kim IA: Molecular
subtype predicts incidence and prognosis of brain metastasis from
breast cancer in SEER database. J Cancer Res Clin Oncol.
144:1803–1816. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Heitz F, Harter P, Lueck HJ,
Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, Traut A and
du Bois A: Triple-negative and HER2-overexpressing breast cancers
exhibit an elevated risk and an earlier occurrence of cerebral
metastases. Eur J Cancer. 45:2792–2798. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Minami CA, Chung DU and Chang HR:
Management options in triple-negative breast cancer. Breast Cancer
(Auckl). 5:175–199. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kennedy BM and Harris RE: Cyclooxygenase
and lipoxygenase gene expression in the inflammogenesis of breast
cancer. Inflammopharmacology. 26:909–923. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Masuda H, Baggerly KA, Wang Y, Zhang Y,
Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD,
Pietenpol JA, Hortobagyi GN, et al: Differential response to
neoadjuvant chemotherapy among 7 triple-negative breast cancer
molecular subtypes. Clin Cancer Res. 19(5533)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hines LM, Risendal B, Byers T, Mengshol S,
Lowery J and Singh M: Ethnic disparities in breast tumor phenotypic
subtypes in hispanic and non-hispanic white women. J Womens Health.
20:1543–1550. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yin L, Duan JJ, Bian XW and Yu S:
Triple-negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22(61)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fitzgibbons PL, Dillon DA, Alsabeh R,
Berman MA, Hayes DF, Hicks DG, Hughes KS and Nofech-Mozes S:
Template for reporting results of biomarker testing of specimens
from patients with carcinoma of the breast. Arch Pathol Lab Med.
138:595–601. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rodríguez-Bautista R, Caro-Sánchez CH,
Cabrera-Galeana P, Alanis-Funes GJ, Gutierrez-Millán E, Ávila-Ríos
S, Matías-Florentino M, Reyes-Terán G, Díaz-Chávez J,
Villarreal-Garza C, et al: Immune Milieu and genomic alterations
set the triple-negative breast cancer immunomodulatory subtype
tumor behavior. Cancers (Basel). 13(6256)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
RStudio Team: RStudio: Integrated
Development of R., 2021.
|
|
25
|
Marini F, Linke J and Binder H: Ideal: An
R/Bioconductor package for Interactive differential expression
analysis. BMC Bioinformatics. 21(565)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bolstad BM, Irizarry RA, Åstrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Banerji S, Cibulskis K, Rangel-Escareno C,
Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY,
Sougnez C, Zou L, et al: Sequence analysis of mutations and
translocations across breast cancer subtypes. Nature. 486:405–409.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Johnson WE, Li C and Rabinovic A:
Adjusting batch effects in microarray expression data using
empirical Bayes methods. Biostatistics. 8:118–127. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Parker JS, Mullins M, Cheang MCU, Leung S,
Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, et al:
Supervised risk predictor of breast cancer based on intrinsic
subtypes. J Clin Oncol. 27:1160–1167. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bareche Y, Venet D, Ignatiadis M, Aftimos
P, Piccart M, Rothe F and Sotiriou C: Unravelling triple-negative
breast cancer molecular heterogeneity using an integrative
multiomic analysis. Ann Oncol. 29:895–902. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hubalek M, Czech T and Müller H:
Biological subtypes of triple-negative breast cancer. Breast Care.
12:8–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brosnan EM and Anders CK: Understanding
patterns of brain metastasis in breast cancer and designing
rational therapeutic strategies. Ann Transl Med. 6:163.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Quattrocchi CC, Errante Y, Gaudino C,
Mallio CA, Giona A, Santini D, Tonini G and Zobel BB: Spatial brain
distribution of intra-axial metastatic lesions in breast and lung
cancer patients. J Neurooncol. 110:79–87. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lv Y, Ma X, Du Y and Feng J: Understanding
patterns of brain metastasis in triple-negative breast cancer and
exploring potential therapeutic targets. Onco Targets Ther.
14:589–607. 2021.PubMed/NCBI View Article : Google Scholar
|