The medical community is constantly concerned about
the rising number of cancer cases. With today's knowledge and
technology, there is high interest in studying the mechanisms
behind cancer activity and finding new treatment options (1). Out of all human cancers, 5% are head
and neck carcinomas, combining cancers of the epithelial lining of
the nasopharynx, oropharynx, oral cavity, hypopharynx and larynx.
The most common types of carcinomas evolving in the head and neck
area are squamous cell carcinoma (SCC) and basal cell carcinoma
(BCC) (2). There are certain risk
factors associated with head and neck carcinoma, such as smoking,
consumption of alcohol, nutrition deficiency, inadequate oral
hygiene, Epstein-Barr virus, human papillomavirus (HPV) or Candida
Albicans infections (3) (Fig. 1). With 60% of the cases already in
advanced stages III or IV (4) by
the time of the first medical examination, recent studies have
focused their research not only on the activity of the cells but
also on the behavior and evolution of the lesion, establishing new
connections between action and reaction (5).
From a treatment perspective, decisions are
frequently the result of multidisciplinary approaches, combining
chemotherapeutic, radiotherapeutic, immunotherapeutic and surgical
procedures (6). For improved
outcomes with higher survival rates, new research has raised
interest in the interaction between the immune system and cancer
cells (7).
SCC of the head and neck is a type of epithelial
neoplasm with 65,000 diagnosed cases in the US in 2019(8). SCC frequently appears in the older
age category (onset at >50 years of age), more common in men
than in women, usually individuals with a light skin color
(9). When located on the head, it
has been discovered that ear and lip areas have higher chances of
developing SCC than BCC and the most important etiologic factor is
chronic exposure to ultraviolet (UV) irradiation, which leads to
free radicals that form thymidine dimers in the DNA, affecting DNA
repair and cellular immunity (10). Other factors, such as ionizing
radiation, smoking, chronic inflammation and arsenic, have a huge
impact as well. HPV has an important part in the epidemiology of
oropharyngeal and head and neck cancers and it is frequently
detected in younger patients. HPV is thought to be a unique entity
with a better prognosis (11).
Immunosuppression has a key role in the onset and evolution of SCC,
and patients who have undergone this type of therapy or simply have
a weak immune status are more likely to develop SCC and have an
aggressive form of the disease (12). SCC appears clinically as a
slow-growing nodule or plaque that may at times be ulcerated
(13), with firm consistency and
pink color or with no alteration of skin color (14). In numerous cases, SCC is associated
with an inflammatory skin condition such as vitiligo, acne
conglobata, lupus vulgaris, burn, scars or actinic keratosis
(15). Actinic keratosis is
described as multiple, small lesions that are erythematous and
hyperkeratotic, usually seen on sun-exposed skin. The lifetime risk
of progression from actinic keratosis to SCC is estimated to be ~8%
(16).
Histological features of invasive SCC have
anepidermal origin. Well-differentiated SCC is distinct with a
keratinizing aspect, an eosinophilic cluster with squamous cells,
intercellular links and minimal nuclear atypia (17).
Moderately-differentiated SCC types have more atypia
and mitoses, while poorly-differentiated SCC types usually have
limited keratinization, making them difficult to be recognized. In
these cases, immunohistochemistry is helpful by using antibodies
against keratins to demonstrate the squamous origin (18).
Statistically, the recurrence risk is 3.7-10.9, and
the metastatic risk is up to 3.3% (19). Risk factors for recurrence and
metastasis are immunosuppression, lymph node status, poor
differentiation, location or perineural invasion (20). Special attention is required for
lesions from burns or radiated areas, chronic ulcers or scars.
Regarding the location, lip SCC is the most aggressive one, with
recurrence and metastasis rates of >10% (21). In the pathophysiology of SCC, the
importance of the TP53 gene has been frequently described. The main
role of the gene is tumor suppression by regulating cell cycle
progression, apoptotic cell death and DNA repair. In cutaneous SCC,
the TP53 gene frequently displays a characteristic UV pattern with
‘C’ to ‘T’ or ‘CC’ to ‘TT’ mutations at dipyrimidine sites
(22). Experimental p53-deficient
mice develop UV-induced SCC after very short exposure, proving not
just the protective role of the gene but also the harmful action of
UV (23). Even if today this
neoplasm is totally curable through surgery (24), the patients' outcome is affected by
the size of the tumor at diagnosis (>2 cm), the depth of
invasion (>4 mm), poor or undifferentiated histological grade
and the presence of metastasis (25). However, it has been discovered that
cutaneous SCC in the head and neck region has a different evolution
compared with its evolution from other origins, such as the oral
cavity, mucosa or lungs, where the chances of metastatic invasion
are higher (26).
BCC, formerly known as basal cell epithelioma, was
first described by Jacob in 1824 and is the most common type of
cutaneous carcinoma on the head and neck area, frequently as a
result of acute, intermittent UV exposure (27). Other important risk factors are
immunosuppression due to certain treatments or infections (human
immunodeficiency virus infection, transplants) and ionizing
radiation. The next affected areas are on the trunk, arms and legs
but have been reported cases of BCC on breasts, axillae, genitalia,
perianal area, palms and soles (28). It has a low mortality rate and
rarely exhibits metastatic behavior, but it has a high recurrence
rate, particularly when the invasion is perineural (29). It usually affects males more than
females, presumably because of the typical male professions, which
imply more exposure to UV. Males are also known to be susceptible
to a greater number of tumors (30). The typical patient is a Caucasian,
light-skinned man, over 40 years of age, with blue eyes and light
or red hair.
Due to the aging of the population all around the
globe and the thinning of the ozone layer, which provides higher UV
exposure, BCC is affecting an increasing number of individuals
(31). The number of cases
increases with age and the peak is known to be between 60 and 70
years of age. This is due to a reduced capacity of DNA repair
ability and a weaker immune system (32). In addition, an important factor in
BCC ethology is individual susceptibility. It refers to each
individual's amount of melanin deposited in the skin and the
capacity to tan when exposed to UV. Usually, darker skin is more
protected by melanin storage. With a locally aggressive
characteristic, it may easily invade cartilage and bone, resulting
in deforming lesions with high morbidity rates (33). The primary location of the tumor is
in the epidermis, and it has a slow-growing evolution (34). Facial BCC, particularly in the nose
and ear areas, may have a recurrent evolution in time, but in
general, metastasis is rare and exhibits no precursor lesions
(35). Clinically, it appears as a
distinct, translucent papule with telangiectasias which may at
times be ulcerated (36). The
subtypes of BCC are nodular, micronodular, superficial,
infiltrative and fibroepithelioma of Pinkus. In the head and neck
area, the nodular one is the most common subtype. This subtype is
distinguished by nodular lobules of tumor cells with mucin-rich
stroma that strip in the epidermal layer with little invasion into
the dermis (37). In most cases,
it takes 15 to 20 years to get from primary UV damage to the
diagnosed lesion. The direct impact is major at the DNA and RNA
level, forming covalent bonding between adjacent pyrimidines and
resulting in mutagenic products (38). Another mechanism involves reactive
oxygen species that directly attack the DNA and slow down the
cutaneous immune system by affecting the local antitumor monitoring
activity of dendritic cells (39).
The outcome of head and neck cutaneous carcinoma
treatment strategies has improved over the past decade.
Multidisciplinary teams frequently obtain better results by
prioritizing toxicity reduction and the patients' quality of life
(40). Early detection and
surgical procedures have higher chances of cancer-free outcomes and
the standard nonsurgical option in most cases is still
chemoradiotherapy with epidermal growth factor receptor cetuximab
treatment (41).
Irradiation of the skin causes tissue damage and
inflammatory cell recruitment by damaging epidermal basal cells,
endothelial cells, vascular components and reducing the number of
Langerhans cells (42). Radiation
dermatitis is a particularly common side reaction to radiotherapy
for head and neck carcinoma. Most patients experience mild to
moderate degrees of skin lesions (such as grades I and II) but up
to 25% develop severe reactions. In severe radiation dermatitis
(grades III or IV), the epidermis is infiltrated with neutrophils
and there are high rates of apoptosis in the deeper layers
(43). The standard administration
of radiation involves successive doses and it frequently prevents
tissue healing due to cellular repopulation, even if the patient
takes a few days to break during cures of daily fractionated
therapy, thereby compromising the result (44). The general management of radiation
dermatitis includes washing once or twice a day with pH-5 soap,
moisturizing shaving to prevent folliculitis, debridement and
checking for systemic inflammation (45). Is it highly recommended to use aloe
vera and to avoid exposure to sunlight, scratching and local
trauma. To prevent severe skin toxicities, patients and physicians
must look for early signs of radiation dermatitis (46).
The development of vaccines is currently the most
widespread method to prevent infections with different germs or the
development of cancer cells (47).
Head and neck squamous cell carcinoma, including cutaneous
locations, is caused by both external conditions and genetic
mutations. Cancer vaccines may be of two types, prophylactic and
therapeutic (48). Therapeutic
vaccines use the host's immune system to recognize and eliminate
cancer cells (49). It acts in the
opposite direction of tumor invasion mechanisms (50), targets antigens associated with
tumors, and induces a widespread mediated immune response (51). Current research includes modified
virus tumor vaccines, DNA, proteins, peptide-based vaccines and
combined strategies, all demonstrating encouraging results
(52).
MiRNAs are single-stranded RNA molecules with a
length of 21-23 nucleotides that control the expression of >50%
of human genes. Each miRNA molecule is able to recognize numerous
mRNA transcripts and regulate a great number of genes downstream
(70). The activity of miRNA
involves modifications of the cell cycle, cellular differentiation,
proliferation, survival, motility, apoptosis and morphogenesis,
which are all involved in the carcinogenesis of head and neck
carcinoma (71). Metabolic
reprogramming is an important indication of cancer, and as a highly
dynamic process, it facilitates the transformation of a normal cell
into a malignant cell (72).
Aerobic glycolysis is an altered metabolic pathway frequently
observed in cancer cells. The mechanism changes the role of cell
energy, making it less efficient but supporting invasion, migration
and drug resistance. For this substantial amount of energy, cancer
cells require a higher glucose intake (73). The glucose transporter (GLUT)
protein family has 14 members, but only GLUT1 and GLUT3 are
aberrantly expressed in carcinomas of the head and neck (74). MiR-218 is a tumor-suppressor mRNA
that downregulates the proliferation, invasion and metastatic
potential of numerous types of cancer cell. Inhibition of miR-218
by activating GLUT1 expression increases proliferation and glucose
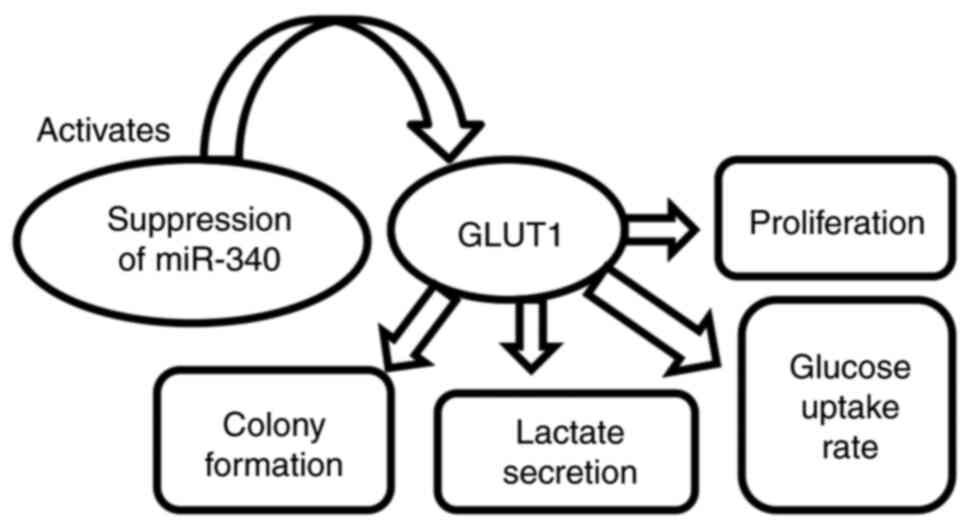
uptake (75). Related to miR-218,
miR-340 regulates GLUT1 expression by increasing glucose absorption
and lactate secretion. Inhibition of miR-340 activates GLUT1 and
promotes proliferation and colony formation with high glucose
uptake. Another mRNA with an oncogenic role in head and neck SCC is
miR-10a, which acts like an upstream regulator of GLUT1(76). The metabolic processes regulated by
miR-340 in head and neck SCC are presented in Fig. 2.
The Cancer Genome Atlas data confirmed the
upregulation of another mRNA in head and neck SCC, miR-31-5p. The
overexpression of miR-31-5p increases free fatty acids aggregation
and lipid droplet formation by targeting acyl-CoA oxidase 1,
enhancing prostaglandin E2(77).
MiR-31-5p promotes migration and invasion of cancer cells (78).
As head and neck SCC is a group of different
cancers, various anatomical areas may serve as a starting point
with diverse characteristics and outcomes. Currently, studies are
devoted to the identification of site-specific miRNA signatures for
every subtype of SCC (79).
Tumor progression and drug resistance are strongly
associated with defects in apoptosis signaling (80). Dysregulation of apoptosis
chaotically prolongs cancer cell life, resulting in gene mutations
(81). The Bcl-2 family of
proteins is relevant in the apoptosis signaling process through
their anti- and pro-apoptotic members, which may interfere with one
another's functions while heterodimerizing and controlling the
death signaling pathway. Members of the Bcl-2 family are frequently
expressed in head and neck SCC, with various locations, including
on the skin, where they have a key role in tumor initiation and
evolution (63). Since
overexpression of Bcl-2, Bcl-XL or survivin is
associated with Cisplatin and Etoposide chemoresistance in head and
neck carcinomas, biological research indicates that antisense
oligonucleotides may be used as a competitor in favor of tumor cell
death (82). Antisense
oligonucleotides have therapeutic potential by inhibiting Bcl-2
proteins and blocking tumor cell progression. In addition,
decreasing Bc-XL expression induces apoptosis in gastric
cancer cells (83). In
vitro experiments demonstrate an increased apoptosis rate and
high drug sensitivity when antisense oligonucleotides are used
against survivin expression (84).
Several studies have been performed with different
strategies regarding the treatment of head and neck carcinoma.
Whether combining drugs with radiation or just using immunotherapy,
recent trials indicated positive results regarding not only the
regression of the tumors, control of metastatic rates and recurrent
behavior, but also severe adverse events (85). The trials looked at the effects of
specific drugs such as HF-10, pembrolizumab, Lenvatinimib,
cetuximab, vismodegib or survivin-2B peptide alone or in
combination with radiotherapy. The most common events were
radiation-induced skin injuries, decreased lymphocyte count,
dysgeusia (86), myalgia and
fatigue (87). Table I provides a summary of the trials
evaluating treatment strategies for head and neck carcinoma.
Not applicable.
Funding: The present study was supported by the ‘Dunarea de Jos’
University of Galati.
Not applicable.
AF and CS revised the manuscript and are the
corresponding authors. ALT summarized the literature findings and
wrote the manuscript. DI, DV, AIP, FB and MIS analyzed the
literature data. AIP, DV, FB and AZ searched and selected the
articles that were included as references. DI, AF, CS, DV, AZ and
MIS reviewed the literature findings, critically revised the
manuscript and approved the review in its current form. All authors
have read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zhu G, Lin JC, Kim SB, Bernier J, Agarwal
JP, Vermorken JB, Thinh DHQ, Cheng HC, Yun HJ, Chitapanarux I, et
al: Asian expert recommendation on management of skin and mucosal
effects of radiation, with or without the addition of cetuximab or
chemotherapy, in treatment of head and neck squamous cell
carcinoma. BMC Cancer. 16(42)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6(92)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jicman Stan D, Niculet E, Lungu M, Onisor
C, Rebegea L, Vesa D, Bezman L, Bujoreanu FC, Sarbu MI, Mihailov R,
et al: Nasopharyngeal carcinoma: A new synthesis of literature data
(review). Exp Ther Med. 23(136)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kang H, Kiess A and Chung CH: Emerging
biomarkers in head and neck cancer in the era of genomics. Nat Rev
Clin Oncol. 12:11–26. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Marur S and Forastiere AA: Head and neck
squamous cell carcinoma: Update on epidemiology, diagnosis, and
treatment. Mayo Clin Proc. 91:386–396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Boussios S, Seraj E, Zarkavelis G,
Petrakis D, Kollas A, Kafantari A, Assi A, Tatsi K, Pavlidis N and
Pentheroudakis G: Management of patients with recurrent/advanced
cervical cancer beyond first line platinum regimens: Where do we
stand? A literature reviews. Crit Rev Oncol Hematol. 108:164–174.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Peng PJ, Lv BJ, Wang ZH, Liao H, Liu YM,
Lin Z, Con YY and Huang PY: Multi-institutional prospective study
of nedaplatin plus S-1 chemotherapy in recurrent and metastatic
nasopharyngeal carcinoma patients after failure of
platinum-containing regimens. Ther Adv Med Oncol. 9:68–74.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tsai MH, Chuang HC, Lin YT, Yang KL, Lu H,
Huang TL, Tsai WL, Su YY and Fang FM: The prognostic value of
preoperative albumin to alkalin phosphatase ratio on survival
outcome for patients with locally advanced oral squamous cell
carcinoma. Technol Cancer Res Treat.
21(15330338221141254)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lubov J, Labbe M, Sioufi K, Morand GB,
Hier MP, Khanna M, Sultanem K and Mlynarek AM: Prognostic factors
of head and neck cutaneous squamous cell carcinoma: A systematic
review. J Otolaryngol Head Neck Surg. 50(54)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nwabudike LC and Tatu AL: Reply to
Gambichler T et al: Altered epigenetic pathways and cell
cycle dysregulation in healthy appearing skin of patients with
koebnerized squamous cell carcinomas following skin surgery. J Eur
Acad Dermatol Venereol. 33:e3–e4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu GFF, Ranck MC, Solanki AA, Cao H,
Kolokythas A, Wenig BL, Chen L, Ard S, Weichselbaum RR, Halpern H
and Spiotto MT: Racial parities in outcomes after radiotherapy for
head and neck cancer. Cancer. 120:244–252. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wiser I, Scope A, Azriel D, Zloczower E,
Carmel NN and Shalom A: Head and Neck cutaneous squamous cell
carcinoma clinicopathological risk factors according to age and
gender: A Population-based Study. Isr Med Assoc J. 18:275–278.
2016.PubMed/NCBI
|
|
13
|
Wang G, Zhang M, Cheng M, Wang X, Li K,
Chen J, Chen Z, Chen S, Chen J, Xiong G, et al: Tumor
microenvironment in head and neck squamous cell carcinoma:
Functions and regulatory mechanisms. Cancer Lett. 507:55–69.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alsahafi E, Begg K, Amelio I, Raulf N,
Lucarelli P, Sauter T and Tavassoli M: Clinical update on head and
neck cancer: Molecular biology and ongoing challenges. Cell Death
Dis. 10(540)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mesia R, Iglesias L, Lambea J,
Martinez-Trufero J, Soria A, Taberna M, Trigo J, Chaves M,
Garcia-Castano A and Cruz J: SEOM clinical guidelines for the
treatment of head and neck cancer (2020). Clin Transl Oncol.
23:913–921. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Burr A, Harari P, Wieland A, Kimple R,
Hartig G and Witek M: Patterns of failure for hypopharynx cancer
patients treated with limited high dose radiotherapy treatment
volumes. Radiat Oncol J. 40:225–231. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kao HF, Huang HC, Liao BC and Hong RL:
Shourt-course pembrolizumab and continuous afatinib therapy for
recurrent or metastatic head and neck squamous cell carcinoma: A
real-world data analysis. BMC Cancer. 22(1228)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jung K, Narwal M, Min SY, Keam B and Kang
H: Squamous cell carcinoma of head and neck: What internists should
know. Korean J Intern Med. 35:1031–1044. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Koh J, Walsh P, D'Costa I and Bhatti O:
Head and neck squamous cell carcinoma survivorship care. Aust J Gen
Pract. 48:846–848. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kitamura N, Sento S, Yoshizawa Y, Sasabe
E, Kudo Y and Yamamoto T: Current trends and future prospects of
molecular targeted therapy in head and neck squamous cell
carcinoma. Int J Mol Sci. 22(240)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vasconcelos L, Melo JC, Miot HA, Marques
ME and Abbade LP: Invasive head and neck cutaneous squamous cell
carcinoma: Clinical and histopathological characteristics,
frequency of local recurrence and metastasis. An Bras Dermatol.
89:562–568. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Oliva M, Spreafico A, Taberna M, Alemany
L, Coburn B, Mesia R and Siu LL: Immune biomarkers of response to
immune-checkpoint inhibitors in head and neck squamous cell
carcinoma. Ann Oncol. 30:57–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kraft S and Granter SR: Molecular
pathology of skin neoplasms of the head and neck. Arch Pathol Lab
Med. 138:759–787. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang J, Chen X, Tian Y, Zhu G, Qin Y, Chen
X, Pi L, Wei M, Liu G, Li Z, et al: Six gene signature for
predicting survival in patients with head and neck squamous cell
carcinoma. Aging (Albany NY). 12:767–783. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rahman S, Kraljević Pavelić S and
Markova-Car E: Circadian (de)regulation in head and neck squamous
cell carcinoma. Int J Mol Sci. 20(2662)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zeng S, Fu L, Zhou P and Ling H:
Identifying risk factors for the prognosis of head and neck
cutaneous squamous cell carcinoma: A systematic review and
meta-analysis. PLoS One. 15(e0239586)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ali SR, Strafford H, Dobbs TD,
Fonferko-Shadrach B, Lacey AS, Pickrell WO, Hutchings HA and
Whitaker IS: Development and validation of an automated basal cell
carcinoma histopathology information extraction system using
natural language processing. Front Surg. 9(870494)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dika E, Scarfì F, Ferracin M, Broseghini
E, Marcelli E, Bortolani B, Campione E, Riefolo M, Ricci C and
Lambertini M: Basal cell carcinoma: A comprehensive review. Int J
Mol Sci. 21(5572)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Szewczyk M, Pazdrowski J, Pabiszczak M,
Wieckowska B, Danczak-Pazdrowska A and Golusinski W: Local
recurrence risk in head and neck basal cell carcinoma. Otolaryngol
Pol. 76:1–5. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shavlokhova V, Vollmer M, Gholam P, Saravi
B, Vollmer A, Hoffmann J, Engel M and Freudlsperger C: Deep
learning on basal cell carcinoma in vivo reflectance confocal
microscopy data. J Pers Med. 12(1471)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang X, Ren H, Guo X, Hu C and Fu J:
Radiation-induced skin injury: Pathogenesis, treatment, and
management. Aging (Albany NY). 12:23379–23393. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bertino G, Muir T, Odili J, Groselj A,
Marconato R, Curatolo P, Kis E, Lonkvist CK, Clover J, Quaglino P,
et al: Treatment of basal cell carcinoma with electrochemotherapy:
Insights from the InspECT Registry (2008-2019). Curr Oncol.
29:5324–5337. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Farah CS: Molecular landscape of head and
neck cancer and implications for therapy. Ann Transl Med.
9(915)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yulek O, Batur S, Ozcan K, Yol C and Aydın
Ülgen Ö: Relationship between PD-L1 expression and prognostic
factors in high-risk cutaneous squamous and basal cell carcinoma.
Bosn J Basic Med Sci. 22:894–900. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hasan A, Rabie A, Elhussiny M, Nasr M,
Kamel MI, Hegab A, El-Kady AS, Nagaty ME, Seleem A, Abbas M, et al:
Recurrent cutaneous basal cell carcinoma after surgical excision: A
retrospective clinicopathological study. Ann Med Surg (Lond).
78(103877)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kara I, Vural A, Unlu M, San F, Benan
Göçer G and Yildiz MG: Management of basal cell carcinomas:
Clinical experience. Turk Arch Otorhinolaryngol. 60:9–15.
2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kasumagic-Halilovic E, Hasic M and
Ovcina-Kurtovic N: A clinical study of basal cell carcinoma. Med
Arh. 73:394–398. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Castanheira A, Boaventura P, Pais Clemente
M, Soares P, Mota A and Lopes JM: Head and neck cutaneous basal
cell carcinoma: What should the otorhinolaryngology head and neck
surgeon care about? Acta Otorhinolaryngol Ital. 40:5–18.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chinem VP and Miot HA: Prevalence of
actinic skin lesions in patients with basal cell carcinoma of the
head: A case-control study. Rev Assoc Med Bras (1992). 58:188–196.
2012.PubMed/NCBI(In English, Portuguese).
|
|
40
|
Otsuka ACVG, Bertolli E, de Macedo AP,
Pinto CAL and Duprat Neto JP: Intraoperative assessment of surgical
margins using ‘en face’ frozen sections in the management of
cutaneous carcinomas. An Bras Dermatol. 97:583–591. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Magnes T, Wagner S, Kiem D, Wiess L,
Rinnerthaler G, Greil R and Melchardt T: Prognostic and predictive
factors in advanced head and neck squamous cell carcinoma. Int J
Sci Mol. 22(4981)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hutchinson MKND, Mierzwa M and D'Silva NJ:
Radiation resistance in head and neck squamous cell carcinoma: Dire
need for an appropriate sensitizer. Oncogene. 39:3638–3649.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zenda S, Ota Y, Tachibana H, Ogawa H,
Ishii S, Hashiguchi C, Akimoto T, Ohe Y and Uchitomi Y: A
prospective picture collection study for a grading atlas of
radiation dermatitis for clinical trials in head-and-neck cancer
patients. J Radiat Res. 57:301–306. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bhatt S, Mandal S, Mehrotra G, Arora V and
Singh U: Multidetector computed tomography perfusion in head and
neck squamous cell carcinomas: Evaluation of a dose reduction
strategy. Indian J Radiol Imaging. 32:451–459. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Haubner F, Ohmann E, Pohl F, Strutz J and
Gassner HG: Wound healing after radiation therapy: Review of the
literature. Radiat Oncol. 7(162)2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ramirez M, Ravichandran S, Ronald L,
Pabon-Ramos WM, Smith TP, Kim CY and Ronald J: Recognition and
management of dermatologic complications from interventional
radiology procedures. Diagn Interv Imaging. 100:659–670.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lecocq M, Poncin A and Sautois B:
Immunotherapy for head and neck squamous cell carcinoma. Rev Med
Liege. 76:398–402. 2021.PubMed/NCBI(In French).
|
|
48
|
Amit M, Takahashi H, Dragomir MP,
Lindemann A, Gleber-Netto FO, Pickering CR, Anfossi S, Osman AA,
Cai Y, Wang R, et al: Loss of p53 drives neuron reprogramming in
head and neck cancer. Nature. 578:449–454. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lim KP, Chun NA, Gan CP, Teo SH, Rahman
ZA, Abraham MT, Zain RB, Ponniah S and Cheong SC: Identification of
immunogenic MAGED4B peptides for vaccine development in oral cancer
immunotherapy. Hum Vaccin Immunother. 10:3214–3223. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jiang H, Zhou L, Shen N, Ning X, Wu D,
Jiang K and Huang X: M1 macrophage-derived exosomes and their key
molecule lncRNA HOTTIP suppress head and neck squamous cell
carcinoma progression by upregulating the TLR5/NF-κB pathway. Cell
Death Dis. 13(183)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Mora Román JJ, Del Campo M, Villar J,
Paolini F, Curzio G, Venuti A, Jara L, Ferreira J, Murgas P,
Lladser A, et al: Immunotherapeutic potential of Mollusk
Hemocyanins in combination with human vaccine adjuvants in murine
models of oral cancer. J Immunol Res. 2019(7076942)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Malonis RJ, Lai JR and Vergnolle O:
Peptide-based vaccines: Current progress and future challenges.
Chem Rev. 120:3210–3229. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Filderman JN and Storkus WJ: Finding the
right help in the tumor microenvironment. J Clin Invest.
132(e161052)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ferris RL: Immunology and immunotherapy of
head and neck cancer. J Clin Oncol. 33:3293–3304. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Albers AE, Qian X, Kaufmann AM, Mytilineos
D, Ferris RL, Hoffmann TK and DeLeo AB: Phenotype of p53 wild-type
epitope-specific T cells in the circulation of patients with head
and neck cancer. Sci Rep. 8(10716)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Leroux-Roels G: Unmet needs in modern
vaccinology: Adjuvants to improve the immune response. Vaccine. 28
(Suppl 3):C25–C36. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chen X, Yang J, Wang L and Liu B:
Personalized neoantigen vaccination with synthetic long peptides:
Recent advances and future perspectives. Theranostics.
10:6011–6023. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Sun Z, Sun X, Chen Z, Du J and Wu Y: Head
and neck squamous cell carcinoma: Risk factors, molecular
alterations, immunology and peptide vaccines. Int J Pept Res Ther.
28(19)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Calvo Tardón M, Allard M, Dutoit V,
Dietrich PY and Walker PR: Peptides as cancer vaccines. Curr Opin
Pharmacol. 47:20–26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bezu L, Kepp O, Cerrato G, Pol J, Fucikova
J, Spisek R, Zitvogel L, Kroemer G and Galluzzi L: Trial watch:
Peptide-based vaccines in anticancer therapy. OncoImmunology.
7(e1511506)2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Shibata H, Zhou L, Xu N, Egloff AM and
Uppaluri R: Personalized cancer vaccination in head and neck
cancer. Cancer Sci. 112:978–988. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Sakakura K, Takahashi H, Motegi SI,
Yokobori-Kuwabara Y, Oyama T and Chikamatsu K: Immunological
features of circulating monocyte subsets in patients with squamous
cell carcinoma of the head and neck. Clin Immunol.
225(108677)2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Miyazaki A, Kobayashi J, Torigoe T,
Hirohashi Y, Yamamoto T, Yamaguchi A, Asanuma H, Takahashi A,
Michifuri Y, Nakamori K, et al: Phase I clinical trial of
survivin-derived peptide vaccine therapy for patients with advanced
or recurrent oral cancer. Cancer Sci. 102:324–329. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Idenoue S, Hirohashi Y, Torigoe T, Sato Y,
Tamura Y, Hariu H, Yamamoto M, Kurotaki T, Tsurama T, Asanuma H, et
al: A potent immunogenic general cancer vaccine that target
survivin, an inhibitor of apoptosis proteins. Clin Cancer Res.
11:1474–1482. 2005.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Farnevo L, Tiefenbock K, Ansell A, Thunell
LK, Garvin S and Roberg K: Strong expression of survivin is
associated with positive response to radiotherapy and improved
overall survival in head and neck squamous cell carcinoma patients.
Int J Cancer. 133:1994–2003. 2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wang C, Zainal NS, Chai SJ, Dickie J, Gan
CP, Zulaziz N, Lye BKW, Sutavani RV, Ottensmeier CH, King EV, et
al: DNA vaccines targeting novel cancer-associated antigens
frequently expressed in head and neck cancer enhance the efficacy
of checkpoint inhibitor. Front Immunol. 12(763086)2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Tseng SH, Liu L, Peng S, Kim J, Ferrall L,
Hung CF and Wu CF: Control of spontaneous HPV16 E6/E7 expressing
oral cancer in HLA-A2 (AAD) transgenic mice with therapeutic HPV
DNA vaccine. J Biomed Sci. 28(63)2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wu A, Zeng Q, Kang TH, Peng S, Roosinovich
E, Pai SI and Hung CF: Innovative DNA vaccine for human
papillomavirus (HPV)-associated head and neck cancer. Gene Ther.
18:304–312. 2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Abd-Aziz N and Poh CL: Development of
peptide-based vaccines for cancer. J Oncol.
2022(9749363)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Shiah SG, Chou ST and Chang JY: MicroRNAs:
Their role in metabolism, tumor microenvironment, and therapeutic
implications in head and neck squamous cell carcinoma. Cancers
(Basel). 13(5604)2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wong TS, Wei G and Chan JY: Interactions
between E-cadherin and microRNA deregulation in head and neck
cancers: The potential interplay. Biomed Res Int.
2014(126038)2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Chen D, Cabay RJ, Jin Y, Wang A, Lu Y,
Shah-Khan M and Zhou X: MicroRNA deregulation in head and neck
squamous cell carcinomas. J Oral Maxillofac Res.
4(e2)2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Derakhshan A, Chen Z and Van Waes C:
Therapeutic small molecules target inhibitor of apoptosis proteins
in cancer with deregulation of extrinsec and intrinsic cell death
pathways. Clin Cancer Res. 23:1379–1387. 2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Schiliro C and Firestein BL: Mechanisms of
metabolic reprogramming in cancer cells supporting enhanced growth
and proliferation. Cells. 10(1056)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Faubert B, Solmonson A and DeBerardinis
RJ: Metabolic reprogramming and cancer progression. Science.
368(eaaw5473)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Greten FR and Grivennikov SI: Inflammation
and cancer. Triggers, mechanisms and consequences. Immunity.
51:27–41. 2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lai YH, Liu H, Chiang WF, Chen TW, Chu LJ,
Wu JS, Chen SJ and Tan BC: MiR31-5p-ACOX1 axis enhances tumorigenic
fitness in oral squamous cell carcinoma via the promigratory
Prostaglandin E2. Theranostics. 8:486–504. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Oshima S, Asai S, Seki N, Minemura C,
Kinoshita T, Goto Y, Kikkawa N, Moriya S, Kasamatsu A, Hanazawa T
and Uzawa K: Identification of tumor suppressive genes regulated by
miR-31-5p and miR-31-3p in head and neck squamous cell carcinoma.
Int J Mol Sci. 22(6199)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Moskovitz J, Moy J and Ferris RL:
Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol
Rep. 20(22)2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Li J, Xie Y, Wang X, Li F, Li S, Li M,
Peng H, Yang L, Liu C, Pang L, et al: Prognostic impact of
tumor-associated macrophage infiltration in esophageal cancer: A
meta-analysis. Future Oncol. 15:2303–2317. 2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Bras L, Driessen DAJJ, de Vries J, Festen
S, van der Laan BFAM, van Leeuwen BL, de Bock GH and Halmos GB:
Patients with head and neck cancer: Are they frailer than patients
with other solid malignancies? Eur J Cancer Care (Engl).
29(e13170)2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Sharma H, Sen S, Lo Muzio L, Mariggio MA
and Singh N: Antisense-mediated downregulation of anti-apoptotic
proteins induces apoptosis and sensitizes head and neck squamous
cell carcinoma cells to chemotherapy. Cancer Biol Ther. 4:720–727.
2005.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Farnebo L, Jedlinski A, Ansell A, Vainikka
L, Thunell LK, Grenman R, Johansson AC and Roberg K: Proteins and
single nucleotide polymorphisms involved in apoptosis, growth
control and DNA repair predict cisplatin sensitivity in head and
neck cancer cell lines. Int J Mol Med. 24:549–556. 2009.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Plavc G and Strojan P: Combining
radiotherapy with immunotherapy in definitive treatment of head and
neck squamous cell carcinoma: Review of current clinical trials.
Radiol Oncol. 54:377–393. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Goel B, Tiwari AC, Pandey RK, Singh AP,
Kumar S, Sinha A, Jain SK and Khattri A: Therapeutic approaches for
the treatment of head and neck squamous cell carcinoma-on update on
clinical trials. Transl Oncol. 21(101426)2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Fortpied C and Vinches M: The statistical
evaluation of treatment and outcomes in head and neck squamous cell
carcinoma clinical trials. Front Oncol. 9(634)2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Kawaguchi M, Kato H, Tomita H, Hara A,
Suzui N, Miyazaki T, Matsuyama K, Seishima M and Matsuo M: Magnetic
resonance imaging findings differentiating cutaneous basal cell
carcinoma from squamous cell carcinoma in the head and neck region.
Korean J Radiol. 21:325–331. 2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Weed DT, Zilio S, Reis IM, Sargi Z,
Abouyared M, Gomez-Fernandez CR, Civantos FJ, Rodriguez CP and
Serafini P: The reversal of immune exclusion mediated by Tadalafil
and an anti-tumor vaccine also induces PDL1 upregulation in
recurrent head and neck squamous cell carcinoma: Interim analysis
of a phase I clinical trial. Front Immunol. 10(1206)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Basset-Seguin N and Herms F: Update in the
management of basal cell carcinoma. Acta DermVenereol.
100(adv00140)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Marzuka AG and Book SE: Basal cell
carcinoma: Pathogenesis, epidemiology, clinical features,
diagnosis, histopathology and management. Yale J Bio Med.
88:167–179. 2015.PubMed/NCBI
|
|
93
|
Firnhaber JM: Basal cell and cutaneous
squamous cell carcinomas: Diagnosis and treatment. Am Fam
Physician. 102:339–346. 2020.PubMed/NCBI
|