Introduction
Chromophobe renal cell carcinoma (CRCC) originates
from the distal convoluted tubule and collecting tubule and is a
distinct subtype of RCC (1),
accounting for ~5-10% of RCC (2)
and is the third most common type of renal cancer (3). Upper tract urothelial carcinoma
(UTUC) is a relatively rare type of uroepithelial malignancy,
including renal pelvic carcinoma and ureteral carcinoma, and the
incidence of UTUC has been reported to account for 5-10% of all
uroepithelial carcinomas in Europe and the United States (4). Multiple primary cancers of the
urinary tract are very rare (5),
and it has been recently reported that renal cancer accounts for 2%
of all multiple primary cancers in the first and 2.4% in the second
(6). The combination of ureteral
urothelial carcinoma (UUC) with CRCC is extremely rare and has
hardly been reported. The treatment of multiple primary cancers of
the urinary tract remains controversial, but surgery remains the
best option. In the present study, a case of CRCC combined with
ipsilateral UUC and full-length resection of the affected kidney
and ureter was presented. It is combined with the relevant
literature to provide relevant reference material for this type of
disease.
Case report
A 59-year-old male patient was admitted to the
Department of Urology of the Affiliated Hospital of Zunyi Medical
University (Guizhou, China) in November 2021 with no apparent cause
of terminal carnal hematuria for >3 months. The patient had no
urinary frequency, urgency, or painful urination and no significant
lumbar pain. He had undergone laparoscopic cholecystectomy 20 years
ago and denied family history of hereditary disease. Renal function
tests showed blood creatinine 101 µmol/l, uric acid 402 µmol/l,
GFR: right kidney, non-functional; and left kidney 62.03 ml/min.
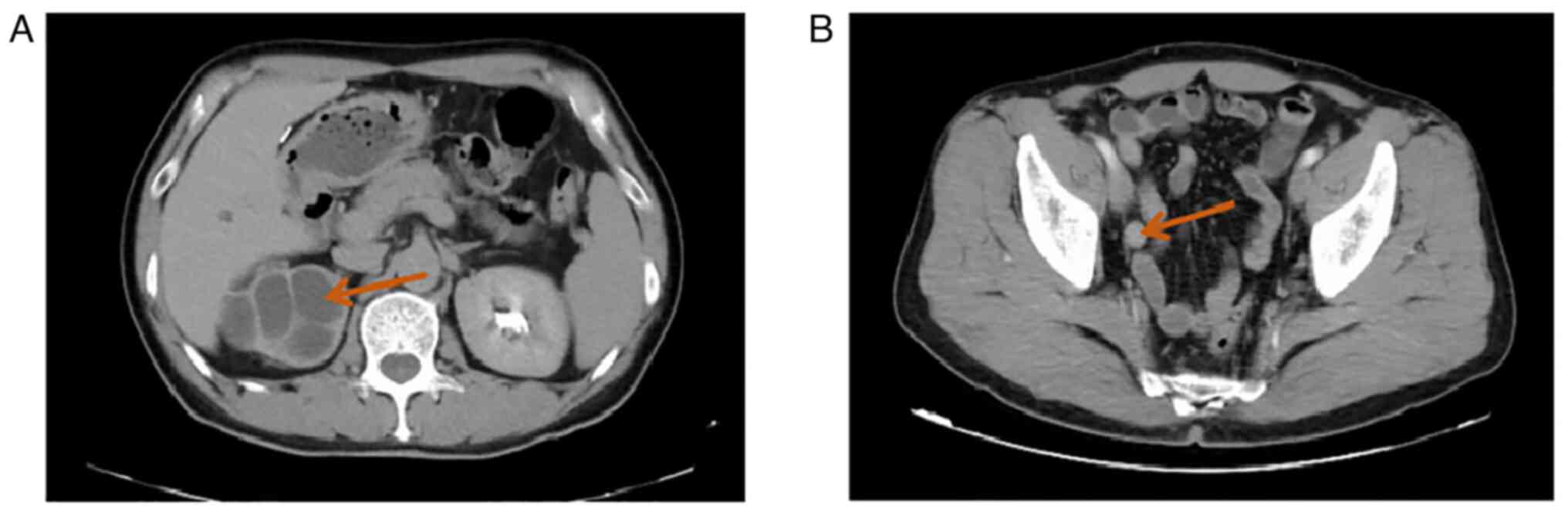
Computed tomography (CT) suggested fluid in the right kidney and
upper right ureter, multiple cysts in the right kidney with partial
marginal calcification (Fig. 1A),
dilatation and fluid in the middle and lower right ureter, slight
thickening of part of the ureteral wall, and occupying lesions
observed in the ureter (Fig. 1B).
Cystoscopy suggested that a persistent hematuric ejection was
observed at the opening of the right ureter. The initial diagnosis
was: i) Right ureteral space-occupying lesion: tumour; ii) right
hydronephrosis with no function and iii) right renal cyst.
Preoperatively, the possibility of renal malignancy was not
considered. After perfect preoperative preparation, laparoscopic
right nephrectomy and right ureteral resection was performed under
general anesthesia, and the resected tissue was sent for
pathological examination.
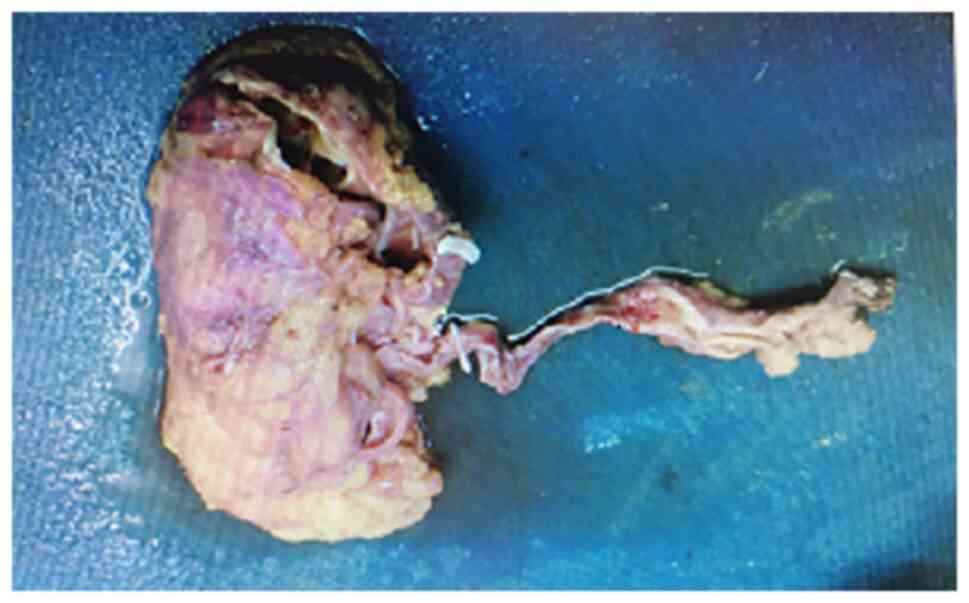
Pathological findings in general: Right post-renal
and ureterectomy specimen with a dilated, cystic nephrectomy with
smooth walls and a greyish-yellow, multi-housed mass at the upper
pole of the kidney, ~3.5x3x2.5 cm in size, with poorly defined
corticomedullary demarcation. A cauliflower-like neoplasm measuring
~5x0.8x0.7 cm was identified 1.8 cm from the ureteral section,
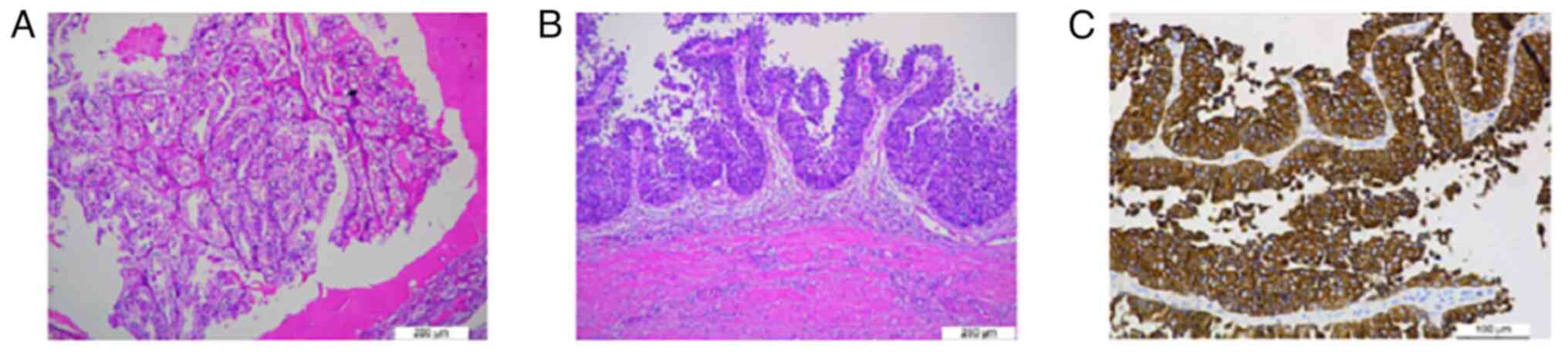
occupying the entire ureteral lumen (Fig. 2). Microscopic examination: i) Low
grade RCC of the right kidney. The tumour cells were tightly
arranged, with abundant cytoplasm, pale and transparent, slightly
reticulated, with clear envelope, moderate or mildly eccentric
nuclei, inconspicuous heterogeneity and rare nuclear division
(H&E staining at room temperature, light microscope, slicing
thickness of 5 µm). The nuclei were moderately or mildly eccentric,
with no obvious heterogeneity. Immunohistochemistry suggested
cancerous tissue: Positive for PAX-8, CA9, creatinine kinase (CK)
7, weakly positive for P504s, partially positive for CD10 and TFE3,
negative for RCC and CD117. Combined with the morphological and
immunohistochemical findings, CRCC was considered with WHO/ISUP
classification of grade 2 (Fig.
3A). 2. Low grade UUC of the right side. The uroepithelium of
the bladder was seen to be papillary with increased cellular
hierarchy and loss of polar disorder, with large deep stained
nuclei and visible nuclear division (H&E staining at room
temperature, light microscope, slicing thickness of 5 µm). There
was local superficial infiltration and the cancer did not invade
the muscular layer of the ureteral wall. No cancerous tissue was
involved in the ureteral section and immunohistochemistry (CK
staining at 37˚C for 30 min, light microscope, slicing thickness of
5 µm) showed positive CK of cancerous tissue (Fig. 3B and C).
After surgery, the patient was treated with
anti-infection, hemostasis, fluid replacement and maintenance of
water-electrolyte balance. The abdominal drainage tube was open and
well positioned, with light red drainage, and was removed in the
afternoon of the third postoperative day; the catheter drained
light yellow urine daily; bladder irrigation chemotherapy was
administered on the fourth postoperative day. The patient was
discharged one week after the operation and was instructed to have
bladder irrigation once a week for eight consecutive times and then
changed to once a month for ten consecutive times. The patient was
asked to repeat cystoscopy and CT of the whole abdomen every three
months, and the results of the follow-up examinations showed no
recurrence and the patient had no particular discomfort.
Discussion
RCC accounts for 3% of adult tumours (7) and UTUC accounts for 5-10% of all
urothelial carcinomas. However, it is very rare for different types
of urological tumours to occur together. Graves and Templeton
(8) reported the first case of
renal cancer combined with UTUC in 1921. The majority of subsequent
studies could be found to be kidney cancer combined with bladder or
pelvis cancer, with the lowest proportion of kidney cancer combined
with ureteral cancer (9). Oka
et al (10) counted 1,352
cases of genitourinary tumours and only one case was RCC combined
with urothelial metastatic cell carcinoma. There are few studies of
CRCC combined with UUC and a small number of studies related to
multiple primary cancers of the urinary tract, whether renal
carcinoma combined with UUC or renal carcinoma combined with
metastatic ureteral cancer, the type of renal carcinoma was almost
always clear cell RCC, the reason for which is not clear. In terms
of the location of tumour presentation, those located on the same
side are slightly more frequently reported than those on different
sides, but remain rare.
Most of the symptoms of different pathological types
of kidney cancer combined with UCC are similar, with hematuria and
back pain as the main symptoms (11). However, the duration of the
patients' history and the maximum diameter of the tumors were found
to be slightly different in the available studies. Patients with
papillary RCC combined with UCC had a shorter history of ~1 month,
with the average maximum diameter of the kidney tumor being ~2 cm
and the average maximum diameter of the ureteral tumor being ~2 cm.
Patients with clear cell RCC combined with UUC had the second
longest history, ~2 months, with an average maximum diameter of ~3
cm for renal tumours and 4 cm for ureteral tumours; patients with
CRCC combined with UCC had a longer history, >3 months on
average, with an average maximum diameter of ~4 cm for renal
tumours and 5 cm for ureteral tumours (Table I) (12-16).
Although the early symptoms of simple kidney cancer are not
obvious, and even ~50% of the patients have no discomfort (17). When the typical triad of kidney
cancer is present, most patients are already in the advanced stage
and have a poor prognosis. The most common symptom of ureteral
cancer is carnal hematuria, and patients often complain of
hematuria. When repeated carnal hematuria, unilateral lumbar pain
and upper urinary tract fluid accumulation are present, and after
examination to exclude the possibility of urinary stones, the
possibility of ureteral occupational lesions needs to be considered
(18).
 | Table IDifferent pathological types of renal
cell carcinoma combined with UUC. |
Table I
Different pathological types of renal
cell carcinoma combined with UUC.
| | Duration of medical
history | Average maximum
diameter of renal tumours | Average maximum
diameter of ureteral tumours |
|---|
| Papillary renal cell
carcinoma combined with UCC | Shorter (~1
month) | ~2 cm | ~2 cm |
| Clear cell renal cell
carcinoma combined with UUC | Medium (~2
months) | ~3 cm | ~4 cm |
| CRCC combined with
UCC | Longer (>3
months) | ~4 cm | ~5 cm |
At this point, the likelihood of an occupying lesion
in the ipsilateral kidney is extremely low, and most clinicians
interrupt their diagnostic thinking at this point, which can easily
lead to a missed or misdiagnosed occupying lesion in the
ipsilateral kidney. Therefore, although the chance of different
types of urological tumours occurring at the same time is very low,
the possibility of the same or different types of tumours in other
parts of the urinary tract should still be noted when a patient is
examined and a detailed preoperative examination and clinical
analysis is essential.
Ureteral carcinoma has been widely reported and may
be primary to the uroepithelium or may result from metastatic clear
cell RCC, thus identification of the source of ureteral carcinoma
is also critical. CK7 and CK20 negative in metastatic clear cell
urothelial carcinoma, are often positive for RCC antigen, Vimentin
and PAX-8, as opposed to pathological findings in primary
uroepithelial carcinoma (19). In
this case, the immunohistochemical staining was positive for CK and
negative for the rest, suggesting that the patient had ureteral
carcinoma of primary uroepithelial origin. The etiology of
co-occurrence of renal and ureteral carcinoma is unclear.
For the treatment of renal cancer combined with
ureteral cancer, total nephro-ureterectomy remains the best
treatment option at present, but in a separate discussion of
surgical options for renal cancer, partial nephrectomy has become
the preferred approach for patients with stage T1(20). The 2020 European Society of Urology
guidelines recommend that for single, <1 cm diameter, urological
enhanced CT urography on which there is no surgery with
preservation of the renal unit can be considered for low-grade
upper urinary tract uroepithelial carcinoma with infiltrative
manifestations, which allows for maximum tumour control while
avoiding the side effects associated with radical resection. For
certain special ureteral cancer patients, such as those with low
malignancy and who must undergo surgery to preserve the renal unit,
complete tumour resection with end-to-end ureteral anastomosis,
ileal substitution ureterotomy or autologous kidney transplantation
can be the main treatment (21-23).
By contrast, when renal cancer is combined with ipsilateral
ureteral cancer, total ureterectomy remains recommended.
The prognosis of patients with multiple primary
urological malignancies has been reported (24) as not being worse than that of
patients with multiple primary malignancies, while Dutta et
al (25) suggested that the
prognosis of patients with multiple primary malignancies is mainly
influenced by the more malignant tumour. It has also been revealed
that their prognosis, although improved than that of metastatic
tumours, remains poor overall (9).
As there are few studies in the literature, low sample sizes and no
systematic statistical analysis, it is not sufficient to draw
definitive prognostic conclusions. However, early diagnosis and
treatment will result in more treatment options and less trauma,
and will certainly help the prognosis of patients.
In conclusion, although CRCC with ipsilateral UUC is
extremely rare but not difficult to diagnose pathologically, it
remains challenging to diagnose and treat early as well as to
maximize the protection of renal function and improve the quality
of life of patients, and more cases still need to be accumulated to
understand their prognosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the special fund for
the cultivation of outstanding young scientific and technological
talents in Guizhou [grant no. Qian Ke He Ren Zi (2015) 31], the
2018 Master's Start-up Fund of the Affiliated Hospital of Zunyi
Medical University [grant no. Yuanzi (2018)31] and the Joint Fund
of Zunyi Science and Technology Bureau [grant no. Zunyi Kehe HZ Zi
(2020) 209].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW and JD were responsible for clinical patient
management. PW was responsible for data collection and article
writing and literature review. LW provided pathology information.
GL was responsible for article review and revision GL and JD acted
as project leaders for the aforementioned grants and financial
support provided. All authors confirm the authenticity of the raw
data in the article. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for the publication of the
patient's clinical information and images was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Casuscelli J, Weinhold N, Gundem G, Wang
L, Zabor EC, Drill E, Wang PI, Nanjangud GJ, Redzematovic A,
Nargund AM, et al: Genomic landscape and evolution of metastatic
chromophobe renal cell carcinoma. JCI Insight.
2(e92688)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ohashi R, Martignoni G, Hartmann A, Caliò
A, Segala D, Stöhr C, Wach S, Erlmeier F, Weichert W, Autenrieth M,
et al: Multi-institutional re-evaluation of prognostic factors in
chromophobe renal cell carcinoma: Proposal of a novel two-tiered
grading scheme. Virchows Arch. 476:409–418. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Weng WH, Chen YT, Yu KJ, Chang YH, Chuang
CK and Pang ST: Genetic alterations of HER genes in chromophobe
renal cell carcinoma. Oncol Lett. 11:2111–2116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Qi N, Chen Y, Gong K and Li H: Concurrent
renal cell carcinoma and urothelial carcinoma: Long-term follow-up
study of 27 cases. World J Surg Oncol. 16(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Feller A, Matthes KL, Bordoni A, Bouchardy
C, Bulliard JL, Herrmann C, Konzelmann I, Maspoli M, Mousavi M,
Rohrmann S, et al: The relative risk of second primary cancers in
Switzerland: A population-based retrospective cohort study. BMC
Cancer. 20(51)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bohosova J, Kubickova A and Slaby O:
lncRNA PVT1 in the pathogenesis and clinical management of renal
cell carcinoma. Biomolecules. 11(664)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Graves RC and Templeton ER: Combined
tumors of the kidney. J Urol. 5:517–537. 1921.
|
|
9
|
Beisland C, Talleraas O, Bakke A and
Norstein J: Multiple primary malignancies in patients with renal
cell carcinoma: A national population-based cohort study. BJU Int.
97:698–702. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Oka H, Kobayashi S, Kobayashi T, Shugino
Y, Matsui Y, Fujikawa K, Iwamura H, Hukuzawa S, Soeda A and
Takeuchi H: Multiple primary cancers limited to the urological
field. Hinyokika Kiyo. 47:405–409. 2001.PubMed/NCBI(In Japanese).
|
|
11
|
Arora HC, Fascelli M, Zhang JH, Isharwal S
and Campbell SC: Kidney, ureteral, and bladder cancer: A primer for
the internist. Med Clin North Am. 102:231–249. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mucciardi G, Galì A, D'Amico C, Muscarà G,
Barresi V and Magno C: Transitional cell carcinoma of the renal
pelvis with synchronous ipsilateral papillary renal cell carcinoma:
Case report and review. Urol Case Rep. 16:93–95. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yun JK, Kim SH, Kim WB, Kim HK and Lee SW:
Simultaneous robot-assisted approach in a super-elderly patient
with urothelial carcinoma and synchronous contralateral renal cell
carcinoma: A case report. World J Clin Cases. 10:7153–7162.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu K, Liu X, Wang Y, Wang X and Li X:
Clinicopathological characteristics and outcomes of synchronous
renal cell carcinoma and urothelial carcinoma: A population-based
analysis. Front Public Health. 10(994351)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Symeonidis A, Tsikopoulos I, Symeonidis
EN, Tsifountoudis I, Michailidis A, Tsantila I, Gkekas C,
Georgiadis C, Malioris A and Papathanasiou M: More than meets the
eye: A case of synchronous ipsilateral clear cell renal cell
carcinoma and urothelial carcinoma of the pelvicalyceal system and
literature review. Acta Biomed. 92(e2021380)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chhajed A, Baraniya J, Chhajed S,
Choudhary A and Jain N: Pathologically diagnosed incidentaloma
transition cell carcinoma (TCC) of renal pelvis in a laproscopic
radical nephrectomy specimen done for a lower pole renal mass. Urol
Case Rep. 37(101607)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang H, Li W, Lv Y, Fan Q, Mao X, Long T,
Xie L, Dong C, Yang R and Zhang H: Exploring the mechanism of clear
cell renal cell carcinoma metastasis and key genes based on
multi-tool joint analysis. Gene. 720(144103)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hung SY, Yang WC, Luo HL, Hsu CC, Chen YT
and Chuang YC: Segmental ureterectomy does not compromise the
oncologic outcome compared with nephroureterectomy for pure ureter
cancer. Int Urol Nephrol. 46:921–926. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Venyo AKG: Clear cell adenocarcinoma of
the urethra: Review of the literature. Int J Surg Oncol.
2015(790235)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rassweiler JJ, Klein J, Tschada A and
Gözen AS: Laparoscopic retroperitoneal partial nephrectomy using an
ergonomic chair: Demonstration of technique and matched-pair
analysis. BJU Int. 119:349–357. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rouprêt M, Babjuk M, Burger M, Capoun O,
Cohen D, Compérat EM, Cowan NC, Dominguez-Escrig JL, Gontero P,
Hugh Mostafid A, et al: European association of urology guidelines
on upper urinary tract urothelial carcinoma: 2020 Update. Eur Urol.
79:62–79. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee KH, Lai WH, Chiu AW, Lu CC and Huang
SK: Robot-assisted retroperitoneoscopic surgery for synchronous
contralateral ureteral metastasis of renal-cell carcinoma. J
Endourol Case Rep. 1:65–67. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ou YC, Hu CY, Cheng HL and Yang WH:
Long-term outcomes of total ureterectomy with ileal-ureteral
substitution treatment for ureteral cancer: A single-center
experience. BMC Urol. 18(73)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fernández Arjona M, Santos Arrontes D, De
Castro Barbosa F, Begara Morillas F, Cortes Aranguez I and González
L: Synchronous renal clear-cell carcinoma and ipsilateral
transitional-cell carcinoma: Case report and bibliographic review.
Arch Esp Urol. 58:460–463. 2005.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
25
|
Dutta G, Silver D, Oliff A and Harrison A:
Synchronous renal malignancy presenting as recurrent urinary tract
infections. Case Rep Urol. 2011(832673)2011.PubMed/NCBI View Article : Google Scholar
|