Introduction
Numerous studies have reported more favorable
survival outcomes of immune checkpoint inhibitor (ICI) therapy,
either alone or in combination with cytotoxic agents, as compared
with cytotoxic agent therapy alone, in patients with non-small cell
lung cancer (NSCLC) (1-5).
However, ICI therapy has also been reported to be relatively less
effective in patients with NSCLC harboring epidermal growth factor
receptor (EGFR) mutations than in those with tumors harboring
wild-type EGFR (2,6).
While tumor programmed death-ligand 1 (PD-L1)
expression may be associated with the efficacy of ICI treatment in
patients with non-squamous NSCLC (2), the reports about the efficacy of ICI
therapy in patients with EGFR-mutant NSCLC are not consistent
(7-12).
Furthermore, although the serum level of lactate dehydrogenase
(LDH), the peripheral blood neutrophil-lymphocyte ratio (NLR), and
serum C-reactive protein (CRP) level may be associated with
survival after the initiation of ICI treatment in patients with
NSCLC (13-16),
it remains unclear if the same associations can also be observed in
patients with EGFR-mutant NSCLC.
Regarding the influence of the tumor
microenvironment in patients with cancer, the number of
tumor-infiltrating macrophages can affect the clinical course in
patients with malignancies (17).
Notably, macrophages contribute to the early elimination of cancer.
However, tumor progression is associated with skewing of macrophage
function (18), and macrophages
recruited by the cancer cells promote the survival and
proliferation of cancer cells (19,20).
Such macrophages can also be a therapeutic target in patients with
cancer (18). However, their
prognostic impact is dependent on the treatment employed (18), and the association between the
tumor-infiltrating macrophage count and the efficacy of ICI
treatment in patients with EGFR-mutant NSCLC has not been
clarified.
We conducted this retrospective study to investigate
the associations of some clinical parameters and tumor-infiltrating
CD68-positive cell counts with the efficacy of ICI therapy in
patients with EGFR-mutant NSCLC.
Patients and methods
Patients
We conducted a retrospective analysis of the data of
consecutive patients who had been diagnosed as having EGFR-mutant
advanced NSCLC and had received ICI monotherapy between 2016 and
2022 at Toyama Prefectural Central Hospital or Toyama University
Hospital. No exclusion criteria were established.
This study was conducted in accordance with the
Declaration of Helsinki and the Ethical Guidelines for Medical and
Health Research Involving Human Subjects (Ministry of Health,
Labour and Welfare, Japan). The requirement for informed consent
was waived and we disclosed the study information on our website to
the patients, under the approval of the Ethics Committee,
University of Toyama (approval number R2019040).
Clinical information
Clinical information on the patient background
characteristics and the clinical course was retrieved from the
medical charts. Results of blood tests performed at the onset of
ICI treatment or the most recent tests performed within the
previous month were evaluated, and the patients were subdivided by
the median value (NLR: 5; serum LDH: 220 U/l; serum CRP: 0.5
mg/dl). The NLR was calculated by dividing the number of
neutrophils by the number of lymphocytes. Tumor PD-L1 expression
was determined using the 22C3 antibody in tumor specimens obtained
at any time during the entire clinical course of the patient,
either before or after the EGFR-tyrosine kinase inhibitor
(EGFR-TKI) therapy. The proportion of PD-L1-positive tumor cells
was calculated as the tumor proportion score (TPS). A positive
history of radiation therapy was defined as radiation therapy
administered within 6 weeks prior to the initiation of ICI therapy
or during the ICI therapy.
Immunohistochemistry
The tumor immunohistochemistry was commissioned to
Mediridge Co., Ltd (Tokyo, Japan) and performed on primary or
metastatic tumor specimens obtained from 11 NSCLC patients who had
received treatment at Toyama University Hospital between 2016 and
2019.
For immunohistochemical staining, formalin-fixed
paraffin-embedded tumor tissues were deparaffinized using xylene
and an 80-100% downgraded ethanol series. Antigen retrieval
treatment was performed with 0.1% trypsin (T4799-25G; Sigma-Aldrich
Corporation, St. Louis, MO, USA)/PBS (pH 7.6) at 37˚C for 15 min.
Endogenous peroxidase was blocked with 3% hydrogen peroxide, and
the non-specific reaction was blocked with Blocking One (#03953-95;
Nacalai Tesque, Kyoto, Japan). For the primary antibody, we
incubated the sections with anti-human CD68 mouse IgG1 monoclonal
antibody (clone: Kp-1, M0814, at 1:200 dilution, Agilent Inc.,
Santa Clara, CA) overnight at 4˚C. Positive reactions were
visualized using horse radish peroxidase-conjugated secondary
antibody (HISTOFINE #424134, Nichirei Bioscience Inc., Tokyo,
Japan) and 3-3' diaminobenzidine as the substrate.
Determination of the tumor-infiltrating cell count
was performed by two investigators who were blinded to the clinical
courses of the patients. The number of CD68-positive cells in the
tumor tissue or stroma in contact with the tumor tissue per field
of view (400x magnification) were counted in up to 10 fields per
section, and the mean CD68-positive cell count was used for the
analysis.
Statistical analysis
The endpoint of the present study was the
progression-free survival (PFS) and overall survival (OS). The PFS
was calculated from the initiation date of ICI therapy to the
detection date of disease progression. Disease progression was
defined according to the clinical judgment or computed tomography
evidence of progressive disease (PD) and censored at the last visit
without disease progression. PD was defined as a 20% or greater
increase in the diameter of the target lesion, emergence of new
lesions, deterioration of the general condition of the patient, or
death. The OS was calculated from the day that ICI therapy was
initiated to the day of death and censored at the last visit during
the life of the patient. Survival curves were drawn by the
Kaplan-Meier method and survival was compared by the log-rank test
between patient groups subdivided according to categorical
variables.
A Cox proportional hazards model was used to
evaluate the associations between the clinical parameters and the
PFS or OS. We included the patient performance status (PS), EGFR
status (9), tumor PD-L1 expression
status (2), values of NLR, LDH,
and CRP (13-16),
and history of radiation therapy (21) as independent variables because they
may be associated with the efficacy of ICI treatment. Fisher's
exact test was used to compare the patient characteristics.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using JMP ver.
14.0.2 (SAS, Cary, NC).
Results
Patient characteristics
Table I shows the
patient characteristics. A total of 46 patients with EGFR-mutant
NSCLC received ICI monotherapy at Toyama University Hospital or
Toyama Prefectural Central Hospital. Of the 46 patients, 43 (93.5%)
were diagnosed with adenocarcinoma, 2 (4.3%) with NSCLC (not
otherwise specified), and 1 (2.2%) with squamous cell carcinoma.
PD-L1 TPS was ≥50% in 10 (21.7%) patients and was not evaluated in
9 (19.6%) patients. Of the 46 patients, 43 (93.5%) had a previous
history of EGFR-TKI therapy prior to ICI therapy, including
gefitinib (n=10), erlotinib (n=4), erlotinib plus bevacizumab
(n=6), afatinib (n=19), or osimertinib (n=4). Of these, 36 patients
had received EGFR-TKI therapy as first-line therapy, and 7 (15.2%)
patients as second-line therapy. The EGFR-TKI therapy was
discontinued because of acquired resistance to the drug in 41
patients and because of the emergence of adverse events in 2
patients. T790M mutation was detected in 12 (30.8%) patients out of
39 patients treated with first- or second-generation EGFR-TKIs
prior to ICI therapy.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variable | No. of patients
(%) |
|---|
| Sex | |
|
Male | 21 (45.7) |
|
Female | 25 (54.3) |
| Age, years | |
|
<70 | 23 (50.0) |
|
≥70 | 23 (50.0) |
| Smoking history | |
|
Yes | 18 (39.1) |
|
No | 28 (60.9) |
| PS | |
|
0-1 | 31 (67.4) |
|
≥2 | 15 (32.6) |
| Histology | |
|
Adenocarcinoma | 43 (93.5) |
|
Others | 3 (6.5) |
| EGFR | |
|
Exon 19
del | 19 (41.3) |
|
L858R | 19 (41.3) |
|
Others | 8 (17.4) |
| PD-L1, % | |
|
<1 | 16 (34.8) |
|
1-49 | 11 (23.9) |
|
≥50 | 10 (21.7) |
|
Unknown | 9 (19.6) |
| History of
radiation therapy | |
|
Yes | 9 (19.6) |
|
No | 37 (80.4) |
| ICI | |
|
Nivolumab | 16 (34.8) |
|
Pembrolizumab | 13 (28.3) |
|
Atezolizumab | 17 (37.0) |
| ICI treatment
line | |
|
2 | 4 (8.7) |
|
3 | 10 (21.7) |
|
4 | 13 (28.3) |
|
5 | 9 (19.6) |
|
≥6 | 10 (21.7) |
| NLR | |
|
<5 | 25 (54.3) |
|
≥5 | 21 (45.7) |
| LDH, U/l | |
|
<220 | 21 (45.7) |
|
≥220 | 25 (54.3) |
| CRP, mg/dl | |
|
<0.5 | 24 (52.2) |
|
≥0.5 | 22 (47.8) |
Clinical parameters
The median (95% confidence interval) PFS and OS
after the initiation of ICI treatment was 1.4 (1.0-1.7) and 6.4
(3.9-19.0) months, respectively. Tables II and III show the results of analyses
performed using the Cox proportional hazards model. The PD-L1
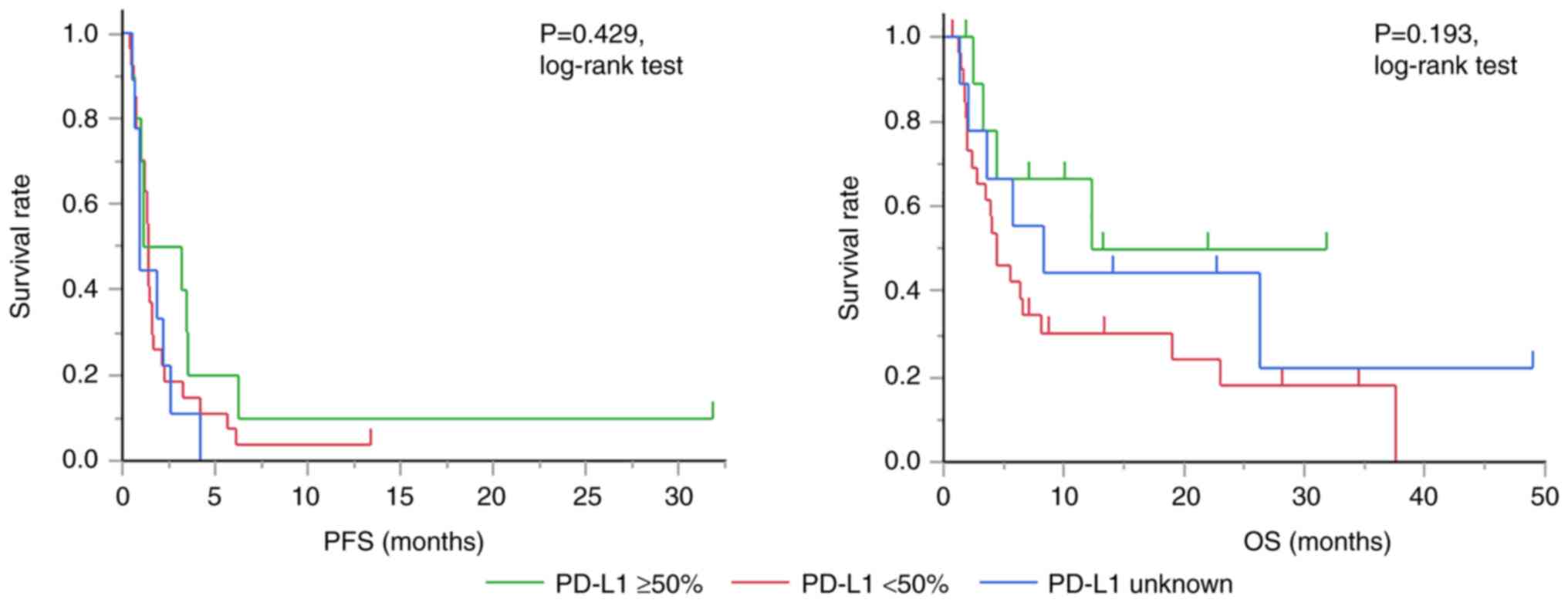
expression level was significantly associated with the OS. Fig. 1 shows the Kaplan-Meier curve
comparing the PFS and OS after the initiation of ICI therapy
according to PD-L1 expression levels.
 | Table IIAssociations between clinical
parameters and progression-free survival after initiation of immune
checkpoint inhibitor therapy according to the Cox proportional
hazards model. |
Table II
Associations between clinical
parameters and progression-free survival after initiation of immune
checkpoint inhibitor therapy according to the Cox proportional
hazards model.
| Variable | HR | 95% CI | P-value |
|---|
| PS | | | |
|
0-1 | 1.00 | | |
|
≥2 | 0.82 | 0.37-1.82 | 0.625 |
| EGFR status | | | |
|
Exon 19
del | 1.15 | 0.54-2.46 | 0.713 |
|
L858R | 1.00 | | |
|
Others | 1.41 | 0.55-3.61 | 0.471 |
| PD-L1, % | | | |
|
≥50 | 0.63 | 0.23-1.72 | 0.368 |
|
<50 | 1.00 | | |
|
Unknown | 0.96 | 0.38-2.43 | 0.926 |
| History of
radiation therapy | | | |
|
Yes | 0.61 | 0.23-1.61 | 0.319 |
|
No | 1.00 | | |
| NLR | | | |
|
<5 | 1.00 | | |
|
≥5 | 2.32 | 1.00-5.38 | 0.051 |
| LDH, U/l | | | |
|
<220 | 1.00 | | |
|
≥220 | 1.02 | 0.51-2.05 | 0.947 |
| CRP, mg/dl | | | |
|
<0.5 | 1.00 | | |
|
≥0.5 | 0.67 | 0.31-1.47 | 0.319 |
 | Table IIIAssociations between clinical
parameters and overall survival after initiation of immune
checkpoint inhibitor therapy according to the Cox proportional
hazards model. |
Table III
Associations between clinical
parameters and overall survival after initiation of immune
checkpoint inhibitor therapy according to the Cox proportional
hazards model.
| Variable | HR | 95% CI | P-value |
|---|
| PS | | | |
|
0-1 | 1.00 | | |
|
≥2 | 2.30 | 0.95-5.60 | 0.066 |
| EGFR status | | | |
|
Exon 19
del | 2.12 | 0.84-5.37 | 0.113 |
|
L858R | 1.00 | | |
|
Others | 1.75 | 0.60-5.14 | 0.308 |
| PD-L1, % | | | |
|
≥50 | 0.16 | 0.04-0.62 | 0.008 |
|
<50 | 1.00 | | |
|
Unknown | 0.36 | 0.10-1.32 | 0.124 |
| History of
radiation therapy | | | |
|
Yes | 2.06 | 0.70-6.06 | 0.188 |
|
No | 1.00 | | |
| NLR | | | |
|
<5 | 1.00 | | |
|
≥5 | 1.68 | 0.73-3.88 | 0.221 |
| LDH, U/l | | | |
|
<220 | 1.00 | | |
|
≥220 | 1.80 | 0.74-4.37 | 0.193 |
| CRP, mg/dl | | | |
|
<0.5 | 1.00 | | |
|
≥0.5 | 0.82 | 0.32-2.11 | 0.676 |
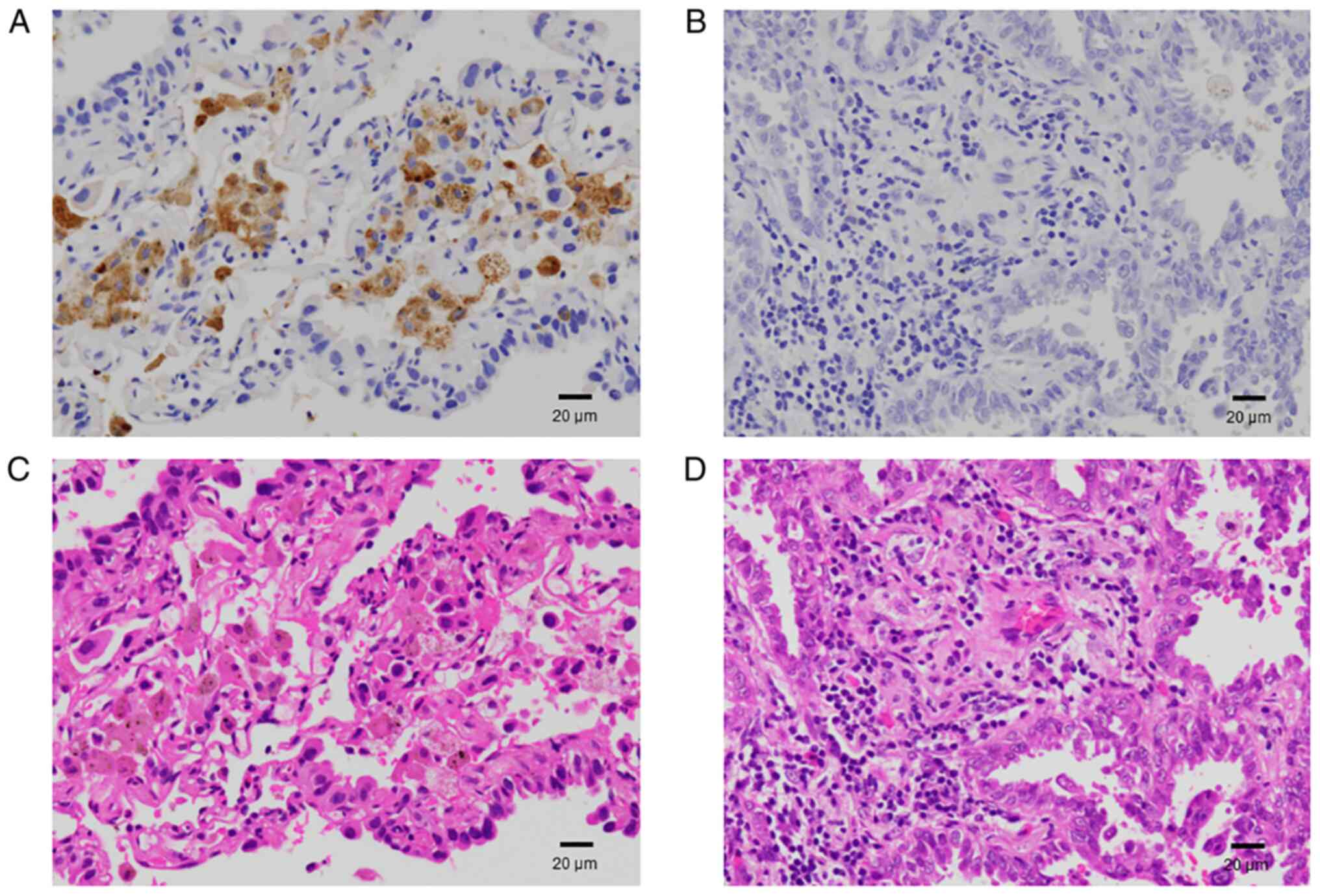
Immunohistochemistry
We conducted an immunohistochemical analysis to
evaluate the degree of infiltration of the tumor tissue and tumor
stroma in contact with the tumor tissue by CD68-positive cells.
Representative images of positive and negative immunohistochemistry
results are shown in Fig. 2. The
patient characteristics are shown in Table IV. The average number of
CD68-positive cells per field of view varied from 1.2 to 23.2 in
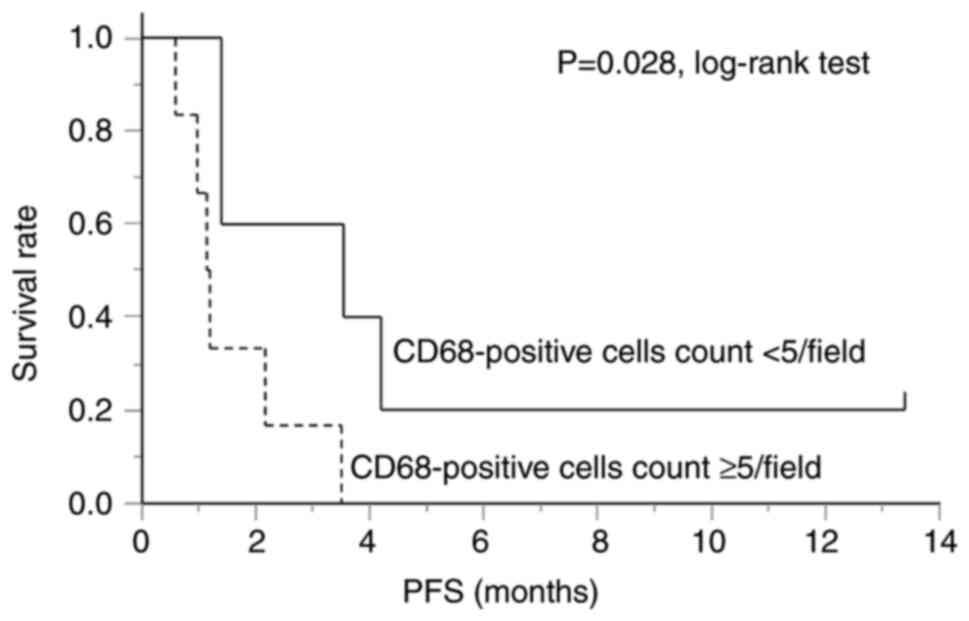
the patients, with a median of 5.4. Fig. 3 shows a comparison of the PFS
between the groups with low (CD68-positive cells <5/field) and
high tumor-infiltrating CD68-positive cell (CD68-positive cells
≥5/field) counts. The PFS was significantly worse in the group with
a high tumor-infiltrating CD68-positive cell count than that in the
group with a low tumor-infiltrating CD68-positive cell count.
 | Table IVInformation on the specimens for
evaluation of the CD68-positive cell count. |
Table IV
Information on the specimens for
evaluation of the CD68-positive cell count.
| Age, years | Sex | Histology | EGFR | Organ | Procedure | Duration,
months | Before/after the
TKI therapy | CD68, /field |
|---|
| 68 | F | Adeno | L858R | Bone | Biopsy | Not
assesseda | Before | 1.2 |
| 81 | M | Adeno | del 19 | Lung | Surgical
resection | 56.1 | Before | 2.0 |
| 74 | M | Adeno | del 19 | Lymph node | Biopsy | 22.3 | Before | 3.1 |
| 60 | F | Adeno | L858R | Lung | Biopsy | 35.1 | After | 4.1 |
| 57 | M | NOS | del 19/ins | Lung | Biopsy | 5.1 | After | 4.7 |
| 80 | F | Adeno | L858R | Bone | Biopsy | 3.2 | After | 5.4 |
| 69 | F | NOS | del 19 | Brain | Surgical
resection | 26.8 | After | 5.8 |
| 87 | F | Adeno | L858R | Lung | Biopsy | 0.7 | After | 6.7 |
| 77 | F | Adeno | L858R | Lung | Biopsy | 67.9 | After | 6.9 |
| 65 | M | Adeno | L858R | Lung | Biopsy | 9.5 | Before | 18.5 |
| 60 | M | Adeno | del 19 | Lung | Biopsy | 18.0 | Before | 23.2 |
Table V shows a
comparison of the patient background characteristics between those
with high and low CD68-positive cell counts. There were no apparent
differences between the two groups, and none of the patients,
except one, had received radiation therapy prior to ICI
therapy.
 | Table VCharacteristics of the patients
evaluated for CD68-positive cell count. |
Table V
Characteristics of the patients
evaluated for CD68-positive cell count.
| Variable | CD68 <5/field, n
(%) | CD68 ≥5 /field, n
(%) | P-value |
|---|
| Sex | | | |
|
Male | 3 (60.0) | 2 (33.3) | 0.567 |
|
Female | 2 (40.0) | 4 (66.7) | |
| Age, years | | | |
|
<70 | 3 (60.0) | 3 (50.0) | >0.999 |
|
≥70 | 2 (40.0) | 3 (50.0) | |
| Smoking
history | | | |
|
Yes | 3 (60.0) | 2 (33.3) | 0.567 |
|
No | 2 (40.0) | 4 (66.7) | |
| PS | | | |
|
0-1 | 3 (60.0) | 3 (50.0) | >0.999 |
|
≥2 | 2 (40.0) | 3 (50.0) | |
| Histology | | | |
|
Adenocarcinoma | 4 (80.0) | 5 (83.3) | >0.999 |
|
Others | 1 (20.0) | 1 (16.7) | |
| EGFR | | | |
|
Exon 19
del | 3 (60.0) | 2 (33.3) | 0.567 |
|
L858R | 2 (40.0) | 4 (66.7) | |
|
Others | 0 (0.0) | 0 (0.0) | |
| PD-L1 TPS, % | | | |
|
<1 | 3 (60.0) | 4 (66.7) | >0.999 |
|
≥1 | 2 (40.0) | 2 (33.3) | |
|
Unknown | 0 (0.0) | 0 (0.0) | |
| ICIs | | | |
|
Nivolumab | 1 (20.0) | 2 (33.3) | >0.999 |
|
Pembrolizumab | 2 (40.0) | 2 (33.3) | |
|
Atezolizumab | 2 (40.0) | 2 (33.3) | |
| NLR | | | |
|
<5 | 2 (40.0) | 3 (50.0) | >0.999 |
|
≥5 | 3 (60.0) | 3 (50.0) | |
| LDH, U/l | | | |
|
<220 | 1 (20.0) | 4 (66.7) | 0.242 |
|
≥220 | 4 (80.0) | 2 (33.3) | |
| CRP, mg/dl | | | |
|
<0.5 | 1 (20.0) | 4 (66.7) | 0.242 |
|
≥0.5 | 4 (80.0) | 2 (33.3) | |
| History of
radiation therapy | | | |
|
Yes | 1 (20.0) | 0 (0.0) | 0.455 |
|
No | 4 (80.0) | 6 (100.0) | |
Discussion
In the present study, the median of PFS after the
initiation of ICI therapy was 1.4 months in patients with
EGFR-mutant NSCLC, suggesting that ICI therapy is relatively less
effective in this subset of NSCLC patients. Conversely, the results
suggested that the PD-L1 expression level and CD68-positive cell
count in the tumor microenvironment are significantly associated
with the efficacy of ICI therapy.
The present study revealed an association between
the OS after the start of ICI therapy and the tumor PD-L1
expression status. Although there is an opposing report (9), several authors also reported the
association between positive tumor PD-L1 expression and survival
benefits in patients with EGFR-mutant NSCLC (7,11,12).
However, it has been reported that the tumor PD-L1 expression
status can change during EGFR-TKI therapy (6,12).
Furthermore, tumor PD-L1 expression may be induced by EGFR
signaling (22) and interferon γ
(23). Given that the CD8-positive
T cell density is significantly higher in PD-L1-positive
EGFR-mutant tumors than in PD-L1-negative or low-positive tumors
after EGFR-TKI treatment (12),
tumor PD-L1 expression may reflect the infiltration of CD8-positive
T-lymphocytes. However, EGFR signaling also suppresses tumor
immunity by increasing the production of C-C motif chemokine 22
(CCL22), which recruits regulatory T cells, and decreasing the
production of C-X-C motif chemokine ligand 10 and CCL5 which are
known to induce CD8+ T cell infiltration (24).
In the present study, the NLR, LDH, and CRP did not
exhibit a significant association with survival after the
initiation of ICI treatment in patients with EGFR-mutant NSCLC.
Alternatively, a meta-analysis of patients with various solid
tumors has shown an association between the NLR and patient
survival across disease stages (25). Furthermore, an elevated NLR has
been reported to be associated with poor survival after ICI
treatment in patients with NSCLC (26). Tumor-infiltrating lymphocytes and
neutrophils (CD15-positive) have been reported as favorable and
poor prognostic factors, respectively, in cancer patients (27,28).
Moreover, cytotoxic T-lymphocyte cell-lytic activity was observed
to be inhibited by neutrophils in-vitro (29). These findings may explain the
association between the NLR and the prognosis in NSCLC patients
treated with ICIs. The results of the present study suggest that
this association may not be found in patients with EGFR-mutant
NSCLC. Therefore, it may be difficult to predict the efficacy of
ICI therapy based on the NLR in patients with EGFR-mutant
NSCLC.
Additionally, γ-irradiation can cause immunogenic
cell death of tumor cells, which reportedly induce the release of
tumor antigens and danger-associated molecular patterns, triggering
tumor immunity (21). However, it
remains unclear if radiotherapy enhances the clinical effectiveness
of ICI therapy. A phase II trial conducted to investigate the
effect of radiotherapy in enhancing the response to pembrolizumab
in patients with NSCLC failed to meet the prespecified endpoint
criteria (30). In contrast, the
results of a subgroup analysis in patients with PD-L1-negative
tumors suggested a beneficial effect of the addition of
radiotherapy. Furthermore, a secondary analysis of the KEYNOTE-001
phase 1 trial suggested that radiotherapy in patients with advanced
NSCLC may yield a longer survival in patients treated with
pembrolizumab (31). We failed to
show any association between radiation therapy and the efficacy of
ICI treatment. However, the possibility of decreased statistical
power due to the small sample size affecting the results of the
analysis cannot be excluded.
In the present study, higher tumor-infiltrating
CD68-positive cell counts were found to be associated with a
shorter PFS after the initiation of ICI therapy. Previously, it was
reported that the number of tumor-infiltrating macrophages can
affect the clinical course in patients with malignancies (17). Furthermore, infiltration of
macrophage was reported to be associated with PFS in patients with
EGFR/ALK wild type NSCLC treated with ICI therapy (32). As for patients with EGFR mutant
NSCLC, it was reported that infiltration of CD8-positive T cells
(7), CD4-positive T cells and
Foxp3-positive cells (10) were
associated with PFS after the initiation of ICI therapy. However,
CD68-positive cells were not investigated in these previous
studies. Macrophages are recruited and polarized to the M2
phenotype to promote the survival and proliferation of cancer cells
(19,20). However, because the timing at which
the specimens were obtained varied among the patients, we could not
evaluate the tumor microenvironment immediately before the start of
the ICI therapy in all cases. Thus, the findings in this study need
to be interpreted with caution, and further studies are warranted
to elucidate the association between the tumor microenvironment and
the efficacy of ICI therapy.
The major limitations of the present study were the
small sample size and retrospective design. Thus, bias or random
error may have affected the statistical analysis.
In conclusion, the present study showed that the
PD-L1 expression level and tumor-infiltrating CD68-positive cell
count might be associated with the efficacy of ICI therapy. Further
investigation is required to verify this association.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MI and KaT contributed to conception and design of
the study. TT, KS and MI contributed to the analysis and
interpretation of data. MI, TT, KS, KH, IM, KoT, CT, SO, KK, SI,
TM, RH, SM, YM and HT contributed to data acquisition. MI and TT
wrote the main manuscript, and KH, IM, KoT, CT, SO, KK, SI, TM, RH,
SM, YM, HT and KaT were involved in revising the manuscript. MI and
TT confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and Ethical Guidelines for Medical and
Biological Research Involving Human Subjects (Ministry of Health,
Labour and Welfare, Japan), and approved by the Ethics Committee,
University of Toyama (Toyama, Japan, approval no. R2019040). The
need to obtain informed consent from the study subjects was waived
under the approval of the Ethics Committee, University of Toyama,
and information about the study was disclosed to the subjects on
the Toyama University Hospital website (http://www.hosp.u-toyama.ac.jp/guide/index.html).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brahmer J, Reckamp KL, Baas P, Crino L,
Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gandhi L, Rodriguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gainor JF, Shaw AT, Sequist LV, Fu X,
Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et
al: EGFR mutations and ALK rearrangements are associated with low
response rates to PD-1 pathway blockade in non-small cell lung
cancer: A retrospective analysis. Clin Cancer Res. 22:4585–4593.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Haratani K, Hayashi H, Tanaka T, Kaneda H,
Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et
al: Tumor immune microenvironment and nivolumab efficacy in EGFR
mutation-positive non-small-cell lung cancer based on T790M status
after disease progression during EGFR-TKI treatment. Ann Oncol.
28:1532–1539. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Inomata M, Tanaka H, Tokui K, Taka C,
Okazawa S, Kambara K, Imanishi S, Yamada T, Miwa T, Hayashi R, et
al: Clinical course after initiation of nivolumab therapy in
patients with egfr-mutated non-small cell lung cancer with or
without Pd-L1 expression. Oncology Therapy. 5:181–185. 2017.
|
|
9
|
Hastings K, Yu HA, Wei W, Sanchez-Vega F,
DeVeaux M, Choi J, Rizvi H, Lisberg A, Truini A, Lydon CA, et al:
EGFR mutation subtypes and response to immune checkpoint blockade
treatment in non-small-cell lung cancer. Ann Oncol. 30:1311–1320.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sato M, Watanabe S, Tanaka H, Nozaki K,
Arita M, Takahashi M, Shoji S, Ichikawa K, Kondo R, Aoki N, et al:
Retrospective analysis of antitumor effects and biomarkers for
nivolumab in NSCLC patients with EGFR mutations. PLoS One.
14(e0215292)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Masuda K, Horinouchi H, Tanaka M,
Higashiyama R, Shinno Y, Sato J, Matsumoto Y, Okuma Y, Yoshida T,
Goto Y, et al: Efficacy of anti-PD-1 antibodies in NSCLC patients
with an EGFR mutation and high PD-L1 expression. J Cancer Res Clin
Oncol. 147:245–251. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Isomoto K, Haratani K, Hayashi H, Shimizu
S, Tomida S, Niwa T, Yokoyama T, Fukuda Y, Chiba Y, Kato R, et al:
Impact of EGFR-TKI treatment on the tumor immune microenvironment
in EGFR mutation-positive non-small cell lung cancer. Clin Cancer
Res. 26:2037–2046. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Taniguchi Y, Tamiya A, Isa SI, Nakahama K,
Okishio K, Shiroyama T, Suzuki H, Inoue T, Tamiya M, Hirashima T,
et al: Predictive factors for poor progression-free survival in
patients with non-small cell lung cancer treated with nivolumab.
Anticancer Res. 37:5857–5862. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Oya Y, Yoshida T, Kuroda H, Mikubo M,
Kondo C, Shimizu J, Horio Y, Sakao Y, Hida T and Yatabe Y:
Predictive clinical parameters for the response of nivolumab in
pretreated advanced non-small-cell lung cancer. Oncotarget.
8:103117–103128. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mezquita L, Auclin E, Ferrara R, Charrier
M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L,
Audigier-Valette C, et al: Association of the lung immune
prognostic index with immune checkpoint inhibitor outcomes in
patients with advanced non-small cell lung cancer. JAMA Oncol.
4:351–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bagley SJ, Kothari S, Aggarwal C, Bauml
JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson
JC, et al: Pretreatment neutrophil-to-lymphocyte ratio as a marker
of outcomes in nivolumab-treated patients with advanced
non-small-cell lung cancer. Lung Cancer. 106:1–7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mantovani A, Schioppa T, Porta C, Allavena
P and Sica A: Role of tumor-associated macrophages in tumor
progression and invasion. Cancer Metastasis Rev. 25:315–322.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan A, Hsiao YJ, Chen HY, Chen HW, Ho CC,
Chen YY, Liu YC, Hong TH, Yu SL, Chen JJ and Yang PC: Opposite
effects of M1 and M2 macrophage subtypes on lung cancer
progression. Sci Rep. 5(14273)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Myers KV, Pienta KJ and Amend SR: Cancer
cells and M2 macrophages: Cooperative invasive ecosystem engineers.
Cancer Control. 27(1073274820911058)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Obeid M, Panaretakis T, Tesniere A, Joza
N, Tufi R, Apetoh L, Ghiringhelli F, Zitvogel L and Kroemer G:
Leveraging the immune system during chemotherapy: Moving
calreticulin to the cell surface converts apoptotic death from
‘silent’ to immunogenic. Cancer Res. 67:7941–7944. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen N, Fang W, Zhan J, Hong S, Tang Y,
Kang S, Zhang Y, He X, Zhou T, Qin T, et al: Upregulation of PD-L1
by EGFR activation mediates the immune escape in EGFR-driven NSCLC:
Implication for optional immune targeted therapy for NSCLC patients
with EGFR Mutation. J Thorac Oncol. 10:910–923. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mandai M, Hamanishi J, Abiko K, Matsumura
N, Baba T and Konishi I: Dual faces of IFNγ in cancer progression:
A role of PD-L1 induction in the determination of pro- and
antitumor immunity. Clin Cancer Res. 22:2329–2334. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sugiyama E, Togashi Y, Takeuchi Y, Shinya
S, Tada Y, Kataoka K, Tane K, Sato E, Ishii G, Goto K, et al:
Blockade of EGFR improves responsiveness to PD-1 blockade in
EGFR-mutated non-small cell lung cancer. Sci Immunol.
5(eaav3937)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Templeton AJ, McNamara MG, Seruga B,
Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106(dju124)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jin J, Yang L, Liu D and Li W: Association
of the neutrophil to lymphocyte ratio and clinical outcomes in
patients with lung cancer receiving immunotherapy: A meta-analysis.
BMJ Open. 10(e035031)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gooden MJ, de Bock GH, Leffers N, Daemen T
and Nijman HW: The prognostic influence of tumour-infiltrating
lymphocytes in cancer: A systematic review with meta-analysis. Br J
Cancer. 105:93–103. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hiramatsu S, Tanaka H, Nishimura J,
Sakimura C, Tamura T, Toyokawa T, Muguruma K, Yashiro M, Hirakawa K
and Ohira M: Neutrophils in primary gastric tumors are correlated
with neutrophil infiltration in tumor-draining lymph nodes and the
systemic inflammatory response. BMC Immunol. 19(13)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Petrie HT, Klassen LW and Kay HD:
Inhibition of human cytotoxic T lymphocyte activity in vitro by
autologous peripheral blood granulocytes. J Immunol. 134:230–234.
1985.PubMed/NCBI
|
|
30
|
Theelen W, Peulen HMU, Lalezari F, van der
Noort V, de Vries JF, Aerts J, Dumoulin DW, Bahce I, Niemeijer AN,
de Langen AJ, et al: Effect of pembrolizumab after stereotactic
body radiotherapy vs pembrolizumab alone on tumor response in
patients with advanced non-small cell lung cancer: Results of the
PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol.
5:1276–1282. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shaverdian N, Lisberg AE, Bornazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of non-small-cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
18:895–903. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li L, Lu G, Liu Y, Gong L, Zheng X, Zheng
H, Gu W and Yang L: Low infiltration of CD8+ PD-L1+ T cells and M2
macrophages predicts improved clinical outcomes after immune
checkpoint inhibitor therapy in non-small cell lung carcinoma.
Front Oncol. 11(658690)2021.PubMed/NCBI View Article : Google Scholar
|