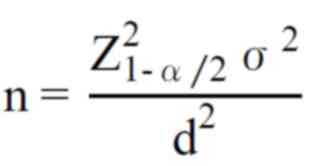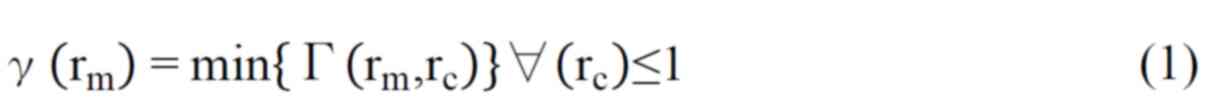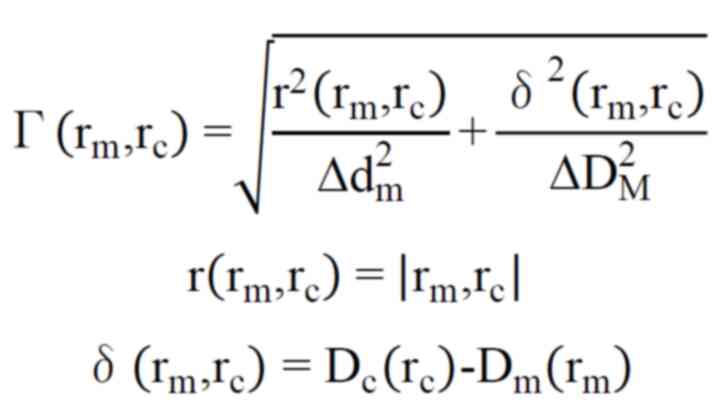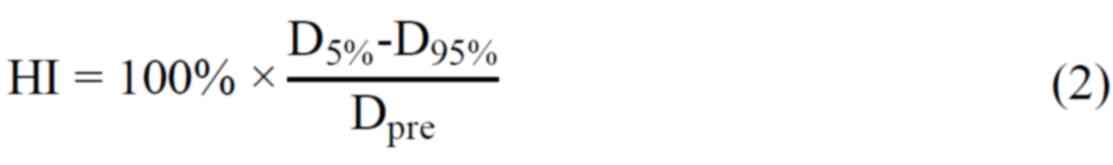Introduction
According to the World Health Organization report in
2018, cancer is the leading cause of death worldwide; of the 9.6
million people who succumbed to cancer in 2018, the number of
people who succumbed to colorectal cancer; colon occupied the
second place with 862,000 cases; and the five common types of
cancer were the following: lung with 2.09 million cases, breast
with 2.09 million cases, colorectal with 1.8 million cases, the
fourth position was prostate with 1.28 million cases and finally
the non-melanoma skin cancer occupied the 5th position (1,042,056
new cases) (1). In 2020, these
cancer's positions were 3rd and 4th (141259 and 1198073 new cases),
respectively (2). Thus, it was
found by the authors that the skin in the pelvis is more often
irradiated than the skin in other areas. Based on the team's 10
years of experience working with radiation therapy using
accelerators as well as reviewing a number of articles on skin care
for radiation therapy patients such as that of Samantha Bostock, in
the British Journal of Nursing issue 4, volume 25, 2016, it was
realized that the most sensitive skin areas on patients' body were
on the groin area, breast, and neck, respectively (3).
Even though there have been numerous studies on skin
care for radiation patients, only a few studies aim to reduce the
dose to the skin or evaluate the dose on the skin for radiation
patients. In addition, the pelvic skin is an active area, difficult
to keep clean, and prone to infection. The appearance of an acute
reaction on this skin area reduces the body's beauty, affects the
quality of life, and even leads to treatment interruption, reducing
treatment quality and there may also be a risk of developing skin
cancer (4,5).
Actually, during the treatment process, patients
treated with radiation therapy are often affected by the side
effects of radiation on their skin. The extent to which the skin
reacts to radiation varies: From erythema, dry peeling,
hyperpigmentation and purulent erosion. The degree of skin response
to radiation depends on numerous factors including the area of the
skin affected by radiation, type of irradiated energy, total
radiation dose and radiation dose division. The present study aimed
to identify the planning optimization of radiotherapy with dose
modulation in the pelvis to reduce the dose on skin.
Materials and methods
Materials
The Unique LINAC has 80 MLC leaves, and only 6 MV
photon energy. According to Varian guideline, Unique accelerator
technology allows radiation therapists to perform a variety of
radiotherapy techniques including: 2D, 3D, IMRT and RapidArc
treatment.
Eclipse 13.5, which is the treatment planning system
of accelerators, and brachytherapy machines, is developed by Varian
Corporation. The eclipse TPS has Anisotropic Analytical Algorithm
(AAA) for photons and Pencil Beam Convolution (PBC) Algorithm.
AAA algorithm is used to calculate the dose of these
IMRT plans. The algorithm is dosimetry for photon beams based on
dose superposition, which was released in 2005 by Varian Medical
System and is used in the Eclipse TPS (6). Complex technique plans are used in
modern radiotherapies including IMRT, RapidArc and Tomotherapy. A
plan may have more than one hundred control points including MLC
movement, gantry angle and collimator angle to induce dose
variation in the patient. Radiotherapy plans are becoming
increasingly complex and require great accuracy. It is extremely
important to control the quality of a plan before starting it.
Samples and sampling methods
The sample size is calculated with the following
formula:
With confidence interval 99% Standard
deviation σ=2,5; Error d=1. Hence the sample size is n=42. A total
of 45 patients (42% male, 58% female) were selected to participate
in the present study as presented in Table I (age range, 30-85 years; average
age, 62 years) from May 2018 to May 2020 at Hanoi Oncology Hospital
(Hanoi, Vietnam). The rate of disease stages T1, T2, T3, T4 are 9,
24, 36, and 31%, respectively. Since the sample size of 45 patients
was small, Fisher's exact test was used in data analysis, and the
data of this research were analyzed with Excel version 2013.
 | Table IThe information of the selected
patients. |
Table I
The information of the selected
patients.
| Patient | Birth year | Sex | Type of cancer | Stages |
|---|
| 1 | 1957 | Male | Rectum | T1N0M0 |
| 2 | 1936 | Male | Rectum | T4N0M0 |
| 3 | 1945 | Female | Rectum | T3N2M0 |
| 4 | 1950 | Male | Rectum | T4An0M0 |
| 5 | 1960 | Male | Rectum | T4N2M1 |
| 6 | 1944 | Female | Rectum | T3N0M0 |
| 7 | 1954 | Male | Rectum | T2bNxM0 |
| 8 | 1972 | Male | Rectum | T4bNxM1 |
| 9 | 1953 | Male | Rectum | T4aN2M0 |
| 10 | 1941 | Female | Rectum | T4aN1M1 |
| 11 | 1954 | Female | Rectum | T4N1M1 |
| 12 | 1949 | Female | Rectum | pT4aN2M0 |
| 13 | 1952 | Male | Rectum | T3N0M0 |
| 14 | 1958 | Female | Rectum | T3N1M0 |
| 15 | 1958 | Male | Rectum | T3N1M0 |
| 16 | 1966 | Male | Rectum | T2N0M0 |
| 17 | 1962 | Male | Rectum | T3N1M0 |
| 18 | 1950 | Male | Rectum | T3N0M0 |
| 19 | 1960 | Male | Rectum | PTaN0M0 |
| 20 | 1954 | Female | Rectum | T3N1M0 |
| 21 | 1967 | Male | Rectum | T3NxM0 |
| 22 | 1954 | Male | Rectum | cT4bNxM0 |
| 23 | 1957 | Female | Rectum | T4bN1Mx |
| 24 | 1951 | Male | Rectum | T4NxM0 |
| 25 | 1944 | Male | Rectum | T4N1M0 |
| 26 | 1935 | Female | Rectum | T4N2M0 |
| 27 | 1961 | Male | Rectum | cT3N0M0 |
| 28 | 1954 | Female | Rectum | T2Bn1M0 |
| 29 | 1985 | Female | Rectum | T3NxM0 |
| 30 | 1958 | Female | Rectum | T2bN9M0 |
| 31 | 1958 | Female | Cervix | T2bN0Mx |
| 32 | 1974 | Female | Cervix | T3N0M0 |
| 33 | 1948 | Female | Cervix | T3bN0M0 |
| 34 | 1959 | Female | Cervix | T2Bn0M0 |
| 35 | 1948 | Female | Cervix | T2bN1M0 |
| 36 | 1967 | Female | Cervix | T2bN0M0 |
| 37 | 1960 | Female | Cervix | T2bN0M0 |
| 38 | 1944 | Female | Cervix | T2bNxM0 |
| 39 | 1988 | Female | Cervix | T2N0M0 |
| 40 | 1972 | Female | Cervix | T3N0M0 |
| 41 | 1990 | Female | Cervix | T4N3M0 |
| 42 | 1960 | Female | Cervix | pT3N0M0 |
| 43 | 1969 | Female | Cervix | T1Bn0M0 |
| 44 | 1967 | Female | Cervix | T1bNxMx |
| 45 | 1978 | Female | Cervix | T3CN0M0 |
Methods
Step 1: A minimum of simulated CT images of 30
preoperative radiotherapy rectal cancer patients and a minimum of
simulated CT images of 15 cervical cancer patients were selected.
These image series, which were not scattered due to metal
artifacts, were contoured all Organ At Risk (OAR) around planning
target volume (PTV) according to the protocol (7). CT simulation image series were
contoured: GTV, CTV, PTV: u + nodes with prescribed dose: 50,4 Gy;
organs at risk: Volume of small intestine 15 Gy ≤120 ml or Dmax of
small intestine <115%; Dmax of bladder: <115% or Dmax <50
Gy and <50% of volume having dose ≥40 Gy; Dmax of femoral
<115% or Dmax <50 Gy and <10% of volume having dose ≥40
Gy; using 6 MV photon energy to make IMRT with Eclipse 13.5.
Step 2: IMRT plan was evaluated including D95% of
PTV; Dmax of plan <107%; 2D dose distribution; analyze Dose
Volume Histogram; homogeneity index (HI) and conformity index (CI);
dose of PTV; dose of OAR (8).
Step 3: The skin was released from the body contour
with depth of 5 mm; IMRT plan was copied in step 2 called Skin
IMRT. Skin IMRT were optimized with all optimization parameters of
IMRT except skin with priority is 60.
Step 4: IMRT was compared with Skin IMRT plan of
each patient in 2D distribution, dose of PTV, dose of OAR, dose
volume histograms (DVH), HI, CI, dose of skin; quality assurance
(QA) plan of IMRT plan and Skin IMRT plan was created with
Delta4.
Step 5: QA plan was implemented with Delta4. The
parameters of the software are distance to agreement (DTA) (3-mm),
dose difference (DD) (3%), and gamma index >95%. DTA 3-mm: The
acceptable interval between a point on the planned control result
and a point with the same absolute dose value on the calculated
result shall not be <3 mm (9).
DD 3%: The calculated absolute dose value of a point with the
measured absolute dose value should not be <3% (9). Gamma index is a combination of DTA
and DD. The gamma index value is used to compare dose distribution
between calculated and measured values. Gamma index is calculated
with the following formula (9).
Where rm is the point in the actual
measured dose distribution, and rc is the point in the
planned dose distribution; Δdm=3 mm; ΔDM=3%;
D(rc) is the absolute dose at rc;
D(rm) is the absolute dose at rm.
Collected dose of PTV includes: 3D maximum dose of
plan (Dmax), 3D maximum dose of PTV
(DmaxPTV), 3D minimum dose of PTV (DminPTV),
3D mean dose of PTV (DmeanPTV), D5% (the dose value at
5% of the PTV volume point on DVH line), D95% (the dose value at
95% of PTV volume point on DVH line), V95% is the volume of PTV
covered with 95% of the prescribed dose, and volume of PTV (VPTV).
The dose of OAR includes: Volume of small intestine gets 15 Gy;
maximum dose and mean dose of the small intestine, bladder, femur,
and skin.
HI has been defined as the following formula
(2): HI is used to evaluate the
uniformity of dose distribution over the PTV volume, the ideal
value of HI is zero. Therefore, the smaller the HI value is, the
more uniform the dose distribution on the PTV is (10).
Where Dpre: Prescribed dose of PTV. CI
has been defined as the following formula (3): This index relates to how well the 95%
isodose conforms to the shape of the PTV. CI ideal value is one.
The plan having CI to be near one has been proven improved than
other plans (10).
Results
Comparing 2D dose distribution
When analyzing plans, the dose distribution on slice
by slice between IMRT plan and Skin IMRT plan of each patient was
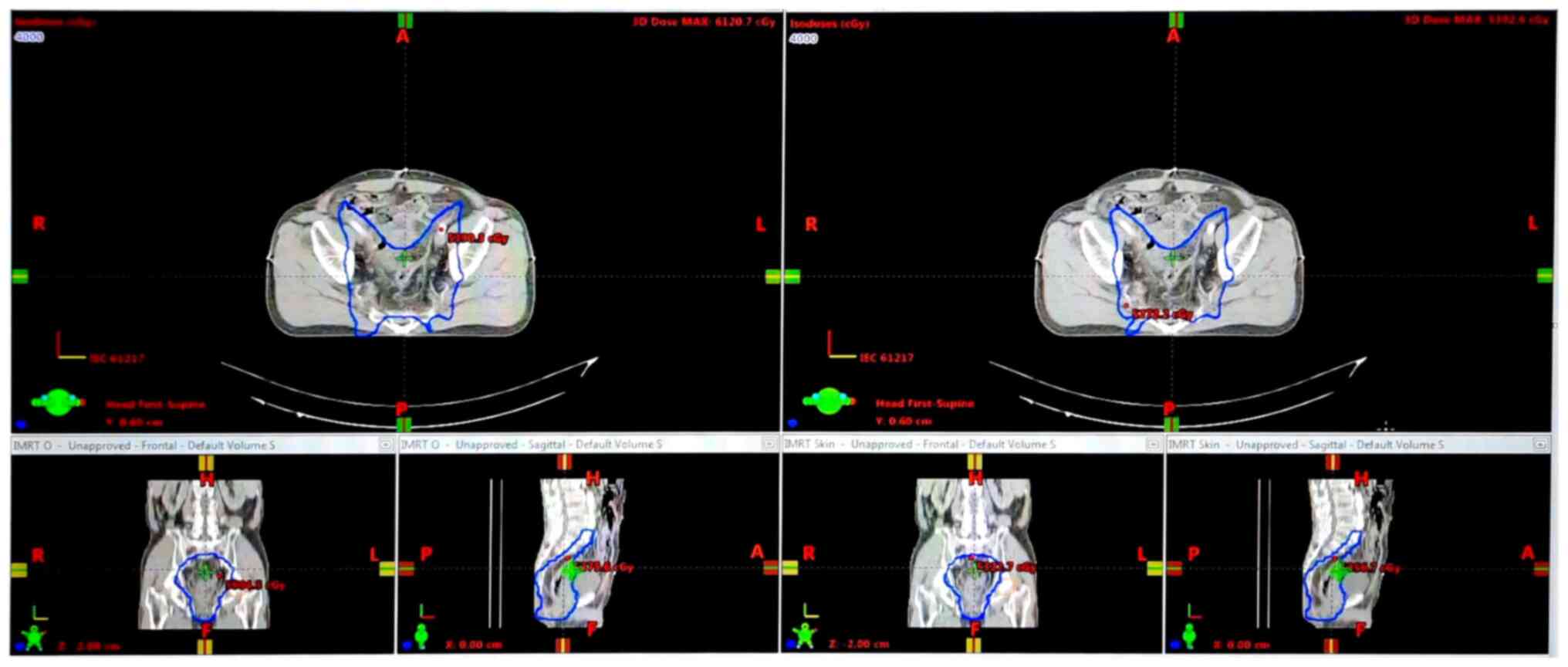
compared. In Fig. 1, the 40 Gy
isodose line is revealed on three images of IMRT plan (left side)
and on three images of Skin IMRT plan (right side).
Dose results of PTV
The relative maximum dose of IMRT and Skin IMRT
plan, the maximum and minimum absolute dose in both types of plan,
the number following the ± sign is a standard deviation are listed
in Table II; P-values were
calculated with Fisher's exact test, the sample size is 45. These
values are compared with the prescribed values.
 | Table IIMaximum dose of plan and dose results
of PTV. |
Table II
Maximum dose of plan and dose results
of PTV.
| | IMRT | Skin IMRT | |
|---|
| | Collected
value | Difference compared
with requested value (%) | Collected
value | Difference compared
with requested value (%) | The requested
values |
|---|
| Dmax
(%) | 108.85±3.15;
P=0.09 | 1.85 | 105.42±0.91;
P=0.31 | -1.58 | <107% |
| DmaxPTV
(cGy) | 5440.33±119.48;
P=0.0023 | 0.88 | 5305.44±48.62;
P=0.0057 | -1.62 | <5393 cGy |
| DminPTV
(cGy) | 3924.24±328.67;
P=0.0009 | -18,04 | 3828.82±347.24;
P=0.0009 | -20.03 | ≥4788 cGy |
| DmeanPTV
(cGy) | 5023.56±26.44;
P=0.011 | -0.33 | 4998.15±26.1;
P=0.01 | -0.83 | 5040 cGy |
As demonstrated in Table III, V95% of the IMRT plan is a
slightly higher than that of the Skin IMRT plan [865.75±379.81
cm3; (P=0.00072) vs. 847.79±370.5 cm3
(P=0.00074)], and both values are suitable with the requested
values. The number following the ± sign is a standard deviation,
P-values were calculated with Fisher's exact test, and the sample
size was 135 because on all the 45 DVHs of 45 plans three values
were received per each DVH. V105% of the Skin IMRT plan was smaller
and closer to the requested value than that of the IMRT plan. These
results mean both plans are matched with the requested plan in
terms of the dose of the PTV in plan, and the Skin IMRT had less
hotpot points than the IMRT plan.
 | Table IIIThe volume of PTV has dose larger
than 95% of the prescribed dose, and the volume has dose larger
than 105% of the prescribed dose in IMRT and Skin IMRT. |
Table III
The volume of PTV has dose larger
than 95% of the prescribed dose, and the volume has dose larger
than 105% of the prescribed dose in IMRT and Skin IMRT.
| | IMRT | Skin IMRT | The requested
values values |
|---|
| V95%
(cm3) | 865.75±379.81;
P=0.00072 | 847.79±370.5;
P=0.00074 | ≥84258.35 |
| V105%
(cm3) | 4.16±8.21;
P=0.041 | 0.24±0.689;
P=0.5 | 0 |
As shown in Table
IV HI and CI values of the IMRT plan were closer to the ideal
values than those of the Skin IMRT plan; the number following the ±
sign is a standard deviation, P-values were calculated using
Fisher's exact test and the sample size was 45. These results
revealed that the PTV of the IMRT plan covers improved and is more
uniform with 95% isodose line than that of the Skin IMRT plan.
 | Table IVHI, CI value of IMRT and Skin
IMRT. |
Table IV
HI, CI value of IMRT and Skin
IMRT.
| | IMRT | Skin IMRT | Ideal value |
|---|
| HI | 6.09±1.19;
P=0.25 | 6.46±1.34;
P=0.21 | 0 |
| CI | 0.976±0.017;
P=0.54 | 0.957±0.026;
P=0.529 | 1 |
Results dose value of OAR
The affection dose on OAR around PTV of both IMRT
and Skin IMRT plans were a little different (Table V). In the aforementioned table, the
number following the ± sign is a standard deviation, P-values were
calculated using Fisher's exact test, and the sample size was 135
because on all the 45 DVHs of 45 plans three values were received
per each DVH. Specifically, the affection dose on the small
intestine of the Skin IMRT plan was smaller than that of the IMRT
plan [2097.82±607.16 cGy (P=0.00046) compared with 2188.68±718.64
cGy (P=0.0004)]. This result indicated that Skin IMRT significantly
decreased the affection dose on the small intestine compared with
the value of IMRT plan.
 | Table VMaximum dose (Dmax) and mean dose
(Dmean) of organ at risk in IMRT and Skin IMRT. |
Table V
Maximum dose (Dmax) and mean dose
(Dmean) of organ at risk in IMRT and Skin IMRT.
| Types | | IMRT | Skin IMRT | The difference
(%) |
|---|
| Small
intestine | DmaxSI
(cGy) | 4791.29±505.7;
P=0.0006 | 4749.19±481.82;
P=0.0007 | -0.88 |
| | DmeanSI
(cGy) | 2188.68±718.64;
P=0.0004 | 2097.82±607.16;
P=0.00046 | -4.15 |
| Bladder | DmaxB
(cGy) | 4655.2±258.91;
P=0.001 | 4609.456±256.65;
P=0.0016 | -0.98 |
| | DmeanB
(cGy) | 2975.22±532.9;
P=0.0005 | 2953.95±497.11;
P=0.0006 | -0.71 |
| Left femur | DmaxLF
(cGy) | 4150.27±769.2;
P=0.0003 | 4051.89±447.69;
P=0.0004 | -2.37 |
| | DmeanLF
(cGy) | 2171.87±395.3;
P=0.0007 | 2169.41±373.9;
P=0.0007 | -0.11 |
| Right femur | DmaxRF
(cGy) | 4150.27±769.2;
P=0.0004 | 4198.92±664.1;
P=0.0004 | 1.17 |
| | DmeanRF
(cGy) | 2320.62±338.74;
P=0.0008 | 2349.72±330.72;
P=0.0009 | 1.25 |
Results of the QA plan and the machine
unit (MU) number of plans
As demonstrated in Table VI, DD, DTA, gamma index, and MU
numbers of both IMRT and Skin IMRT plans were quite similar. In the
aforementioned table, the number following the ± sign is a standard
deviation, P-values were calculated with Fisher's exact test, and
the sample size was 45. The results pointed out that the optimal
processing to reduce dose to skin doesn't affect the difference
between the calculated dose and measured dose, and the MU number of
the plans.
 | Table VIThe results of quality assurance
plan, and the MU number of IMRT and Skin IMRT. |
Table VI
The results of quality assurance
plan, and the MU number of IMRT and Skin IMRT.
| Type plan | IMRT | Skin IMRT |
|---|
| DD (%) | 96.08±1.63;
P=0.17 | 96.09±1.66;
P=0.17 |
| DTA (%) | 98.77±1.15;
P=0.25 | 98.87±0.89;
P=0.31 |
| Gamma index
(%) | 99.04±0.92;
P=0.33 | 99.09±0.78;
P=0.36 |
| MU | 1384.17±222.51;
P=0.001 | 1380.17±220.54;
P=0.001 |
The volume of skin gets 10, 20, 30, 40
and 50 Gy in IMRT and Skin IMRT plan
The skin volumes receiving doses of ≥10, ≥20, ≥30,
≥40 and ≥50 Gy of the Skin IMRT plan were all smaller than those of
the IMRT plan (Table VII). In
the aforementioned table, the number following the ± sign is a
standard deviation, P-values were calculated using Fisher's exact
test, and the sample size was 135 because on all the 45 DVHs of 45
plans three values per each DVH were received. The skin volume
receiving a dose of ≥10 Gy in Skin IMRT was 2.23% smaller compared
with this volume of the IMRT plan. Particularly, the skin volume
receiving doses ≥20, ≥30, ≥40 and ≥50 Gy of the SKIN IMRT plan
decreased significantly compared with that of the IMRT plan. The
reduction values were 8.76, 18.83, 46.84 and 100%, respectively.
Furthermore, the skin in SKIN IMRT plan was no longer affected by
the 50 Gy dose.
 | Table VIIThe volume of skin gets 10 Gy (V10
Gy), 20 Gy (V20 Gy), 30 Gy (V30 Gy), 40 Gy (V40 Gy) and 50 Gy (V50
Gy) in IMRT and Skin IMRT plan. |
Table VII
The volume of skin gets 10 Gy (V10
Gy), 20 Gy (V20 Gy), 30 Gy (V30 Gy), 40 Gy (V40 Gy) and 50 Gy (V50
Gy) in IMRT and Skin IMRT plan.
| | IMRT | SKIN IMRT | Reduction volume
(%) |
|---|
| V10Gy
(cm3) | 294.02±105.93;
P=0.0023 | 287.45±102.7;
P=0.0027 | -2.23 |
| V20Gy
(cm3) | 52.64±30.3;
P=0.009 | 48.03±28.85;
P=0.0098 | -8.76 |
| V30Gy
(cm3) | 11.79±11.33;
P=0.025 | 9.57±9.44;
P=0.029 | -18.83 |
| V40Gy
(cm3) | 1.9±3.45;
P=0.09 | 1.01±2.3;
P=0.15 | -46.84 |
|
V50Gy(cm3) | 0.046±0.18;
P=1.98 | 0 | -100 |
Discussion
Comparing dose distribution between the slice of
IMRT plans with the slice of Skin IMRT, the results revealed that
more isodose lines in IMRT plan cover on skin than those of Skin
IMRT, such as in Fig. 1; the 40 Gy
isodose line of IMRT overlaid more skin area in compared with that
of Skin IMRT plan.
The results of Tables
II, III, IV and V
pointed out that the IMRT was performed with more optimization to
decrease the effective dose on the skin, small intestine, bladder
and right femur. This optimization also decreased the hot pot of
plan, the maximum dose on PTV; however, the mean dose of PTV also
decreased. The decreasing in the mean dose of PTV could be
acceptable (from -0.33 to -0.83%). The results of Table VI pointed to the number MU of the
plan and the QA plan results of IMRT plan and Skin IMRT were the
same. It means that the optimization for decreasing the effective
dose of skin made decreasing the dose on other organs at risk,
however, the optimization did not affect the quality of the
plan.
There are numerous types of studies on the skin's
dose in radiotherapy (1-5,11-23)
including the previous study of Anscher (4), changes in the skin caused by
radiation may appear from the first days of irradiation. Acute
effects of radiation may occur within 6 weeks of radiation, while
late effects appear after radiation therapy from a few months to a
few years. The acute effects of radiation therapy are often
considered transient because cells are usually capable of
self-repairing. Late effects are usually long-lasting and are
likely to become more severe over time. The severity of acute and
late effects depends on radiation dose, duration of exposure, total
irradiated dose, and location of the irradiated skin. From the
presence and severity of acute effects on the skin, late effects
can be predicted. Late skin effects including fibrosis or necrosis
may occur with acute skin reactions. Side effects of radiotherapy
on the skin, both acute and late, are local and limited to the
irradiated site (5). Acute skin
reactions to radiation include erythema, dry desquamation,
hyperpigmentation and purulent exfoliation as shown in Table VIII (5). Not all patients develop an acute
reaction. However, it is possible to have different reactions
occurring simultaneously in the irradiation field.
 | Table VIIIAcute effects of radiation on the
skin (5). |
Table VIII
Acute effects of radiation on the
skin (5).
| Tissue
response | Onset/Duration | Clinical
presentation |
|---|
| Erythema | Onset within 4-14
days of first treatment (dose 10-30 Gy), peaks at 4-5 weeks.
Resolves 2-6 weeks after last treatment | Faint to brisk
redness that outlines treatment field. Intensifies as treatment
continues. Increased skin temperature, slight edema. |
| Dry
desquamation | As early as 3rd-4th
week (40 Gy), but typically by 5th-6th; earlier with accelerated RT
or chemotherapy. Resolves 3-4 weeks after completion of
treatment | Dryness, flaking,
and peeling often accompanied by itching, a layer of dry, dead,
dark skin can accumulate over part or all of the treatment field
and will eventually slough off. Mild pain |
|
Hyperpigmentation | As early as 2-3
weeks of standard fractionated radiation therapy, depending on
baseline skin pigmentation. Usually resolves 3 months-1 year
following completion of treatment; occasionally chronic | Tanned
appearance |
| Moist
desquamation | Following 40-50 Gy
or with trauma/excess friction, bolus material, or chemotherapy.
Recovery usually 2-6 weeks after completion of treatment | Bright erythema,
sloughing skin, exposed dermis, serous exudates and mucus oozing
from skin surface moderate pain |
Archambeau et al (5) described early and late skin changes
as dose-dependent and as a reflection of changes in cellular
components including the epidermis, dermis and blood vessels. In
terms of classifying the acute effects of radiation therapy on
cancer patients' skin, Cox et al (11) identified 5 levels: Grade 0, no
response; Grade 1 (mild erythema, dry scaling, hair loss and
decreased sweating); Grade 2 (moderate to strong erythema, patchy
exudative dermatitis and moderate edema); Grade 3 (exudative
dermatitis, in addition to skin folds and intense edema); and Grade
4 (ulcer, hemorrhage and necrosis) (11).
As pointed out in Table VII, the skin volume receiving a
dose of ≥10 Gy in Skin IMRT decreased by only 2.23% compared with
this volume of the IMRT plan. However, the skin volumes receiving
higher doses (20, 30, 40 and 50 Gy) sharply declined compared with
these volumes of the IMRT plan. As aforementioned in the method
section, Skin IMRT plans were optimized with all optimization
parameters of IMRT except skin with a priority was 60. This
priority is a little larger than the default priority value (50 is
the default value), and very smaller than the priority of PTV,
which was set 300. Thanks to these chosen priorities the quality of
plans was not affected, but the skin volume receiving high dose was
sharply decreased.
Comparing the results of Table VII with the results of Table VIII, it can be observed that if
using the Skin IMRT plan in treatment, patients' skin is less
affected by the high dose, and skin symptoms including dryness,
flaking, scaly often with exudate, a layer of dry, dead, dark skin
thickens during treatment and may peel off, moderate pain, tanning
erythema, skin death, severe exudate, oozing from the skin surface,
moderate pain, were significantly reduced. Radiation induced skin
reactions were limited to moderate and mild erythema. The
significant reduction of clinical symptoms will help enhance the
quality of treatment and improve the patients' quality of life.
In conclusion, the present study identified the
parameters to optimize the dose reduction effect on the skin in the
pelvic IMRT plan. The dermal dose optimized plan has reduced the
dose of dermal effects compared with the original plan.
Particularly, the skin in the dermal dose optimization plan is no
longer affected by the 50 Gy dose. Optimizing the dose reduction
effect on the skin also contributes to reducing the average dose on
the small intestine, and the planned hotpot but still maintaining
the dose of PTV.
Acknowledgements
The authors would like to thank Dr Nguyen Thanh Hang
from International Cooperation and Scientific Research Unit of
Hanoi Oncology Hospital, for checking English language of the
manuscript. The research team would like to thank all doctors of
Radiotherapy Department, Hanoi Oncology Hospital for their support
during this research.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QBV purposed, designed the research, collected data
and analyzed data, and reviewed and edited the final manuscripts.
SDQ designed collecting data's protocol, collected and analyzed
data, wrote the draft of the manuscript and prepared the documents
for submission. THV, TV and TPT collected data and reviewed the
manuscript. QBV and SDQ confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval nos.
629/QĐ-BVUB and 2088/QĐ-BVUB) and sponsored by Hanoi Oncology
Hospital (Hanoi, Vietnam).
Patient consent for publication
All participants were explained and informed about
the study, and oral informed consent was provided by all patients
for participation in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bostock S and Bryan J:
Radiotherapy-induced skin reactions: Assessment and management. Br
J Nurs. 25(S18, S20-4)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Anscher MS: Radiation Toxicity-A Practical
Guide. Int J Radiat Oncol Biol Phys. 70(1611)2008.
|
|
5
|
Archambeau JO, Pezner R and Wasserman T:
Pathophysiology of irradiated skin and breast. Int J Radiat Oncol
Biol Phys. 31:1171–1185. 1995.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Varian Medical Systems. Inc: Eclipse
Algorithms Reference Guide. Palo Alto, CA, 2009.
|
|
7
|
Lee NY, Riaz N and Lu JJ: Target Volume
Delineation for Conformal and Intensity-Modulated Radiation
Therapy. Springer International Publishing, Cham, 2015.
|
|
8
|
International Commission on Radiation
Units and Measurements: Prescribing, recording, and reporting
photon beam therapy. Internat. Comm. on Radiation Units and
Measurements, Bethesda, MD, 1993.
|
|
9
|
Li H, Dong L, Zhang L, Yang JN, Gillin MT
and Zhu XR: Toward a better understanding of the gamma index:
Investigation of parameters with a surface-based distance method.
Med Phys. 38:6730–6741. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kataria T, Sharma K, Subramani V,
Karrthick KP and Bisht S: Homogeneity Index: An objective tool for
assessment of conformal radiation treatments. J Med Phys.
37:207–213. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation Therapy Oncology Group (RTOG) and the
European organization for research and treatment of cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hollinworth H and Mann L: Managing acute
skin reactions to radiotherapy treatment. Nurs Stand. 24:53–54, 56,
58 passim. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pires AM, Segreto RA and Segreto HR: RTOG
criteria to evaluate acute skin reaction and its risk factors in
patients with breast cancer submitted to radiotherapy. Rev Lat Am
Enfermagem. 16:844–849. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lavery BA: Skin care during radiotherapy:
A survey of UK practice. Clin Oncol (R Coll Radiol). 7:184–187.
1995.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McQuestion M: Evidence-Based skin care
management in radiation therapy. Semin Oncol Nurs. 22:163–173.
2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schratter-Sehn AU, Brinda K, Kahrer M and
Novak M: Improvement of skin care during radiotherapy. Onkologie.
24:44–46. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Salvo N, Barnes E, van Draanen J, Stacey
E, Mitera G, Breen D, Giotis A, Czarnota G, Pang J and De Angelis
C: Prophylaxis and management of acute radiation-induced skin
reactions: A systematic review of the literature. Curr Oncol.
17:94–112. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wei J, Meng L, Hou X, Qu C, Wang B, Xin Y
and Jiang X: Radiation-induced skin reactions: Mechanism and
treatment. Cancer Manag Res. 11:167–177. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
D'Haese S, Bate T, Claes S, Boone A,
Vanvoorden V and Efficace F: Management of skin reactions during
radiotherapy: A study of nursing practice. Eur J Cancer Care
(Engl). 14:28–42. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Harper JL, Franklin LE, Jenrette JM and
Aguero EG: Skin toxicity during breast irradiation: Pathophysiology
and management. South Med J. 97:989–993. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hymes SR, Strom EA and Fife C: Radiation
dermatitis: Clinical presentation, pathophysiology, and treatment
2006. J Am Acad Dermatol. 54:28–46. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Roy I, Fortin A and Larochelle M: The
impact of skin washing with water and soap during breast
irradiation: A randomized study. Radiother Oncol. 58:333–339.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schreck U, Paulsen F, Bamberg M and Budach
W: Intraindividual comparison of two different skin care
conceptions in patients undergoing radiotherapy of the
head-and-neck region. Creme Or Powder? Strahlenther Onkol.
178:321–329. 2002.PubMed/NCBI View Article : Google Scholar
|